The aim of this position statement is to provide health professionals with an updated and evidence-based guideline for the pharmacologic management of irritable bowel syndrome (IBS) in Mexico.
Material and methodsA literature review was conducted that included relevant guidelines and studies, up to the date of its publication. The mechanism of action, specific indications in IBS, safety profile, and availability of each therapeutic class were evaluated. The recommendations were developed by 14 experts, considering the clinical reality of IBS patients in Mexico.
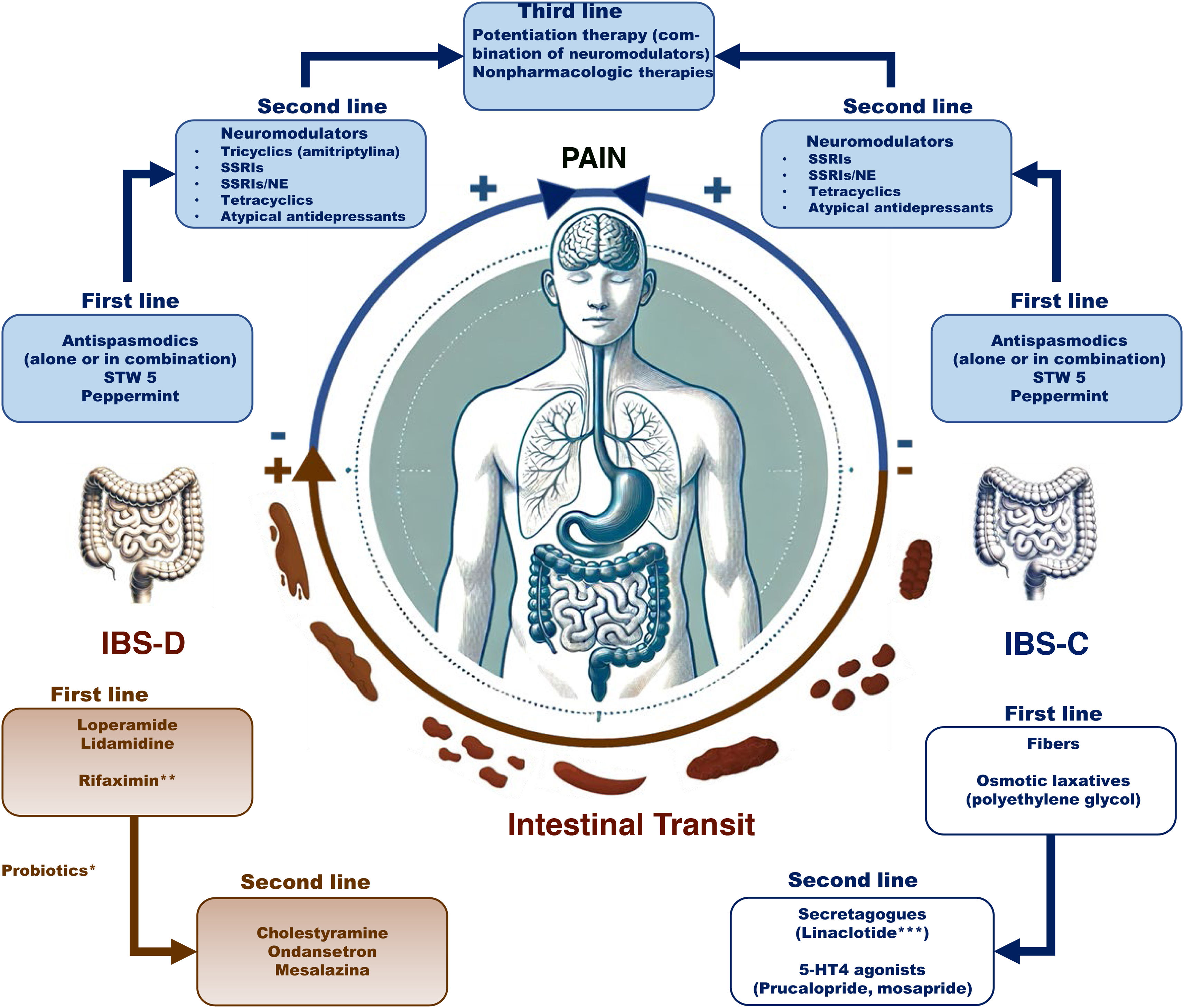
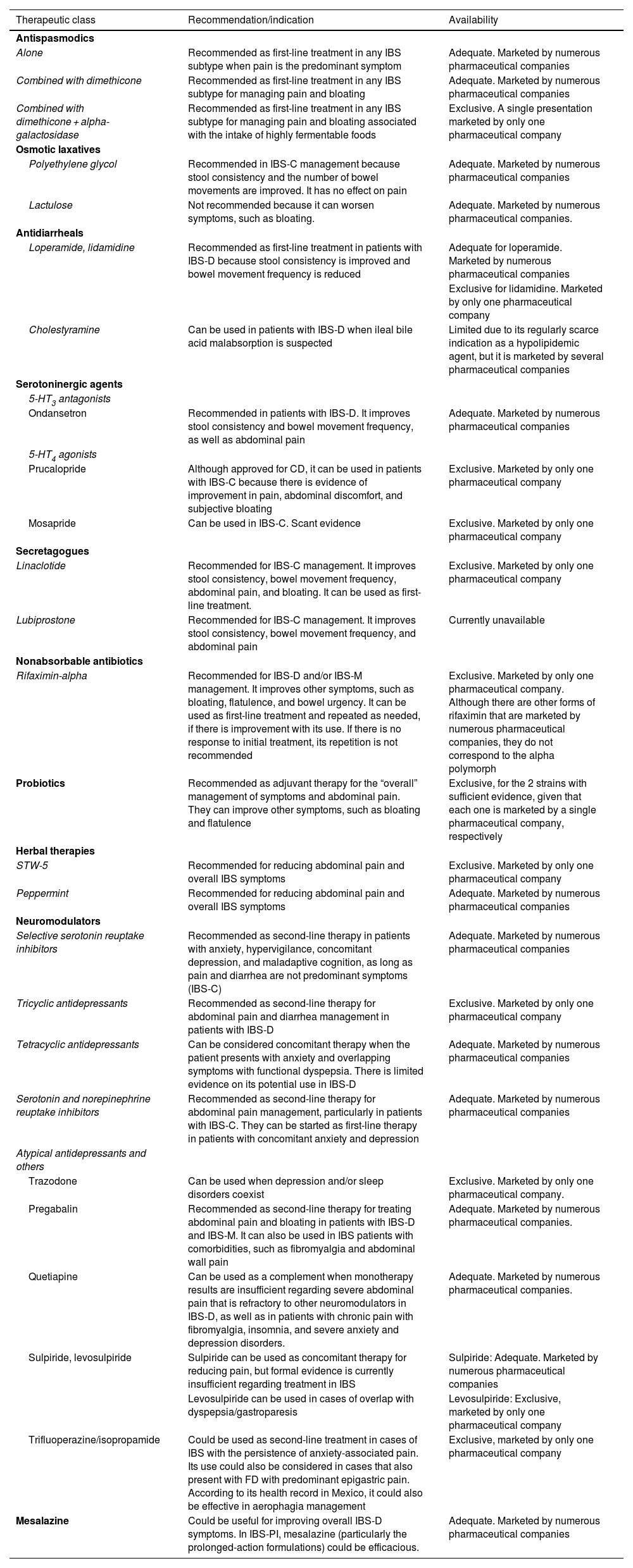
ResultsSpecific recommendations were issued for each class. Antispasmodics (alone or combined) are used as first-line therapy for pain management, whereas antidiarrheals, such as loperamide, are used for reducing diarrhea in diarrhea-predominant IBS (IBS-D) and laxatives are used for constipation in constipation-predominant IBS (IBS-C). 5-HT4 agonists (prucalopride and mosapride) are recommended in IBS-C and 5-HT3 antagonists (ondansetron) are recommended in IBS-D. Linaclotide is the only secretagogue available in Mexico and is used in IBS-C. Rifaximin-alpha stands out for its efficacy in a subgroup of patients with IBS-D or mixed IBS. Probiotics are conditionally recommended as adjuvant therapy due to heterogeneous evidence. Neuromodulators (tricyclic antidepressants, selective serotonin reuptake inhibitors, etc.) are recommended as second-line treatment for pain management. Mesalazine can be used in IBS-D, but the corresponding evidence is weak.
ConclusionOverall, these recommendations provide a solid framework for personalizing treatment, based on the clinical characteristics of the Mexican patient with IBS.
El objetivo de este posicionamiento es proporcionar a los profesionales de la salud una guía actualizada y basada en evidencia para el manejo farmacológico del síndrome del intestino irritable (SII) en México.
Material y métodosSe realizó en una revisión de la literatura, incluyendo guías y estudios relevantes hasta la fecha de su publicación. En cada clase terapéutica se evalúo su mecanismo de acción, indicaciones específicas en SII, perfil de seguridad y disponibilidad. Las recomendaciones fueron desarrolladas por 14 expertos, considerando la realidad clínica de los pacientes con SII en México.
ResultadosSobre cada clase se emitieron recomendaciones específicas. Los antiespasmódicos (solos o en combinación) se usan como primera línea para el manejo del dolor, mientras que los antidiarreicos, como la loperamida, para reducir la diarrea en SII con diarrea (SII-D) y los laxantes para el estreñimiento en SII con esta variedad (SII-E). Los agonistas 5-HT4 (prucaloprida y mosaprida) se recomiendan en SII-E y los antagonistas 5-HT3 (ondansetrón) en SII-D. El único secretatogogo disponible en México es linaclotida y se usa en SII-E. La rifaximina alfa destaca por su eficacia en un subgrupo de pacientes con SII-D o Mixto. Los probióticos son recomendados como adyuvantes y de manera condicional debido a la evidencia heterogénea. Los neuromoduladores (tricíclicos, inhibidores de recaptura de serotonina, etc.) son recomendados como segunda línea para el manejo del dolor. Aunque se puede utilizar mesalazina en SII-D, la evidencia es débil.
ConclusiónEn conjunto, estas recomendaciones proporcionan un marco sólido para la personalización del tratamiento en función de las características clínicas del paciente mexicano con SII.
Irritable bowel syndrome (IBS) is a benign disorder of gut-brain interaction (DGBI), with episodes of exacerbation and remission, that affects quality of life and is characterized by the presence of abdominal pain related to altered stool frequency and consistency.1 Traditionally, it had been considered a “systemic functional disorder” by not being associated with structural or biochemical alterations, but distinct pathophysiologic mechanisms that can explain the symptomatology are currently recognized to be involved, greatly aiding in its more accurate treatment.2
In Mexico, the prevalence of IBS is estimated at between 4 and 35%, making it one of the most common gastrointestinal diseases in the population.3–5 Its pharmacologic treatment has been the subject of continuous research and debate. There is a wide range of pharmacologic options in our country, with varying degrees of access and availability in recent years. The aim of this position statement is to provide healthcare professionals with a practical, up-to-date, evidence-based guideline for the pharmacologic management of IBS in Mexico, addressing relevant aspects, such as the efficacy of different drugs, their safety profiles, recommendations for their use, and the limitations regarding drug accessibility.
Material and methodsThe present position statement was jointly requested by the 2023 Scientific Committee and the Board of Directors of the Asociación Mexicana de Gastroenterología (AMG), to provide timely guidance on a theme with great clinical importance for the members of the AMG. The proposal to carry out a didactic, detailed, and complete review of the pharmacologic treatment for IBS available in Mexico was made, designating 2 coordinators (ECA and JMRT), who then summoned 12 experts in the area of IBS treatment. The work was divided into 14 sections, according to the therapeutic classes available for IBS management. The author of each section carried out a cross-database search (up to June 2024) in PubMed and IMBIOMED of the terms related to the drugs for managing IBS. The corresponding mechanisms of action, indications, clinical evidence, adverse events, and availability in Mexico were provided in each section.
The participants were summoned on July 1, 2023, and a virtual meeting was held on July 10 to explain the work process. Within a 3-month period, the authors sent the material to the coordinators, who organized it to be presented at a face-to-face meeting on November 16, 2023. At said meeting, the material collected was discussed in each section, deciding upon the content of the present document. The most relevant information in each section is described below.
AntispasmodicsAntispasmodics, also called spasmolytics, are a group of medications that have traditionally been used for managing pain in IBS.6,7
Mechanisms of actionAntispasmodics are divided into several subgroups, according to their chemical structure and mechanism of action:
- a)
Direct smooth muscle relaxants (e.g., mebeverine, trimebutine, derivatives of papaverine)
- b)
Anticholinergic scopolamine derivatives (e.g., butylhyoscine, hyoscine, levsin, hyoscyamine, dicycloverine, cimetropium bromide, propantheline bromide, butylscopolamine)
- c)
Ammonia derivatives (e.g., otilonium bromide, prifinium bromide)
- d)
Calcium antagonists (pinaverium bromide, alverine citrate, fenoverine, rociverine, pirenzepine, mint or peppermint oil).7–13
Some agents can have several mechanisms of action by acting on one or more receptors, presenting a calcium antagonist, anticholinergic, antimuscarinic effect, or having an effect on 5-hydroxytryptamine (5-HT) receptors. For the majority of these molecules, the exact mechanism of action is not completely established, but they are thought to have mixed mechanisms of action. The typical example is otilonium bromide, a quaternary amine with a calcium antagonist effect that prevents excessive intestinal contractions. However, it interferes with muscarinic responses and tachykinin receptors, which results in a motor modulation effect but also one in which antinociceptive properties have been described. Another example is peppermint oil, which, in addition to its antispasmodic effect, has other mechanisms, and so is discussed in the “herbal therapies” section of this review.14–16
IndicationsThey are recommended in any IBS subtype (diarrhea: IBS-D, constipation: IBS-C, mixed: IBS-M), when pain is the predominant symptom. Due to the anticholinergic effects, some antispasmodics (e.g., calcium antagonists) can induce changes in bowel habit toward constipation, and so could be of greater use in IBS-D, as well as in IBS-M.16–18
Clinical evidenceThe effectiveness of antispasmodics has been evaluated in open studies and controlled clinical trials (CCTs). At least 7 systematic reviews and meta-analyses have been published that evaluate the utility of antispasmodics in combination in IBS.17,19–24 In the Cochrane meta-analysis,21 antispasmodics as a group were superior in abdominal pain improvement, (p < 0.001) and overall symptom improvement (p < 0.001), with a therapeutic gain of 12% (58 vs 46%) and a number needed to treat (NNT) of 7 for abdominal pain, 5 for overall improvement, and 3 for symptom score improvement. Other meta-analyses have reported the NNT for each antispasmodic: otilonium bromide 5; pinaverium bromide 4; hyoscine butylbromide 3; cimetropium bromide 3; dicyclomine 4; alverine 4; and mebeverine 5.17,20 On the other hand, the number needed to harm (NNH) was 17.5,20 In a Mexican meta-analysis that included 23 studies and 2,585 patients, antispasmodics were superior to placebo in overall improvement (odds ratio [OR] 1.55, 95% confidence interval [CI] 1.33-1.83) and pain (OR 1.52, 95% CI 1.28-1.80).22 In that study, otilonium bromide and the combination of alverine with simethicone were significantly associated with overall improvement, whereas pinaverium bromide with simethicone was associated with bloating improvement. Lastly, a network meta-analysis published 3 years ago ranked antispasmodics as a group in second place, behind tricyclic neuromodulators, in terms of pain improvement at 4-12 weeks.24
In the latest AMG consensus on IBS, antispasmodics were assigned an A1 evidence level and a strong recommendation in favor of the intervention.25
Adverse eventsEven though antispasmodics are prescribed as safe drugs, the most common adverse effects are those related to their anticholinergic effect (dry mouth, dizziness, blurry vision). Fenoverine has been reported to cause rhabdomyolysis, and so given the broad availability of antispasmodics, opting for those with a better safety profile is recommended.26
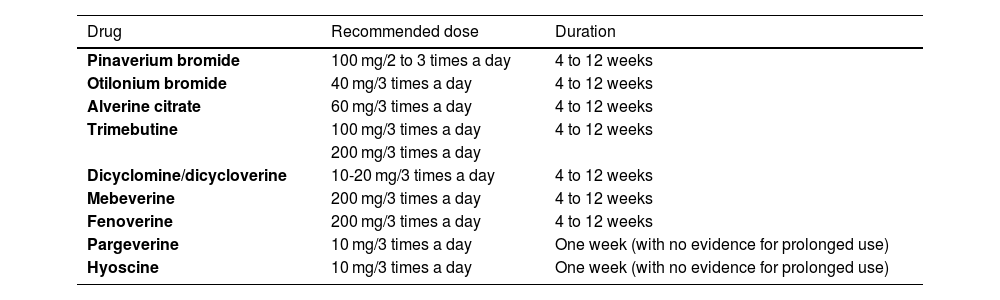
Availability, recommended dose, and treatment durationTable 1 shows the antispasmodics available in Mexico and their recommended doses. The duration of treatment with antispasmodics is initially at least 4 weeks. However, they can be prescribed for 8 to 12 weeks and there is evidence suggesting that some (e.g., otilonium, pinaverium) can be effective for up to 15 weeks.
Antispasmodics available in Mexico for IBS management
| Drug | Recommended dose | Duration |
|---|---|---|
| Pinaverium bromide | 100 mg/2 to 3 times a day | 4 to 12 weeks |
| Otilonium bromide | 40 mg/3 times a day | 4 to 12 weeks |
| Alverine citrate | 60 mg/3 times a day | 4 to 12 weeks |
| Trimebutine | 100 mg/3 times a day | 4 to 12 weeks |
| 200 mg/3 times a day | ||
| Dicyclomine/dicycloverine | 10-20 mg/3 times a day | 4 to 12 weeks |
| Mebeverine | 200 mg/3 times a day | 4 to 12 weeks |
| Fenoverine | 200 mg/3 times a day | 4 to 12 weeks |
| Pargeverine | 10 mg/3 times a day | One week (with no evidence for prolonged use) |
| Hyoscine | 10 mg/3 times a day | One week (with no evidence for prolonged use) |
IBS: irritable bowel syndrome.
For the purpose of improving associated symptoms (e.g., bloating, flatulence, etc.) or favoring carbohydrate digestion, some antispasmodics are administered in combination with antifoaming agents, such as simethicone, or with enzymes, such as alpha-galactosidase. From this point on, we will refer to antispasmodics in combination, as those combined with simethicone or enzymes, but first clarifying that even though in Mexico antispasmodics in combination with anti-inflammatory agents (e.g., with lysine clonixinate) are available, we do not recommend their use in IBS.
Mechanisms of actionSimethicone, or activated dimethicone, is a mixture of dimethicone (dimethylpolysiloxane) and silicon dioxide. This compound has antifoaming properties, and so reduces the superficial tension of mucus and gas bubbles, causing their coalescence. In animal models, it has also been shown to reduce stress-induced colonic paracellular permeability.27,28 Due to its exclusive effect on the intestinal lumen, it is considered a very safe drug.
On the other hand, alpha-D-galactosidase (an enzyme that decomposes different nonabsorbable oligosaccharides), also called agalsidase alpha or melibiase, is an enzyme derived from the selective fermentation of the Aspergillus niger fungus that hydrolyzes terminal alpha-galactosyl groups from glycolipids and glycoproteins.29 This enzyme hydrolyzes 3 complex carbohydrates – raffinose, stachyose, and verbascose – and converts them into the monosaccharides, glucose, galactose, and fructose, as well as into the disaccharide, sucrose, which are easily absorbed.30 These complex sugars are thus prevented from arriving at the colon, where fermentation by gas-producing bacteria occurs, and so, theoretically, the symptoms of bloating and flatulence are prevented.31
IndicationsCombinations with simethicone are recommended in any IBS subtype, when in addition to pain, the patient has associated bloating. The combination with alpha-D-galactosidase is recommended when, in addition to pain, the patient has bloating and other gas-related symptoms (e.g., flatulence), especially if abundant highly fermentable carbohydrates have been consumed.
Clinical evidenceCurrently, the antispasmodics that have been combined with simethicone are pinaverium bromide, alverine citrate, trimebutine, and more recently, mebeverine. Pinaverium bromide with simethicone has been studied in Mexico. A CCT conducted for 12 weeks that included 285 patients found that said combination was superior to placebo for improving pain and subjective bloating in patients that met the Rome III criteria for IBS.32 It also reported improvement in stool consistency, particularly in IBS-C and IBS-M. Another CCT conducted for 4 weeks that included 412 patients that met the Rome III criteria for IBS showed that the combination of alverine citrate with simethicone was superior to placebo for improving overall symptoms, such as controlling pain and bloating.33 A third study compared 2 strategies: the use of on-demand alverine with simethicone versus conventional treatment prescribed by first-contact physicians. The results showed that quality of life at 6 months was superior in the patients that received alverine with simethicone.34 The NNT for overall symptom improvement with the alverine/simethicone combination was 8.22
Trimebutine maleate combined with simethicone, alone, and with simethicone and alpha-D-galactosidase, is marketed in Mexico. Alpha-D-galactosidase, alone, has been shown to improve IBS symptoms. For example, in a study on 125 patients with IBS that received alpha-D-galactosidase (400 units of galactosidase [Ga] IU 3 times a day [TID]) or placebo with meals, for 12 weeks, showed that the enzyme had a tendency to more prominently reduce symptoms.35 In another study, Tuck et al.36 administered alpha D-galactosidase at a dose of 300 Ga IU TID, or 150 Ga IU TID, or placebo to 31 subjects with IBS that were hydrogen producers in breath tests, as they ate a high oligosaccharide diet for 3 days. The addition of foods with a high oligosaccharide content resulted in a significant increase in general symptoms, with 21 patients presenting with sensitivity to those foods (increase > 10 mm for general symptoms). Of those patients, a complete dose of the enzyme reduced general symptoms (p = 0.006) and bloating (p = 0.017).
A crossover trial was recently conducted in Mexico, in which patients with functional abdominal distension (some with IBS symptoms) and controls underwent a diet rich in fermentable foods and then randomly received one tablet of trimebutine + simethicone + alpha-D-galactosidase (154 mg/75 mg/450 Ga IU) or placebo.37 The study showed that the triple combination significantly prevented objective abdominal distension and reduced the intensity of flatulence and burping in the control subjects. The combination reduced pain intensity in the patients with abdominal distension.
Lastly, the combination of mebeverine with simethicone is the most recently available combination in Mexico, but there is not yet any clinical evidence on it.
Adverse eventsIn some cases, high doses of simethicone are associated with diarrhea. No serious adverse events have been described regarding simethicone or alpha-D-galactosidase.
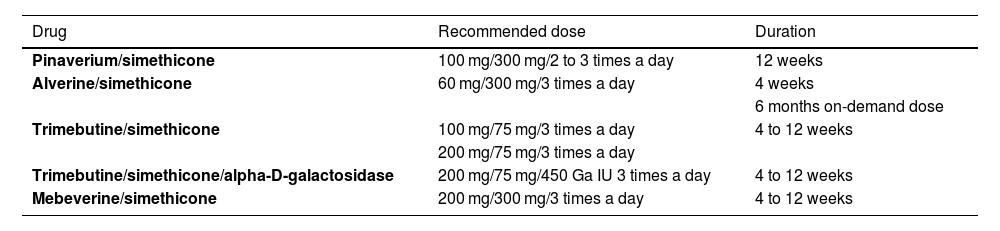
Availability, recommended dose, and treatment durationTable 2 shows availability in Mexico and how the combinations are prescribed.
Antispasmodic combinations available in Mexico for IBS management
| Drug | Recommended dose | Duration |
|---|---|---|
| Pinaverium/simethicone | 100 mg/300 mg/2 to 3 times a day | 12 weeks |
| Alverine/simethicone | 60 mg/300 mg/3 times a day | 4 weeks |
| 6 months on-demand dose | ||
| Trimebutine/simethicone | 100 mg/75 mg/3 times a day | 4 to 12 weeks |
| 200 mg/75 mg/3 times a day | ||
| Trimebutine/simethicone/alpha-D-galactosidase | 200 mg/75 mg/450 Ga IU 3 times a day | 4 to 12 weeks |
| Mebeverine/simethicone | 200 mg/300 mg/3 times a day | 4 to 12 weeks |
IBS: irritable bowel syndrome.
Laxatives are foods or drugs that, upon consumption, directly act on the intestine to increase stool frequency and facilitate bowel movements by improving stool consistency. In IBS management, not all laxatives are indicated, nor is there sufficient evidence for recommending their use. For example, stimulants are recommended for acute or occasional constipation, but not for IBS. Even though dietary fibers have a laxative effect as bolus formers, in the present document, our particular focus is on the use of osmotic laxatives (polyethylene glycol and lactulose).
Mechanisms of actionPolyethylene glycol (macrogol 3350) is a nonabsorbable, highly soluble, synthetic polymer that does not produce salt absorption (in contrast to polymer 4000 of this compound), is not toxic in large quantities, and can produce an osmotic effect, retaining water in the intestinal lumen, increasing stool volume and bowel transit.38
Lactulose is a synthetic disaccharide that is undigestible in the gastrointestinal tract. It arrives undigested in the colon and through the process of fermentation, colonic acidification is produced, creating an irritative effect with the potential to promote colonic contractility. The most well-known effects of its action are osmotic retention, stool hydration, and bowel transit acceleration.39 Its action is expected to start in 24-48 h.
IndicationsPolyethylene glycol is indicated for the management of constipation associated with IBS-C, but it has no effect on abdominal pain. Lactulose is indicated for the management of chronic constipation, but it can also be used in the management of constipation associated with IBS-C. However, because it is a nonabsorbable disaccharide, it can produce bloating, thus worsening symptoms. In addition, it is important to state that there is no evidence for recommending stimulating laxatives or emollients in the treatment of patients with IBS-C, but they may be used as rescue therapy.
Clinical evidenceThere is a high level of evidence (grade 1A) supporting macrogol 3350 in the treatment of chronic constipation.40 Nevertheless, clinical evidence on the use of macrogol 3350 in IBS-C is scarce and comes from a single 4-week clinical trial. Said trial compared macrogol 3350 with placebo and its primary endpoint was the mean number of bowel movements. Macrogol increased the number of bowel movements per week compared with placebo (4.40 ± 2.5; placebo, 3.11 ± 1.9, p < 0.0001). There were slightly lower values of pain/bloating in the patients that used macrogol, but they were not statistically significant. Abdominal pain and diarrhea were the most frequent adverse events.41 Given the above, we conclude that macrogol 3350 can be used in the treatment of IBS-C.
On the other hand, there are no studies with adequate quality regarding the use of lactulose in patients with IBS-C.
Adverse eventsMacrogol 3350 is well-tolerated and is associated with mild-to-moderate adverse effects, compared with placebo (38.8 vs 32.9%, respectively), that sometimes lead to treatment discontinuation. The most frequent adverse effects are diarrhea, bloating, and abdominal pain.41 No severe adverse effects with the use of macrogol 3350 have been reported.
Availability, recommended dose, and treatment durationIn Mexico, there are 2 presentations of macrogol 3350 for the treatment of chronic constipation. One form is in packets with 17 grams of powder that are dissolved in a glass of water, titrating the dose to patient requirements. Starting with one dose per day and increasing it to 3 times a day is recommended. The other form is in a bottled powder, with which varying quantities can more adequately be used for titrating the dose, in situations in which the patient requires a higher or lower dose to get a response. Evidence of response in IBS-C is at 4 weeks, and long-term safety (52 weeks) has also been shown in chronic constipation. Lactulose is available in suspension, and a commercial form of lactulose plus paraffin is also available. The recommended dose is 1-3 tablespoons a day.
AntidiarrhealsThe use of these medications can benefit some patients, especially in improving stool frequency and consistency.
Mechanisms of actionLoperamide is a synthetic peripheral µ opioid receptor agonist that inhibits peristalsis and antisecretory activity and increases bowel transit time with limited penetration of the blood-brain barrier.42 Lidamidine is an antidiarrheal that acts as an alpha-2 adrenergic receptor agonist, thus inhibiting intestinal secretion and modifying bowel transit time.43 Cholestyramine is insoluble and is not absorbed by the gastrointestinal tract. Its mechanism of action consists of interchanging chloride ions with carboxyl groups of bile acids in the small bowel, binding to them and interfering in their reabsorption by the enterohepatic circulation, which is why it is used as a bile acid sequestrant, in this way forming ion complexes that are excreted in the stool.44
IndicationsLoperamide and lidamidine are indicated in patients with IBS-D to decrease stool frequency and improve stool consistency. Cholestyramine can be used in patients with IBS-D with suspected ileal bile acid malabsorption.45
Clinical evidenceLoperamide: CCTs have been published that evaluate the efficacy of loperamide in patients with IBS-D, but they have few patients and utilize old criteria.46,47 Compared with placebo, loperamide was associated with adequate abdominal pain relief (relative risk [RR] 0.41; 95% CI 0.2-0.84), stool consistency improvement (RR 0.06; 95% CI 0.01-0.43), and overall symptom improvement (RR 0.73; 95% CI 0.29-1.86). No improvement has been reported, with respect to urgency symptoms, and there is no information about the impact on quality of life. Therefore, clinical evidence with the use of loperamide is very low.
Lidamidine: The efficacy of lidamidine in IBS-D is evaluated in some published CCTs. One of them is a cross-over trial on 72 patients with IBS. One group underwent a 2-week washout phase and then were randomized to receive 8 mg/day of lidamidine for 2 weeks, after which the dose was increased to 16 mg/day; the other group received placebo. The groups were then switched.48 The results showed no benefit from lidamidine in IBS-D regarding improvement in stool frequency and consistency or in overall symptoms. A double-blind clinical trial controlled with placebo conducted in Mexico many years ago included 40 patients with normal Manning criteria, ova and parasite exam, rectosigmoidoscopy, and barium enema results. They were randomly placed into 4 treatment groups: lidamidine with group psychotherapy, lidamidine without group psychotherapy, placebo with group psychotherapy, and placebo without group psychotherapy for 6 weeks, after which the groups were switched.49 Thirty-eight patients had a favorable response: 97% that received lidamidine alone, 68.4% that received placebo alone, 84.3% that received lidamidine and psychotherapy, and 63.2% that received placebo and psychotherapy. The difference with and without psychotherapy was not statistically significant. Overall, response was better with lidamidine than with placebo (89.5 vs 65.8%, p = 0.02). Thus, we conclude that the clinical evidence on lidamidine is very low, and its effectiveness is modest in the control of IBS symptoms.
Cholestyramine: Some patients with IBS-D can have overlap with bile acid malabsorption (approximately 30%).50 Specifically, this group of patients could receive a certain benefit from this medication. However, there is no direct scientific evidence, given that there are no studies that specifically evaluate the usefulness of cholestyramine in IBS-D, and on the other hand, diagnostic tests are not available in all parts of Mexico, and they are expensive. Nevertheless, assuming that there could be bile acid malabsorption, its use is recommended, starting with a therapeutic test.
Adverse eventsLoperamide: The most frequent adverse effects are constipation, nausea, vomiting, dry mouth, bloating, asthenia, somnolence, dizziness, and exanthematous eruptions. In <1% of children, and at high doses, it can cause central nervous system (CNS) depression (somnolence, myosis, respiratory depression, and ataxia). In the case of overdose, it can cause respiratory depression. Loperamide above the recommended doses can cause serious cardiac events, including QT interval prolongation, torsades de pointes, other ventricular arrythmias, cardiac arrest, syncope, and death.
Lidamidine: At therapeutic doses, dry mouth, nausea, headache, dizziness, and mild, transitory constipation have been reported.
Cholestyramine: Adverse effects, such as constipation, abdominal pain, flatulence, vomiting, diarrhea, skin eruptions, and steatorrhea, have been described but are generally infrequent. More frequently, patients report intolerance to the cholestyramine suspension presentation.
Availability, recommended dose, and treatment durationTable 3 shows the antidiarrheals that are available in Mexico and the recommended doses.
Antidiarrheals available in Mexico for IBS management
| Drug | Recommended dose | Duration |
|---|---|---|
| Loperamide | 2 mg to adjust according to response (maximum dose 16 mg/day) | 4 to 12 weeks |
| Lidamidine | 4-8 mg/3 times a day | 4 to 6 weeks |
| Cholestyramine | 4 g one to 4 times a day (powder for suspension) | According to response |
IBS: irritable bowel syndrome.
Serotoninergic agents are drugs that act through serotonin (5-HT) receptor agonism or antagonism. They can be used in the management of IBS-D and IBS-C, depending on which receptors they stimulate.
Mechanisms of actionSerotonin (5-HT) is a cell transmission signaler and neurotransmitter synthesized from tryptophan through the tryptophan hydroxylase enzyme (TPH). Ninety-five percent of 5-HT production is carried out in the intestinal enterochromaffin cells and a lower percentage in the serotonergic neurons of the myenteric plexuses.51 The limiting step of 5-HT activity is the serotonin reuptake transporter (SERT) because it removes the 5-HT from the interstitial space in the lamina propria into the enterocytes of the mucosa and presynaptic neurons responsible for its catabolism.52 On the other hand, there are 7 5-HT receptor subtypes. Of those, the 5-HT3 and 5-HT4 receptors are therapeutically important for IBS. The 5-HT3 receptors are located in the intestinal plexuses, sensory nerves, and the parasympathetic and sympathetic nerves; by binding to the 5-HT3 receptors in the parasympathetic ganglia, serotonin stimulates smooth muscle contraction, intestinal secretion mediated by acetylcholine release, and visceral sensitivity.53 The 5-HT4 receptors are located in the neurons of the myenteric plexuses, in primary afferent neurons, smooth muscle cells, and enterochromaffin cells. These receptors are mediators of locally released neurotransmitters that stimulate the peristaltic reflex, as well as mediators of circular smooth muscle contraction and relaxation, and have variable effects on the longitudinal muscle and on fluid secretion in the small bowel; they are less extended in the colon.54 In IBS, there are several serotonergic signaling elements that are altered, including the number of enterochromaffin cells, serotonin content, TPH levels, 5-hydroxyindoleacetic acid levels, and SERT expression. SERT variants are genetically determined and can contribute to their lower expression in IBS-D, especially reducing serotonin reuptake and consequently resulting in greater serotonin availability.55
Various 5-HT3 antagonists have been utilized in IBS-D, given that they produce inactivation of the neurons that express those receptors, reducing motor reflex activity and secretion, and they decrease the depolarization of the extrinsic sensory neurons that transmit signals to the brain.
Indications5-HT3 antagonist use is indicated in IBS-D because they reduce stool frequency, improve stool consistency, and reduce symptoms, such as abdominal pain. On the other hand, 5-HT4 agonists are recommended in IBS-C.
Clinical evidence5-HT3 antagonists: Currently these antagonists are alosetron, ramosetron, and ondansetron,56 but only this last one is available in Mexico. In the most recent CCT (the TRITON study), ondansetron was evaluated. It was administered at a dose of 4.0 mg/day, and after 2 weeks, was adjusted, by increasing to 8.0 mg/3 times a day every 3 days or decreasing to a minimum of 4.0 mg every 3 days, and then compared with placebo.57 The primary endpoint was the combination of abdominal pain and diarrhea (according to the Food and Drug Administration [FDA]), carrying out an intention-to-treat (ITT) analysis. Four hundred patients were calculated, but due to the difficulty in recruiting them during the pandemic, the study ended before its anticipated time, randomizing only 80 patients (37 to ondansetron and 43 to placebo). After a review of the literature, the data from that trial were pooled with data from other placebo-controlled trials on ondansetron, and a separate meta-analysis was carried out to estimate the RR, 95% CI, and NNT.58,59 In the ITT, 40.5% (95% CI 24.7-56.4%) achieved the primary endpoint with ondansetron vs 27.9%; (14.5-41.3%) con placebo, (p = 0.19). Ondansetron improved stool consistency, compared with placebo (p < 0.001). Likewise, ondansetron increased the total whole gut transit time between the baseline and week 12 (mean [SD] difference 3.8 [9.1] hours vs placebo –2.2 [10.3] hours [p = 0.01]). With respect to the separate meta-analysis, one of the 2 additional studies identified had a 10-week crossover period, but the authors of that trial only obtained data from the first 5 weeks of the trial.59 With the TRITON study patients and those from the 2 additional trials, a total of 327 patients were analyzed in the meta-analysis. Ondansetron was found to be superior to placebo in the FDA composite endpoint (RR of symptoms not responding: 0.86; 95% CI 0.75-0.98, NNT = 9) and stool consistency response (RR: 0.65; 0.52-0.82, NNT = 5). There were no differences, with respect to abdominal pain (RR: 0.95; 0.74-1.20).
5-HT4 agonists: Mosapride citrate is a selective serotonin 5-HT4 receptor agonist whose main metabolite is a weak 5-HT3 antagonist. In a pilot study on 10 patients with IBS-C, based on the Rome III criteria, the relation of intestinal transit time with symptoms was analyzed, before and after treatment with 15 mg of mosapride, once a day after breakfast, for 4 weeks.60 The primary endpoint was the correlation of the changes in IBS-C symptoms with the changes in transit time. The symptom changes were in abdominal pain severity, the Bristol Stool Scale, and bowel movement times. After 4 weeks, abdominal symptom frequency decreased (from 3.7 to 2.6) abdominal pain severity decreased (from 3.8 to 2.0), according to the scales employed. Likewise, stool consistency increased from 2.5 to 3.5, according to the Bristol Stool Scale, and stool frequency increased predominantly in the patients that reported at least one bowel movement a day. Likewise, those changes significantly correlated with gastric transit, but not with bowel transit.60 Another study evaluated sensorimotor function in 37 patients with Rome II IBS and 17 controls, all of whom underwent barostat testing to determine pain perception.61 The IBS patients were then randomized to take mosapride 15 mg (n = 19) or placebo (n = 18), administered orally with 200 ml of water. Perception and motility were again evaluated 60 min after treatment. Rectosigmoid colon tone and contractility were determined every 10 min. The pain threshold was significantly lower in the IBS patients than in the controls, but there were no differences in the sensorimotor parameters between them. However, bag volume decreased, and the number of contractions increased, compared with placebo, but perception was not modified. Specifically in the patients with IBS-C assigned to mosapride (9/19), there was a significant increase in rectosigmoid colon tone and contractions, compared with placebo.61 Those data suggest that mosapride has the potential to manage constipation in patients with IBS-C.
Another study randomized 285 IBS patients without diarrhea, according to the Rome III criteria, to a combination of probiotics (Bacillus subtilis and Streptococcus faecium) in one of 4 doses and mosapride (10 mg/day in the 2 groups with low doses of probiotics and 15 mg/day in the 2 groups with high doses of probiotics) or placebo, for 4 weeks.62 Compared with placebo, adequate improvement was significantly superior in all the treatment groups (53.6 to 55.2%), compared with placebo (35.1%). Likewise, complete overall improvement or considerable improvement were superior in the treatment groups, compared with placebo. Abdominal pain/discomfort significantly improved in the higher dose treatment group versus placebo, and stool frequency and consistency improvement in the IBS-C patients was superior in the highest and lowest dose treatment groups but not in the intermediate dose groups.
Prucalopride is another selective serotonin 5-HT4 receptor agonist with colokinetic effects that has been approved for chronic constipation.63 However, a retrospective review conducted in Sheffield, Great Britain, analyzed all the patients that had received prucalopride for at least 4 weeks to determine whether there was any association between the response and the type of constipation (slow transit constipation: 44%, obstructive constipation: 29%, the combination: 12%, or IBS-C: 15%).63 They identified 69 treated patients, 59 of whom were women, and reported that 65% of the prescriptions were from colorectal surgeons. Responses were considered positive when there was patient satisfaction and treatment continuation. At 4 weeks, 31, 59, 43, and 44% of the abovementioned groups, respectively, reported symptom improvement, indicating that the type of constipation did not predict the positive response. In 2017, a diagnosis and management analysis of 878 consecutive patients with constipation was published in Italy; the information was collected by 52 Italian gastroenterologists.64 The patients were classified into chronic constipation, IBS-C, and constipation not related to the Rome criteria. Prucalopride was prescribed to 14.4% of the patients, with no differences in the groups. The low prescription percentage could be attributed to the fact that, in the year the study was conducted, prucalopride had only just become available in Italy and was expensive, and therefore was used as second-line treatment. Nevertheless, that respective review did not determine treatment response predictors, and so no conclusions can be drawn regarding its effectiveness in IBS-C.
In 2014, data accumulated from phase 3 trials on prucalopride in chronic constipation on women treated with 2 mg/day of this prokinetic were published.65 Data from 936 women showed that prucalopride had a large effect size (>0.8) on all the Patient Assessment of Constipation Symptoms (PAC-SYM) scales, including abdominal pain, abdominal discomfort, subjective bloating, straining, and painful bowel movements. For abdominal symptoms and stool symptoms, the effect size with prucalopride 2 mg was 1.3 to 2.3-times larger than with placebo. Much more recently, a post hoc analysis of patients with chronic constipation and moderate-to-severe subjective abdominal bloating in 6 phase 3 and 4 studies showed that the number of responders (≥1 point of improvement on the subjective bloating score at week 12) was higher in the patients that received prucalopride than in those that received placebo (62.1 vs 49.6%).66
The abovementioned data show that prucalopride is frequently used in clinical practice in patients with IBS-C. Even though there are no clinical trials specifically on IBS-C, the improvement in pain, abdominal discomfort, and subjective bloating in patients with chronic constipation, suggests that it also has the potential to improve those key symptoms of IBS-C. Furthermore, the Rome IV criteria consider chronic constipation and IBS-C as spectrum extremes and that their differentiation is artificial. Therefore, it cannot be determined whether many of the patients included in those clinical trials really have IBS-C. Finally, due to the fact the trials are not specifically on IBS-C, a NNT cannot be provided.
Adverse eventsIn the TRITON study, no serious adverse effects were reported with ondansetron, but a higher number of patients treated with the drug presented with constipation, compared with placebo (45.9 vs 25.6%), even though, in general, it was mild; only 3% of patients treated with ondansetron and one with placebo reported severe constipation. Likewise, one patient in each group discontinued treatment due to constipation. In addition, through direct questioning, rectal bleeding was reported in 3 patients with ondansetron and in 7 with placebo. It was considered a minor effect, except in one of the cases with placebo, but rectosigmoidoscopy was not deemed necessary in any of them.
With respect to 5-HT4 agonists, in the studies with mosapride analyzed above, the 2 mechanistic studies showed no adverse effects, but they were trials with a single dose. Regarding prucalopride, in the pivotal trials on chronic constipation, side effects were very frequent (71.4 to 80.2 vs 67.1 to 78.4%); headache was the most frequent, presenting in up to 29% of patients and abdominal pain in one out of every 5 patients.
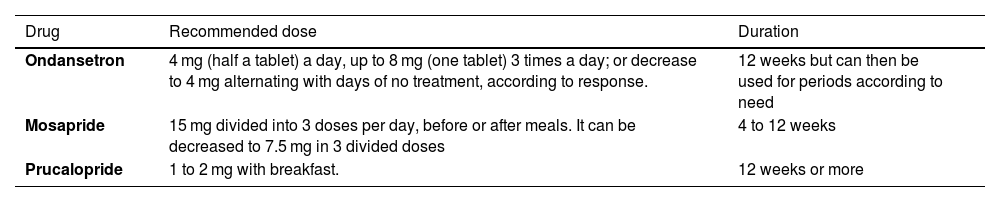
Availability, recommended dose, and treatment durationTable 4 shows the serotonergic agents available in Mexico and the recommended doses.
Agents that act on serotonin receptors available in Mexico for IBS management
| Drug | Recommended dose | Duration |
|---|---|---|
| Ondansetron | 4 mg (half a tablet) a day, up to 8 mg (one tablet) 3 times a day; or decrease to 4 mg alternating with days of no treatment, according to response. | 12 weeks but can then be used for periods according to need |
| Mosapride | 15 mg divided into 3 doses per day, before or after meals. It can be decreased to 7.5 mg in 3 divided doses | 4 to 12 weeks |
| Prucalopride | 1 to 2 mg with breakfast. | 12 weeks or more |
IBS: irritable bowel syndrome.
Secretagogues are a group of drugs specifically used in IBS-C and chronic constipation. These medications increase fluid secretion in the intestine, which helps soften stools, promoting more regular and easier bowel transit. Secretagogues include lubiprostone, linaclotide, and plecanatide. Only the first 2 have been marketed in Mexico, but linaclotide is currently the only one available.
Mechanisms of actionLinaclotide is a 14-amino acid peptide, structurally similar to the human endogenous hormones, guanylin and uroguanylin, and functionally analogous to the heat-stable enterotoxin of the pathogenic strains of Escherichia coli (E. coli). Linaclotide acts as a potent, highly selective agonist of guanylate cyclase-2C (GC-2C); its active metabolites bind to the GC-C transmembrane receptors and function locally on the luminal surface of the mucosa, in the epithelial lining of the intestine.67,68 GC-C activation conditions elevated levels of intracellular and extracellular cyclic guanosine monophosphate (cGMP). Elevated intracellular cGMP levels stimulate electrolyte, chloride, and bicarbonate secretion into the intestinal lumen, mainly through activating the ion channel known as the cystic fibrosis transmembrane conductance regulator (CFTR), and also inhibit sodium absorption, producing an increase in intestinal fluid content and accelerating transit. On the other hand, elevated extracellular cGMP levels inhibit colonic nociceptors, improving abdominal pain.69
Lubiprostone is a bicyclic fatty acid metabolite of the prostaglandin E1 (PGE1) metabolite that activates a type 2 specific chloride channel (ClC-2) in the apical membrane of the enterocyte.70,71 Once the channels are opened, chloride enters the enterocyte in the basal membrane through the action of active Na+ K+ 2Cl cotransporters that create the driving force that favors chloride secretion. Specifically, a sodium ion and a potassium ion enter the cell, together with every 2 chloride ions. The isoelectric and isotonic balances are maintained when the sodium ions and water, respectively, follow the chloride ions into the intestinal lumen through the paracellular route, resulting in a general increase in intestinal fluid secretion that is concentration-dependent, without altering serum sodium and potassium levels.72 This flow of chloride ions, in turn, leads to the net secretion of fluid into the intestinal lumen, increasing the fluid content of stool and improving transit. There is sufficient evidence on the primary mechanism of action of lubiprostone as a pro-secretion agent in constipation, but its exact mechanism(s) for improving symptoms (including abdominal pain) in IBS-C, are only partially characterized.73
IndicationsLinaclotide and lubiprostone are approved for IBS-C management in Mexico.
Clinical evidenceLinaclotide: There is adequate evidence for the use of this drug, and the majority of the guidelines consider it an intervention with an A1 level of evidence. A CCT conducted for 12 weeks included 420 patients with IBS-C, evaluating the efficacy and safety of oral linaclotide at doses of 75, 150, 300, or 600 mcg. All the doses of linaclotide significantly improved bowel habit, including spontaneous bowel movement frequency, straining severity, and stool consistency.74 Abdominal pain decreased significantly from the start, compared with placebo; the mean changes in abdominal pain (evaluated on a 5-point scale) from the start were –0.71, –0.71, –0.90, and –0.86 for the linaclotide doses of 75, 150, 300, and 600 mcg, respectively, compared with –0.49 for the placebo. In a phase 3 trial, linaclotide efficacy and safety were evaluated in 804 patients with IBS-C, for 26 weeks.75 They were randomly assigned to receive placebo or 290 mcg of linaclotide once a day. During the first 12 weeks, 33.7% of patients showed significant symptom improvement (defined by the FDA as an increase ≥ 1 complete spontaneous bowel movement per week from the start of treatment and a reduction ≥ 30% in the mean abdominal pain score per week for 50% of the treatment weeks) in the linaclotide group versus 13.9% in the placebo group (p < 0.0001), with a NNT of 5.1 (95% CI 3.9-7.1).75 In subsequent trials, linaclotide, at a dose of 290 mcg for 12 weeks, has been shown to significantly reduce abdominal pain (≥ 30%) and consistently increase the number of spontaneous bowel movements.76
Lubiprostone: The therapeutic efficacy of lubiprostone has been evaluated in numerous trials, including a study conducted on a Mexican population.77 In the pivotal study by Johanson et al.,78 195 patients received a dose of 16 mcg (8 mcg twice a day [BID]), 32 mcg (16 mcg BID), or 48 mcg (24 mcg BID) of lubiprostone or placebo BID for 3 months. After 2 months, all the lubiprostone groups showed significantly higher mean improvement scores for abdominal discomfort/pain (p ≤ 0.039), but the doses above 16 mcg were associated with more nausea. In all later studies, and according to a meta-analysis of 9 trials, with a total of 1,468 subjects that received lubiprostone and 841 that received placebo, lubiprostone was shown to significantly improve constipation symptom intensity, stool consistency, and quality of life.79 The estimated NNT with lubiprostone is 4 (95% CI 3-6).80 In a Mexican study that included 211 patients, there was better response within 24 h after the first dose with lubiprostone, compared with placebo (60.0 vs 41.5%; OR 2.08; 95% CI 1.19-3.62; p = 0.009). Lubiprostone also showed significant improvement with respect to straining, stool consistency, and bloating.77
Adverse eventsLinaclotide: The most common adverse effect is diarrhea.81,82 Cases of severe diarrhea associated with dehydration during post-marketing surveillance have been reported. Dehydration manifested as tachycardia, hypotension, dizziness, syncope, and electrolyte imbalance (hypokalemia, hyponatremia), requiring hospitalization and intravenous fluid therapy. Diarrhea generally begins within the first 2 weeks from having started therapy with linaclotide. The frequency of severe diarrhea is greater in patients that receive higher doses and need to suspend the dose, reduce the dose, or interrupt treatment with linaclotide. Other common side effects are abdominal pain, flatulence, bloating, bowel urgency, fecal incontinence, viral gastroenteritis, and headache.
In general, the most common adverse effect is nausea. In one study, the incidence of nausea in patients treated with lubiprostone varied between 11.4 and 31.1%. Patients reported that the severity of nausea ranged from mild to moderate and that nausea was more frequent within the first 5 days of treatment.83 Nausea appears to be dose-related and can be due to delayed gastric emptying.84 When lubiprostone is administered with foods, nausea appears to decrease. The incidence of nausea was also found to be lower in men and advanced-age patients (8.2 and 18.8%, respectively).85
Availability, recommended dose, and treatment durationIn Mexico, linaclotide is marketed in the form of hard gel capsules of 290 mcg for oral administration, which is the dose for IBS, whereas the dose for chronic constipation is 145 mcg. However, that lower dose is no longer available in Mexico, making dose adjustment difficult, especially in patients that present with excessive diarrhea with the 290 mcg dose.
The recommended dose of lubiprostone for IBS-C is 8 mcg BID for 4 to 12 weeks, but the drug is not available in Mexico.
Nonabsorbable antibiotics (rifaximin)Rifaximin-alpha is a nonabsorbable antibiotic that has shown safety and efficacy in IBS management.86
Mechanism of actionDerived from rifamycin, rifaximin-alpha is a nonabsorbable, synthetic, broad-spectrum, bactericidal antibiotic that inhibits the synthesis of bacterial RNA through its binding to the beta subunit of the bacterial DNA-dependent RNA polymerase.87 Rifaximin has distinct polymorph crystals named with the Greek letters: α, β, γ, δ, ε, which are hydrates of rifaximin with different water content. The distinct rifaximin polymorphs display different solubility and bioavailability profiles that result in predictable absorption variations. Because gastric secretions do not activate rifaximin-alpha, due to its scant oral absorption, adjustments are not required in patients with liver failure or kidney failure. Its bioavailability is <0.4%. After oral administration, approximately 97% of the dose is excreted in stool, unchanged, with 0.32% of the dose detected in urine and no detectable levels in bile or breast milk.88 One of the rational bases for using rifaximin-alpha in IBS is the fact that some patients can present with associated small intestinal bacterial overgrowth. In addition to the traditional antibiotic effect, rifaximin has been described to have positive modulating effects (eubiotic effects) on the gut microbiota. For example, with metagenomic techniques, Soldi et al.89 evaluated the effect of 1,650 mg daily of rifaximin for 14 days on the fecal microbiota in 15 patients with IBS. They found an increase in the abundance of Faecalibacterium prausnitzii, Bacteroidaceae, and Prevotellaceae and a decrease of Clostridiaceae and Streptococcaceae, with no significant impact on the overall composition of the gut microbiota. Likewise, Ponziani et al.,90 studied the composition of the microbiota, through metagenomic techniques, in patients with different gastrointestinal disorders. They found a significant change in the total composition of the microbiota and an increase in the abundance of Lactobacilli.
IndicationsRifaximin-alpha is indicated in patients with IBS-D and/or IBS-M. In addition to bloating, it has also been reported to improve flatulence and bowel urgency.
Clinical evidenceRifaximin-alpha has been widely studied in the TARGET 1 and TARGET 2 phase 3 clinical trials. They have shown that, in patients with IBS and no constipation, treatment with rifaximin for 2 weeks was associated with the significant relief of IBS symptoms, bloating, abdominal pain, and loose or watery stools.91 A meta-analysis of 5 CCTs with placebo (1,803 subjects with IBS/IBS-D) that included TARGET 1 and TARGET 2 data, reported that 42.2% of the patients treated with rifaximin, compared with 32.4% that received placebo, had overall IBS symptom improvement (OR 1.57).92 Based on those studies, the estimated NNT is 1 in 10. The TARGET 3 study showed that retreatment with rifaximin was effective and well-tolerated in patients with recurrent IBS symptoms.93 A sub-analysis of the TARGET 3 study also showed that 56.8% of the 2,438 patients had abdominal pain response to rifaximin (≥30% improvement from the baseline in the mean weekly abdominal pain score during ≥ 2 of the first 4 weeks after treatment).94 In addition, after the first treatment, significantly more patients treated with rifaximin were abdominal pain responders (53.9%), compared with placebo (44.4%), with similar results after the second treatment (52.9 vs 44.7%, respectively). After the TARGET 3 study, another trial on 2,579 patients with IBS broadened those findings by showing that repeated treatment with rifaximin (550 mg BID for 2 weeks) improved IBS-related quality of life.95
Adverse eventsSufficiently accurate pharmacologic evaluations and experiments have enabled adequate assessment of the toxicity of the alpha polymorph of rifaximin (but not of other polymorphs), particularly in view of its very limited oral absorption. There are very few adverse events (<1%) during brief treatment with the drug, and the most frequent are gastrointestinal (flatulence, nausea, abdominal pain, and vomiting).
An evaluation of the safety of rifaximin in clinical trials reported that, according to data from retrospective and prospective studies, there were no significant differences in the incidence of adverse effects between rifaximin and the drug it was compared with.91–95 In general, only around 6% of the adverse events described were severe, and of those, only 0.1% were related to rifaximin.
Availability, recommended dose, and treatment durationRifaximin-alpha is available in Mexico in tablets of 200, 400, and 550 mg. In IBS with no constipation, the recommended dose is 550 mg TID for 14 days. When there is symptom improvement with the first treatment and symptom recurrence within 18 weeks, treatment can be repeated as often as necessary.
ProbioticsNumerous studies have recently demonstrated the importance of the gut microbiota in the pathophysiology of IBS and have promoted the use of treatments, such as prebiotics, probiotics, synbiotics, antibiotics (reviewed above), and fecal microbiota transplantation, whose aims are to modulate the composition and/or functions of the gut microbiota.96 Probiotics are live microorganisms that, administered in adequate quantities, confer health benefits on the host.97 On the other hand, prebiotics is the name given to undigestible dietary components, generally fibers, that promote the growth and/or activity of beneficial microorganisms in the gut.97 Synbiotics are combinations of probiotics and prebiotics that act synergically.97 Fecal microbiota transplantation consists of the transfer of stool from a healthy donor to a recipient for the purpose of reversing dysbiosis. Because prebiotics and synbiotics are not considered drug therapies and there is no evidence on their use in IBS, they are not addressed in this document. Even though there are studies on fecal microbiota transplantation in IBS, the use of this non-pharmacologic therapy is not yet approved. Therefore, only the evidence on probiotics is addressed herein.
Mechanisms of actionThe mechanisms through which probiotics can influence the pathophysiology of IBS include regulating intestinal motility, reducing visceral hypersensitivity, decreasing mucosal immune activation, improving intestinal permeability, and increasing gut-brain communication.98 The majority of those effects have been shown in in vitro studies or in animal models. Very few mechanistic studies on probiotics in humans have been conducted. Bifidobacterium lactis DN‐173 has been described to improve symptoms and orocecal transit in patients with IBS-C.99Lactobacillus paracasei NCC2461, Lactobacillus acidophilus NCFM, and E. coli Nissle 1917 improved abdominal pain and reduced visceral hypersensitivity in humans and animals, modulating the expression of neurotransmitters, such as substance P, or the receptors involved in nociception, such as μ-opioid 1 or cannabinoid 2.100,101Bifidobacterium infantis 35624 improved IBS symptoms and increased the relation of the anti-inflammatory interleukin (IL)-10/proinflammatory IL-12 in patients with IBS.102,103 The combination of probiotics, such as VSL#3 (L. casei subsp. paracasei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, B. longum, B. infantis, B. breve, and Streptococcus thermophilus) reduced intestinal cytokine secretion and improved gut barrier function in animal models of intestinal inflammation,104 whereas E. coli Nissle 1917 restored intestinal permeability in vitro, induced by fecal supernatants from IBS patients.105Bifidobacterium longum NCC3001 improved depression scores in patients with IBS-D or IBS-M associated with reduced cerebral amygdala activity demonstrated in neuroimaging studies.106
Restoration of the microbiota in patients with IBS is a potential mechanism of probiotics.22–28 However, there are very few studies that have evaluated the role of probiotics in restoring normal gut bacteria in IBS.29–31 Therefore, the possible mechanism of action of probiotics for modulating the gut microbiota in IBS patients is not yet well defined and requires further research.32
IndicationsDue to heterogeneity and the methodological rigidity with which many of the studies have been conducted, the use of probiotics in clinical practice in the treatment of IBS is still considered controversial and the studies are low-quality analyses. Table 5 lists the Clinical Practice Guidelines (CPGs) of the main gastroenterology associations on the use of probiotics in IBS published in the last 5 years in the Western world.107 In summary, the American CPGs of the American College of Gastroenterology (ACG)108 and the American Gastroenterological Association (AGA)109 do not recommend the use of probiotics in IBS, whereas the British110 and Canadian111 CPGs and the Mexican consensuses25,112 recommend their use for the management of overall symptoms and abdominal pain for a period limited to 4 or 12 weeks, as well as their suspension if there is no clinical response.
Recommendations for the use of probiotics, according to different clinical practice guidelines
| Clinical practice guidelines | Recommendations | Level of evidence |
|---|---|---|
| 2019 CAG guideline on IBS | It suggests offering probiotics to patients with IBS to improve IBS symptoms (for one month) | GRADE focus |
| Conditional recommendation | ||
| Very low level of evidence | ||
| 2020 AGA guideline on probiotics | Probiotics are recommended only in the context of one clinical trial | GRADE focus |
| No recommendations | ||
| 2021 BSG guideline on IBS | For overall symptoms and abdominal pain | GRADE focus |
| Weak recommendation | ||
| Very low level of evidence | ||
| 2021 ACG guideline on IBS | Against the use of probiotics for overall symptoms | GRADE focus |
| Conditional recommendation | ||
| Very low level of evidence | ||
| 2023 WGO guideline on probiotics | For relief from bloating and flatulence Some specific strains for abdominal pain | Oxford: 2 and 3 |
ACG: American College of Gastroenterology; AGA: American Gastroenterological Association; BSG: British Society of Gastroenterology; CAG: Canadian Association of Gastroenterology; IBS: irritable bowel syndrome; WGO: World Gastroenterology Organisation.
Various systematic reviews and meta-analyses have shown that probiotics have a limited but significantly superior effect, compared with placebo, in the management of IBS symptoms.113 Ford et al.114 evaluated 53 CCTs in a total of 5,545 patients with IBS. Thirty-seven of those trials were selected for analysis (21 evaluated the combination of probiotics), with a total of 4,403 patients, ranging from 16 to 391 subjects per study. The combinations of probiotics had a beneficial effect on symptom persistence that was superior to placebo (RR = 0.79; 95% CI 0.68‐0.91), but with significant heterogeneity (I2 = 72%) and a NNT of 7. Compared with the combined species, single probiotic species had a lower impact on the treatment of IBS. In 33 trials, the impact on abdominal pain was evaluated. A modest effect was observed with the combination of probiotics and there were no differences with the placebo in the trials. Twenty-four studies reported the effect on bloating. There was a tendency toward a reduced bloating score with the combination of probiotics. In 11 trials, the combination of probiotics significantly reduced the flatulence score, but not with any of the other probiotics studied. Bowel urgency was evaluated in 8 trials and no apparent beneficial effects with any probiotic were observed. Only a few studies have a large patient sample, well-defined endpoints, and utilize specific probiotic strains. One such study is a CCT by Whorwell et al.115 that evaluated 3 different doses of Bifidobacterium infantis 35624 versus placebo, in 362 primary care patients with IBS, for 4 weeks. The results showed overall improvement of symptoms, abdominal pain, and bloating at a dose of 1 × 108 colony-forming units (CFUs), compared with placebo. In another study, Spiller et al.116 analyzed the effect of Saccharomyces cerevisiae I-3856 (1,000 mg daily) in 379 patients with IBS versus placebo, for 12 weeks. The authors found no beneficial effect of the probiotic, compared with placebo, in the population studied, but in a sub-analysis of patients with IBS-C, the S. cerevisiae strain was superior to placebo, regarding the improvement of pain and bloating. Importantly, not all probiotics are similar, nor do they produce the same results. Their effectiveness is strain-specific and symptom-specific.
Given the evidence, establishing accurate recommendations for the use of probiotics in IBS is difficult due to the heterogeneity of the clinical trials, the numerous probiotic combinations and strains used, and the inconsistency of their benefits on individual symptoms, as well as the lack of studies with rigorous outcomes based on the criteria of the FDA or the European Medicines Agency (EMA) for IBS.
Nevertheless, in real-world clinical practice, physicians recommend probiotics for the treatment of IBS, without taking the low levels of evidence for their use into account. For example, Rangan et al.117 surveyed 302 American physicians, gastroenterologists, and general physicians that treat patients with IBS and 3,254 subjects with Rome III criteria for IBS. The results showed that 77% of the patients with IBS used treatments without a medical prescription and only 15% were “very satisfied” with said treatment. Interestingly, 70% of the physicians surveyed recommended probiotics for the management of IBS, most likely because of their low cost, good safety profile, and perceived efficacy, despite a low quality of evidence. Valdovinos et al.118 surveyed 997 Mexican gastroenterologists and nutritionists on the use of probiotics in clinical practice. A total of 64.9% frequently used probiotics, 31.7% rarely used them, and only 3.6% never recommended them. A total of 81.2% of the gastroenterologists and nutritionists considered probiotics efficacious in the management of IBS and 7% stated they were not aware of any scientific evidence on the use of probiotics in gastrointestinal disorders.
Adverse eventsEven though probiotics are perceived as innocuous and safe, and no major incidence of adverse effects has been reported in clinical trials, when compared with placebo, certain precautions should be taken with their use. For example, sepsis and endocarditis associated with some Saccharomyces and Lactobacillus probiotic species have been reported, when used in immunocompromised patients or when there is vascular access contamination.119,120 Symptoms, such as brain fog and chronic fatigue, as a possible consequence of increased lactic acid production, have recently been described.121
Availability, recommended dose, and treatment durationEven though many formulations of probiotics are available in Mexico, Table 6 shows the specific strains that have been efficacious in good-quality studies. The recommended doses vary, according to each strain, and the recommended treatment duration is between 4 and 12 weeks. If probiotics are opted to be used, it is important to underline that they are recommended as adjuvant therapy and not as monotherapy.
Probiotics recommended in Mexico for IBS management
| Probiotic strain | Recommended dose | Duration |
|---|---|---|
| Bifidobacterium longum subsp. longum 35624 | 108 CFUs once a day | 4 to12 weeks |
| Saccharomyces cerevisiae I-3856 | 500 mg (8 × 109 CFUs) once a day | 4 to12 weeks |
CFUs: colony-forming units; IBS: irritable bowel syndrome.
Among the options that have been explored for the management of IBS, there is a group of interventions, considered alternative therapies, that are based on plant extracts (alone or in combination), of which STW5 and peppermint oil stand out.
Mechanism of actionSTW 5 is a phytopharmaceutical that contains hydro-ethanolic extracts from 9 herbs combined in a fixed proportion (Iberis amara totalis recens, Angelicae radix, Cardui mariae fructus, Chelidonii herba, Liquiritiae radix, Matricariae flos, Melissae folium, Carvi fructus, and Menthae piperitae folium) that has been marketed in Europe as an over-the-counter medication for dyspepsia and IBS relief since the 1960s.122,123 STW 5 is considered a multipurpose therapeutic agent because it has been shown to simultaneously act on different therapeutic targets.124,125 In in vitro pharmacologic models, it has been shown to have a dual effect (relaxing and toning) on small bowel smooth muscle,126 produce prosecretory,127 anti-inflammatory, and antioxidant effects on the intestine,128 and improve visceral hypersensitivity.129 More recent studies have shown that STW 5 has beneficial effects on intestinal dysbiosis-induced models through 3 different routes: greater microbial production of short-chain fatty acids, microbial production of potentially bioactive metabolites of the phytopharmaceutical components, and the proliferation of beneficial bacteria.130,131
Peppermint is a plant that is a hybrid of water mint (Mentha aquatica) and spearmint (Mentha spicata) that belongs to the Lamiaceae family. It is widely distributed in temperate regions of the world. It has an ample variety of applications in traditional medicine and is also used as an aromatizing agent and a functional tea.132 Peppermint oil is volatile and its main active ingredient is menthol, which has antispasmodic properties due to its capacity to block intestinal smooth muscle calcium channels.133 Its clinical benefits have been attributed to its antispasmodic effect, but there is evidence of other possible mechanisms of action, among which central and visceral sensitivity, antioxidant effects, antiparasitic effects, antifungal effects, microbiota modulation, and direct anti-inflammatory effects stand out.134–136 There are studies on humans that have shown that inhaling the aroma of mint improves attention, and studies on rodents suggest that menthol has dose-dependent anti-anxiety effects through the dopamine pathways.137 Mint oil has effects on esophageal, gastric, small bowel, gall bladder, and colon functions, which is why its clinical application in gastroenterology is potentially broad and rapidly expanding.138,139
IndicationsSTW 5 is indicated in the symptomatic control of IBS and functional dyspepsia (FD).122–124 Therefore the profile of the patient that can most benefit from the phytopharmaceutical agent is that of the patient with IBS and FD overlap. Clinical studies have shown that STW 5 is significantly better than placebo for reducing abdominal pain and the composite indices of overall symptoms in IBS. The clinical studies conducted with STW 5 do not differentiate between IBS subtypes.
Peppermint oil is indicated for the control of general symptoms and abdominal pain. Recent consensuses and guidelines recommend its use as a therapeutic agent separate from antispasmodics, and it has not been indicated specifically for any IBS subtype.108,111,140 Because it has shown a good clinical effect on FD, it is reasonable to assume that the patients most likely to experience a greater therapeutic benefit from peppermint oil use are those presenting with FD and IBS overlap.
Clinical evidenceSTW 5: Twelve noncontrolled or observational studies published between 1980 and 1990 reported on the efficacy of STW 5 for gastrointestinal symptom relief in different clinical settings.141 At present, the largest published study on IBS is a CCT that evaluated the efficacy and safety of STW-5 in 208 patients with different IBS subtypes in the United States.142 The phytopharmaceutical was significantly better than placebo for reducing abdominal pain and the overall symptom score (flatulence, meteorism, bloating, and incomplete evacuation sensation). A real-world study on 2,500 IBS patients that received STW 5 for a maximum of 4 weeks, showed a 65 to 80% decrease in the individual abdominal symptom score.143 In that work, 80% of physicians and patients evaluated STW 5 efficacy as very good or good. Different meta-analyses and systematic reviews involving this phytopharmaceutical point out that there is evidence on beneficial effects in modern phytotherapy in IBS, while at the same time stressing the need for more and better studies with high-quality trials.144,145
Peppermint: Five systematic reviews and meta-analyses have been published that only include moderate-to-good quality randomized CCTs that are compared with placebo.20,21,146–148 All of them have shown that peppermint oil is superior to placebo, with respect to abdominal pain relief, with a NNT of 4 to 7. The main critique of those meta-analyses is the great heterogeneity of their clinical trials, mainly regarding the definition criteria of IBS, the subgroups studied, doses utilized, drug presentation, and treatment duration. More recent studies, not included in the abovementioned meta-analyses, have reported findings that were less promising and confirm the need for further research. A CCT that compared the administration of 182 mg intestinal-release peppermint oil, 182 mg of ileocolonic-release peppermint oil, and placebo, for 4 weeks, found no statistically significant response regarding reduced abdominal pain or general symptom relief.149 However, compared with placebo, peppermint oil released in the small bowel produced significant improvement in the secondary results, including the abdominal pain score, discomfort, and IBS severity. Another randomized and controlled clinical trial that compared the administration of 180 mg TID and placebo, for 6 weeks, found no statistically significant differences between the two groups, with respect to overall relief of symptoms.150 The cost-effectiveness of treatment with small intestine-release peppermint oil was evaluated in an 8-week multicenter, randomized, placebo-controlled trial on IBS patients.151 The study showed that, when using abdominal pain as the response parameter, peppermint oil had a high probability of cost-effectiveness and its use could be justified, given the modest increase in quality-of-life scales.
Adverse eventsSTW 5: Its safety has been evaluated in nonintervention and retrospective clinical and preclinical controlled trials that included chronic, sub-chronic, and acute toxicity, specifically focused on liver toxicity, reproductive toxicity, fertility, embryonic and fetal toxicities, mutagenicity, and cytotoxicity, finding no relevant safety effects for its use in humans. It produced no severe adverse effects, nor did studies find significant clinical deviations from normal-range laboratory values. STW 5 was well-tolerated in the populations analyzed, regardless of concomitant diseases, and there were no medication interactions.152 Hypersensitivity reactions are rare and may present as pruritus, dyspnea, or skin reactions in predisposed patients.153 There is only one published study on severe liver toxicity, leading to liver transplantation, associated with STW-5.154
Peppermint: Peppermint oil has been shown to have a good safety and tolerance profile in clinical studies. Adverse effects, albeit generally mild and transitory, have been significantly more frequent, compared with placebo.20,21,146–148 According to the results of different meta-analyses, the RR of presenting with any adverse effect is 1.4 to 1.57-times higher, compared with placebo, and the NNH is 125.146–148 The effects on esophageal function and the lower esophageal sphincter have been reported to cause the development of reflux symptoms. This is where the different presentations and release forms (in the small bowel or ileocolonic release) could be relevant.
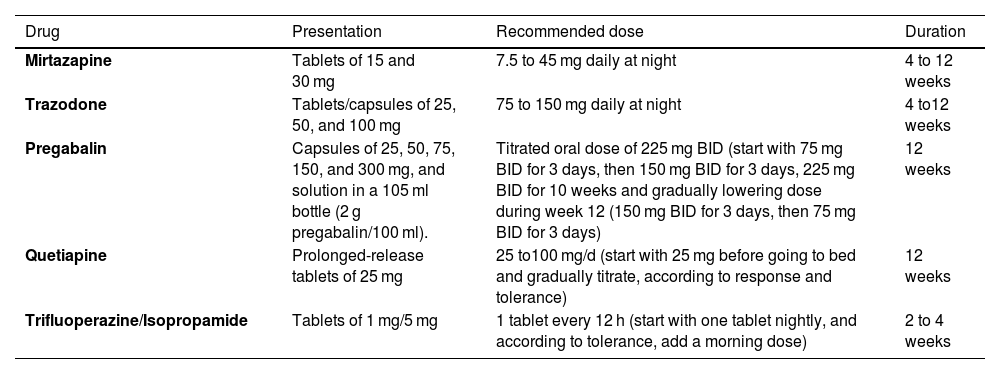
Availability, recommended dose, and treatment durationSTW 5 is available in Mexico, in 20, 50, or 100-ml dropper bottles. The dose for adults recommended by the manufacturer is 20 drops in a small quantity of liquid before or with meals, 3 times a day. The mechanism of action is fast, with a maximum of 4 weeks. According to the manufacturer, the phytopharmaceutical can be used for prolonged periods.
Peppermint: At least 2 presentations are available in Mexico. One over-the-counter presentation is in the intestinal-release form. The dose for adults recommended by the manufacturer is one capsule taken before meals, 3 times a day. There is no consensus on adequate treatment duration, but the available information varies from 2 to 12 weeks. Recently, a prescription-based presentation is again being marketed in the form of capsules containing a combination of 90 mg of menta piperita essential oil and 50 mg of Carum carvi (caraway) 50 mg, and prescribed TID for at least 12 weeks.
NeuromodulatorsThe term neuromodulator has been proposed by the Rome Foundation for substituting “antidepressant”, given that this improves patient acceptance and reduces the stigma on the part of clinicians to this drug group.155 The international guidelines have recommended this group of medications for more than 40 years for the management of patients with IBS, with or without psychiatric comorbidities.156 Their use is based on the effect they have on peripheral visceral sensitivity and the central processing of pain, in addition to having an effect on the psychiatric comorbidity.
This medication group began to be used more frequently in DGBI when pain predominated, and they are considered second-line drugs for IBS management. It is important to underline the fact that, since these medications can take a few weeks to achieve their therapeutic effect, they can be combined with first-line therapies (e.g., spasmolytics). Information on the different categories of neuromodulators that can be used for treating IBS in Mexico follows below.
Selective serotonin reuptake inhibitors (SSRIs)Serotonin, norepinephrine (NE), dopamine, and epinephrine affect digestive tract function due to their action on receptors in the intestinal wall and thus have an effect on intestinal motility and visceral sensitivity.157 SERTs are also found in the intestine. The high plasma levels of serotonin in plasma in patients with IBS-D and post-infection IBS (IBS-PI), as well as the low levels in patients with IBS-C, can be explained in the context of serotonin recapture inhibition.158 SSRIs are a group of medications whose first indication is in the treatment of depression in adults and children, as well as in other psychiatric conditions (anxiety, obsessive-compulsive disorder, post-traumatic stress, panic disorder, and social phobia). Fluoxetine, sertraline, paroxetine, fluvoxamine, citalopram, and escitalopram are included in this class of medications.
Patients can be surprised by the fact that physicians indicate SSRIs for the treatment of IBS. There are numerous reasons for their use in the context of IBS. Said reasons are not limited to the coexistence of anxiety and depression disorders (they can be prescribed in the absence of those disorders); for example, SSRIs can be used for their effect on chronic pain and the correction of intestinal motility disorders.159
Mechanisms of actionSSRIs correct serotonin deficiency, which has been postulated as a cause of depression in the monoamine hypothesis.160 Their mechanism of action is based on the inhibition of serotonin recapture in the nerve terminal, which ends up increasing serotonin activity on postsynaptic receptors. These drugs inhibit the SERT in the presynaptic axon terminal, increasing serotonin concentrations in the synapsis, which in turn, can have an effect on contractility. For example, citalopram has been shown to increase colonic contractility and decrease colonic tone before and after a meal.161 This therapeutic class has little activity on other neurotransmitters, such as dopamine or NE. By having little effect on histamine, acetylcholine, and adrenaline receptors, their side effects are limited.162 SSRIs do not act on norepinephrine receptors, unlike other neurotransmitters, and therefore have no action on pain. They are indicated in clinical pictures in which anxiety, obsessive-compulsive disorders, and phobic symptoms predominate.
IndicationsSSRIs are indicated in IBS when patients present with anxiety, hypervigilance disorders with somatic symptoms, visceral anxiety, and maladaptive cognition, as long as pain and diarrhea are not predominant symptoms. SSRIs have no effect on pain and are more useful in individuals with constipation because their propensity to produce diarrhea is a secondary effect.
Empirically, there are no specific studies on medication combinations. Another neuromodulator can be added, in cases of partial symptom improvement. For example, a SSRI can be added if a patient is treated with tricyclic antidepressants and achieves improvement of pain, but not of anxiety, because the dose of the antidepressant used was insufficient for treating anxiety or depression.155
Clinical evidenceNumerous studies have shown the efficacy of SSRIs. For example, in a CCT on citalopram, compared with placebo, the scores for abdominal pain and bloating unrelated to anxiety or depression were lower.161 In another study, paroxetine produced improvement of general wellbeing in individuals with IBS,163 and fluoxetine reduced abdominal discomfort in IBS-C.164 Based on a meta-analysis of 7 CCTs with 356 participants, SSRI use could be considered when anxiety was predominant in the clinical picture and pain or diarrhea were not important problems. The RR in favor of SSRIs was 0.74; (95% CI 0.58-0.95) and the NNT was 6.8.
Adverse eventsCommon adverse effects of SSRIs are sexual dysfunction, sleep alterations, weight gain or loss, anxiety, dizziness, xerostomia, headache, and gastrointestinal discomfort.165 In 2004, the FDA issued a warning about an increased suicide risk in adolescents and adults up to 25 years of age. SSRIs, particularly citalopram, can cause a prolonged QT interval on electrocardiogram, which can be associated with lethal arrhythmias, such as torsades de pointes.166 Two other effects to be taken into consideration are coagulopathy and serotonin syndrome; the latter occurs when other medications are used that have effects on serotonin. Several of the adverse effects of this group of medications improve through adaptation, after several doses (tolerance), with the exception of sexual dysfunction, which can often be a long-term event. The effect on sexual function can be mediated by 5-HT2A and 5-HT2C receptor stimulation. SSRIs are contraindicated for use with monoamine oxidase inhibitors, linezolid, and other medications that increase serotonin levels. Paroxetine is teratogenic and contraindicated during pregnancy.159 SSRI overdose is rare due to their chemical structures but would be more probable with citalopram or escitalopram than with other members of this therapeutic group. Serotonin syndrome can occur when the patient takes several medications that can elevate serotonin levels, and is characterized by changes in mental state, autonomic dysfunction, and dystonia.
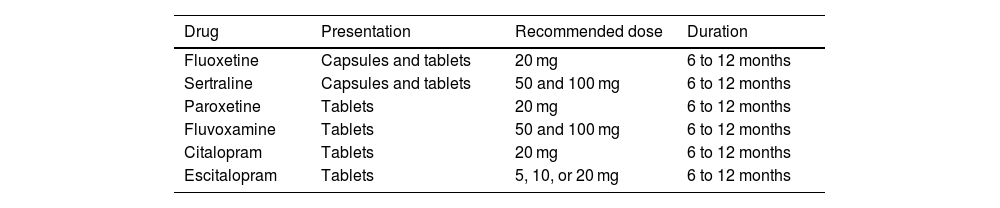
Availability, recommended dose, and treatment durationTable 7 shows the SSRIs that are available in Mexico, their presentation, daily dose, and treatment duration. Starting with low doses (e.g., half the dose) and then scaling it according to patient tolerance is a very important recommendation. In general terms, treatment should be administered for 6 to 12 months to prevent relapses, and it is important to understand that it takes from 2 to 4 weeks from the start of treatment for symptom benefit to become apparent. A very interesting concept is central neurogenesis. The formation of altered conduction circuits due to changes in brain structure may be part of the pathophysiology of IBS symptom persistence. Therefore, SSRIs should be administered for a sufficient period of time to achieve long-term symptom remission.
Selective serotonin reuptake inhibitors available in Mexico
| Drug | Presentation | Recommended dose | Duration |
|---|---|---|---|
| Fluoxetine | Capsules and tablets | 20 mg | 6 to 12 months |
| Sertraline | Capsules and tablets | 50 and 100 mg | 6 to 12 months |
| Paroxetine | Tablets | 20 mg | 6 to 12 months |
| Fluvoxamine | Tablets | 50 and 100 mg | 6 to 12 months |
| Citalopram | Tablets | 20 mg | 6 to 12 months |
| Escitalopram | Tablets | 5, 10, or 20 mg | 6 to 12 months |
Note: Administration can start with half the recommended dose, and then increasing the dose 1-2 weeks later, according to patient tolerance.
In addition to their primary psychiatric indications (anxiety, depression), TCAs are medications used at low doses as visceral analgesics in chronic pain-related diseases, such as fibromyalgia, lumbalgia, and neuropathic pain. In that context, their use in IBS is for pain management, as well as for reducing diarrhea and stool frequency, due to their anticholinergic effects155; these drugs include amitriptyline, nortriptyline, and imipramine.
Mechanisms of actionTCAs act through several mechanisms that contribute to their therapeutic effects. They mainly inhibit the reuptake of neurotransmitters, such as serotonin and NE in the presynaptic neurons, increasing their levels in synapsis and improving neuronal transmission, which is essential for their antidepressive effect. In addition, they block the α1-adrenergic receptors, which can cause orthostatic hypotension, and they antagonize the histamine H1 receptors, contributing to the sedative effects and weight gain that some patients experience. They also block the muscarinic receptors, causing the anticholinergic effects of dry mouth, blurry vision, constipation, and urinary retention. Albeit less prominently, TCAs can also interact with other neurotransmitter receptors, such as those of dopamine and glutamate. These different mechanisms of action make TCAs “dirty” drugs, which not only explains their therapeutic benefits in the treatment of depression and other disorders, but also their secondary effect profile.155 The antimuscarinic effect that TCAs produces makes them ideal for managing the patient with IBS-D, due to the fact that pain and bowel habit are controlled with a single drug.
TCAs are sub-classified into secondary amines, such as desipramine and nortriptyline, and tertiary amines, such as amitriptyline and imipramine. These tertiary amines have greater antimuscarinic and antihistamine actions. Both amine types can be effective in IBS-associated pain, but the secondary amines are preferred, if constipation is a predominant symptom.167
IndicationsTCAs are recommended as second-line therapy for abdominal pain management in the patient with IBS-D and IBS-M (tertiary amines) or in IBS-C (secondary amines). The following are the 4 general recommendations for the use of these drugs: 1) utilize them at low doses; 2) before adding a second drug, the first drug dose should be increased; if adverse effects are produced, the first drug can be combined with others (quetiapine, α2δ ligands), always being aware of interactions and adverse effects; 3) if there is treatment response, treatment should be maintained for 6 to 12 months; and 4) the physician must be skilled at effective communication with the patient, as this will improve patient treatment adherence and drug acceptance.
Clinical evidenceThe evidence supporting TCA use is based on data from meta-analyses.20,21 For example, in a 2012 meta-analysis by Chao et al.,168 they reported a RR of 4.18 (95% CI 2.00-8.77; p = 0.0001). Other meta-analyses have replicated those results, showing that, with low doses of TCAs, there is a reduction on the symptom scale of 44.15 (95% CI 53.27-35.04; p = 0.0001), especially regarding abdominal pain.169 In the meta-analysis by Ford A et al.170 that included 12 CCTs (787 patients), of the 436 patients that received active therapy, 186 (42.7%) did not present with symptom improvement after treatment, compared with 224 (63.8%) of the 351 that received placebo. The RR for IBS symptoms not improving with TCAs, compared with placebo, was 0.65 (95% CI 0.55-0.77). The NNT with TCAs was 4.5 (95% CI 3.5-7).
The recently published ATLANTIS study perhaps provides the evidence that was lacking in the literature for supporting the use of amitriptyline in IBS. In that CCT, the intervention was amitriptyline as second-line therapy for IBS patients with any clinical subtype, at a starting dose of 10 mg and increased weekly up to 30 mg, according to patient tolerance. Treatment was maintained for 6 months. The primary endpoint was symptom improvement evaluated through the Irritable Bowel Syndrome-Symptom Severity Scale (IBS-SSS).171 The study included 463 patients from 55 general medicine practices in Great Britain; 232 of the patients were blinded and randomized for the intervention. At 6 months, there was a mean decrease of 27 points on the IBS-SSS (95% CI –46.9 to –7.10) (p = 0.0079) in the amitriptyline group versus the control group, and the drug was also superior in adequate symptom improvement (OR 1.56; 95% CI 1.20-2.30) (p = 0.008). The symptom with the greatest response was abdominal pain but there was no effect on bloating. The authors of that trial concluded that amitriptyline is superior to placebo for the management of IBS patients, regardless of clinical subtype. A NNT of 4 has been calculated for abdominal pain improvement.172
Adverse eventsThe induction of serotonin function causes agitation, anxiety, insomnia, sexual dysfunction, nausea, and vomiting and the induction of NE can cause alterations in arterial pressure, heart rate, motor activation, and agitation. Because TCAs are nonselective, they act on other neurotransmitters, as described above, and so they have numerous adverse effects: an antimuscarinic effect that explains constipation, dry mouth, blurry vision, and somnolence; α1 adrenergic antagonism that causes dizziness, somnolence, and orthostatic hypotension; H1 antagonism that can produce weight gain and somnolence; and sodium channel blockade. This last effect explains some of the most feared complications of these drugs (arrythmias, convulsions, and coma), especially when doses above therapeutic ones are taken. Therefore, we must be certain that the patient receiving the drug does not have a prolonged corrected QT interval (QTc) on electrocardiogram. To improve treatment adherence, it is important to explain to the patient that adverse effects can appear before the benefits and clarify that the adverse effects disappear within 2 to 4 weeks and the benefits persist. Given the above, it is also recommended to start treatment with the lowest dose possible and progressively scale it until reaching the therapeutic effect with the fewest side effects.
Availability, recommended dose, and treatment durationAmitriptyline and imipramine are available in Mexico. Nortriptyline is only available in combination with fluphenazine, and desipramine is not available. The commonly used doses of amitriptyline are 6.25 mg (1/4 of a 25 mg tablet, which is the lowest presentation in Mexico) every 24 h and increasing the dose to 50 mg, if needed. Later, 6 to 12-month maintenance treatment is recommended, to prevent relapses. Notably, if during treatment there is relapse with the dose achieved, it can be adjusted, when possible. The initial dose of imipramine is 6.25 mg (1/4 of the 25 mg tablet) every 24 h, increasing the dose to 25 mg, if needed, and maintaining treatment for 6 to 12 months.
Tetracyclic antidepressantsTetracyclic antidepressants are a class of medications primarily used for treating depression and include maprotiline and mianserin. Mirtazapine is classified as a tetracyclic antidepressant but has additional characteristics that distinguish it from others in that class of antidepressants. Specifically, mirtazapine belongs to the noradrenergic specific serotonergic antidepressants (NaSSAs) and there is evidence on its use in IBS management.
Mechanism of actionMirtazapine is a 6-aza derivative of mianserin that has a dual mechanism of action on the CNS. On the one hand, it is a NaSSA that antagonizes the adrenergic α-2 autoreceptors and α-2 heteroreceptors, and on the other, it is a postsynaptic 5-HT2 and 5-HT3 receptor blocker, which stimulates 5-HT1A-mediated serotonergic transmission. Mirtazapine has a low affinity for the dopaminergic and muscarinic-cholinergic receptors. This dual mechanism is responsible for its fast-acting action onset. After one dose, it is rapidly absorbed, reaching peak plasma concentrations (Cmax) after 1 to 2.1 h. It binds to plasma proteins (85%) in a nonspecific and reversible manner. It has 50% bioavailability due to first-pass liver metabolism. It is mainly metabolized in the liver (CYP P450 isoenzymes: CYP1A2, CYP2D6, and CYP3A4), with an elimination half-life that varies from 20-40 h, reaching a state of equilibrium after 4 days in adults and 6 days in older adults.173,174
IndicationsAt present, there is no precise indication for the use of tetracyclic antidepressants in the context of IBS as monotherapy. However, given its effects on anxiety, early satiety, nausea, and other symptoms associated with esophageal and gastroduodenal disorders, they can be used in patients with IBS with FD and nausea and chronic vomiting syndrome overlap. Their use could be recommended for IBS-D management, according to the clinical evidence.175
Clinical evidenceA search conducted on mirtazapine in IBS produced some case reports and a randomized, placebo-controlled study on IBS-D management due to the drug’s anti-HT3 effect.174,176 Khalilian A et al.,175 in a placebo-controlled study on patients with Rome IV IBS-D, evaluated 67 patients that were randomly assigned to receive mirtazapine (n = 34) or placebo (n = 33). The patients started with 15 mg/day of mirtazapine before going to bed, for one week; afterwards, the dose was increased to 30 mg/day for 7 more weeks. The results showed that, compared with placebo, mirtazapine was more efficacious in reducing IBS symptom severity (p = 0.002). Additionally, at the end of the treatment period, all the symptoms, except bloating, had significantly higher improvement in the subjects treated with mirtazapine, compared with those that received placebo. Mirtazapine was well-tolerated and also significantly improved patient quality of life (p = 0.04) and anxiety (p = 0.005). Similarly, but in an open study, Sanagapalli et al.177 evaluated the efficacy of mirtazapine in the treatment of IBS-D in 16 patients: 11 received 15 mg and 5 received 30 mg, for 12 weeks. Sixty-nine percent were considered responders, due to a reduction of >50 points on the IBS-SSS. There was also a significant decrease in anxiety and depression on the Hospital Anxiety and Depression Scale (HADS). Likewise, there was a significant decrease in the scores for abdominal pain, urgency, diarrhea, and bloating (p ≤ 0.01).
Adverse effectsThe majority of adverse effects are mild and transitory. The effects associated with blocking the H1 histamine receptor, such as sedation and weight gain, are more marked when a low dose is utilized. Unlike the SSRIs, mirtazapine has no side effects on sexuality. Elevated alanine aminotransferase (ALT) can be produced in 2% of patients, as well as elevated cholesterol and triglycerides in 3-4%. It is well-tolerated in older adults, with dizziness and dry mouth as its most frequent adverse events.
Availability, recommended dose, and treatment durationTable 8 shows the presentations of mirtazapine available in Mexico and the recommended doses.
Tetracyclic and atypical antidepressants and other neuromodulators that can potentially be used in IBS management in Mexico
| Drug | Presentation | Recommended dose | Duration |
|---|---|---|---|
| Mirtazapine | Tablets of 15 and 30 mg | 7.5 to 45 mg daily at night | 4 to 12 weeks |
| Trazodone | Tablets/capsules of 25, 50, and 100 mg | 75 to 150 mg daily at night | 4 to12 weeks |
| Pregabalin | Capsules of 25, 50, 75, 150, and 300 mg, and solution in a 105 ml bottle (2 g pregabalin/100 ml). | Titrated oral dose of 225 mg BID (start with 75 mg BID for 3 days, then 150 mg BID for 3 days, 225 mg BID for 10 weeks and gradually lowering dose during week 12 (150 mg BID for 3 days, then 75 mg BID for 3 days) | 12 weeks |
| Quetiapine | Prolonged-release tablets of 25 mg | 25 to100 mg/d (start with 25 mg before going to bed and gradually titrate, according to response and tolerance) | 12 weeks |
| Trifluoperazine/Isopropamide | Tablets of 1 mg/5 mg | 1 tablet every 12 h (start with one tablet nightly, and according to tolerance, add a morning dose) | 2 to 4 weeks |
BID: twice daily; IBS: irritable bowel syndrome.
These drugs bind to the SERT and NE transporters with different levels of potency and binding affinity, with no significant influence on other neurotransmitters (acetylcholine, adrenalin, dopamine, histamine).178 Unlike the SSRIs, these antidepressants have an ascending dose-response curve, rather than a flat one. The serotonin and NE reuptake inhibitors are duloxetine, venlafaxine, desvenlafaxine, and milnacipran. They are approved for depressive disorders, anxiety, diabetic peripheral neuropathic pain, fibromyalgia, and skeletal muscle pain. However, evidence on their use in IBS is limited, and at present there is only scant information on the use of duloxetine and venlafaxine.
Mechanism of actionDuloxetine is a stronger inhibitor than the rest of this drug group, with a more balanced binding profile of approximately 10:1 for binding to the 5-HT and NE transporters.178 It is also a moderate CYP2D6 inhibitor, making moderate dose reduction and careful control necessary when using it in combination with medications that are metabolized by that pathway. It is absorbed by the digestive tract and reaches its Cmax at 6 h, without being affected by foods. It also has a long plasma half-life (12 h, range: 8-17 h) and elevated binding to plasma proteins (>90%). Its main elimination route is through urine (>70%), as metabolites.
Venlafaxine, a phenylethylamine, is a relatively weak 5-HT and a weaker NE uptake inhibitor, with a 30-fold difference in binding of the 2 transporters. As a result, the drug has a clear dose progression, with low doses predominantly binding to the 5-HT transporter; as the dose increases it binds more to the NE transporter.178 Venlafaxine is also metabolized by CYP2D6. It has a short half-life of 5 h and the metabolite of 12 h, and it has low protein binding. Thus, it is a potential option if drug interactions are a concern.
IndicationsThe same as with the SSRIs, this drug class is recommended as second-line therapy, when pain is the predominant symptom, or when TCAs limit SSRI use, and when there is a psychologic comorbidity.
Clinical evidenceDuloxetine efficacy has been shown in several pilot studies and clinical trials. Three pilot studies have described significant improvement in several aspects of IBS.179–181 In an open study with 15 patients, only 8 completed the 12-week follow-up, with duloxetine at a dose of 60 mg/24 h. There was significant improvement in pain, disease severity, quality of life, stool consistency, and anxiety.179 Another 12-week open study with 17 patients (only 11 completed it) started with duloxetine at a dose of 30 mg, increasing to a maximum of 120 mg, with a mean dose of 60 mg. There was improvement in the overall clinical scale, severity, anxiety, and quality of life.180 A third 12-week pilot study with 17 patients (only 10 completed it) employed an initial dose of 20 mg, followed by 30 mg, and ending with 60 mg, and also reported positive results.181
In addition, 2 CCTs have confirmed those findings.182,183 One study compared the therapeutic effects of duloxetine and fluoxetine in 182 patients with Rome III IBS-C criteria, for 8 weeks. The group that received duloxetine showed significant improvement in flatulence, abdominal pain intensity, quality of life, and stool frequency. Another 12-week study with 60 patients with IBS-D, according to the Rome IV criteria, compared 135 mg of mebeverine plus placebo, with 135 mg of mebeverine plus 30 mg of duloxetine.183 The patients that received duloxetine presented with significant improvement in IBS symptoms, severity, and quality of life, with initial adverse effects that decreased after the fourth week.
With respect to venlafaxine, a randomized double-blind study was conducted on 33 patients with IBS, according to the Rome III criteria. The patients received venlafaxine at an initial dose of 37.5 mg/24 h for 2 weeks, increasing to 75 mg for 2 more weeks, and reaching a final dose of 150 mg/24 h for a follow-up period of 12 weeks, compared with a placebo group.184 The results showed significant improvement in IBS symptom severity, as well as in levels of depression, anxiety, stress, intestinal symptoms (abdominal pain, bloating, and satisfactory bowel movements), and quality of life. However, at the follow-up 3 months after the study had ended, the treated patients had symptom relapse.
Adverse eventsIn the case of duloxetine, adverse effects were reported in less than 2% of patients and the most common was nausea. Nevertheless, the adverse events were the main reason the patients left the pilot studies. The most common side effects identified in the clinical trials were nausea, dry mouth, dizziness, constipation, insomnia, asthenia, hypertension, and fatigue.
In the case of venlafaxine, at low doses, the adverse effect profile is similar to that of a SRI, with nausea, diarrhea, fatigue or somnolence, vomiting, and sexual side effects, whereas venlafaxine at higher doses can produce mild increases in arterial pressure, diaphoresis, tachycardia, trembling, and anxiety.
Availability, recommended dose, and treatment durationIn Mexico, both duloxetine and venlafaxine are available. Duloxetine comes in 30 mg and 60 mg prolonged-release presentations. The recommended initial dose is 30 mg daily, increasing to 60 mg daily after 2 weeks; 60 mg is the recommended maximum dose. On the other hand, venlafaxine is available in presentations of 37.5 mg and 75 mg. The initial dose is 37.5 mg daily, and after 2 weeks, increasing to 75 mg daily, which is the recommended maximum dose for IBS management. These doses are designed to improve initial tolerance to treatment, reduce IBS symptoms, including abdominal pain and anxiety, and improve patient quality of life. Treatment duration is at least 12 weeks and up to 12 months.
Atypical antidepressants, antipsychotics, and other neuromodulatorsTrazodoneMechanism of actionTrazodone is a drug catalogued as an atypical antidepressant and is a derivative of triazolopyridine, with a dual mechanism of action: a 5-HT2 receptor antagonist and a serotonin antagonist and reuptake inhibitor (SARI). This simultaneous activity of antagonizing the 5-HT2A/2C receptors and inhibiting the SERT increases the antidepressive effect and improves treatment tolerance. In addition, it has antagonist properties against α1 and α2-adrenergic receptors and histamine H1 receptors, with minimal anticholinergic effects. After the oral administration of 100 mg of trazodone, the Cmax is reached in 1 h and the mean elimination half-life is relatively short, at 6.6 h. It is extensively metabolized in the liver, primarily by the microsomal oxidation pathway.185
IndicationsTrazodone is not specifically indicated for IBS management. Even though it is an atypical antidepressive mainly utilized to treat depression and insomnia, trazodone’s efficacy has not been widely established for treating IBS symptoms, but when said conditions coexist with IBS, the drug can be used concomitantly.
Clinical evidenceThe most solid evidence for using trazodone in the context of DGBI is in esophageal pain of presumable esophageal origin but it could also be used in the absence of availability of other neuromodulators.
Adverse eventsThe most frequent adverse events are somnolence, vertigo, headache, and dry mouth. In older adults, the risk for orthostatic hypotension can increase, and in toxic plasma concentrations, it can cause QTc interval prolongation. It can also be associated with rare cases of priapism.
Availability, recommended dose, and treatment durationTable 8 shows the presentations and recommended dose of trazodone in Mexico.
PregabalinMechanism of actionPregabalin is a peripheral neuromodulator of the group of second-generation alpha2-delta (α2δ) ligands that blocks the α2δ protein subunit of voltage-dependent calcium channels at the presynaptic level, decreasing the depolarization-induced calcium influx at nerve terminals, and as a result, inhibits the release of different excitatory neurotransmitters, such as glutamate, NE, acetylcholine, substance P, and the peptide related to the calcitonin gene. These are all involved in the pain pathways, with analgesic and anxiolytic effects, as well as in the decreasing of visceral hypersensitivity in patients with IBS.186–188
Pregabalin is 2 to 10-times stronger and has more predictable pharmacologic effects than its α2δ ligand prototype, gabapentin. Some researchers consider pregabalin to be a gamma-aminobutyric acid (GABA) agonist, due to its chemical similarity with said acid, but it should be emphasized that functionally, it does not bind to GABAA receptors.186
IndicationsPregabalin can be used in the treatment of pain and bloating in patients with IBS-D and IBS-M, as second-line therapy. It can also be used in patients with IBS and comorbidities, such as fibromyalgia and abdominal wall pain.
Clinical evidenceThere are 2 CCTs on pregabalin that evaluate visceral sensitivity in patients with IBS.186,189 In the first study, 26 patients with Rome II IBS criteria, without specifying the subtype, and with rectal hypersensitivity to balloon distention (pain threshold ≤ 28 mmHg), received oral pregabalin for 3 weeks (titrated: 50 mg TID days 1 to 3, 100 mg TID days 4 to 7, 150 mg TID days 8 to 11; fixed 200 mg TID days 12 to 21 ± 4) or placebo.186 Compared with placebo, pregabalin significantly increased the first sensation thresholds, resulting in visceral sensitivity improvement (p = 0.045) and increased the desire to defecate (p = 0.008) and pain thresholds, with an effect on allodynia (p = 0.048); it also significantly increased rectal compliance (p < 0.0001), meaning pregabalin could have both a sensory and a motor satisfactory response in patients with IBS. However, in the second study on 18 patients with IBS-C that were given a single 200 mg dose of pregabalin versus placebo, sensation and left colonic compliance thresholds measured through barostat-controlled ascending distensions (16, 24, 30 and 36 mmHg) were evaluated. Pain at distention did not decrease, nor were fasting or postprandial colonic tone or the pre and postprandial motility index modified.189
Saito et al.190 conducted the first CCT for evaluating the efficacy of pregabalin on gastrointestinal symptom improvement in patients with IBS. A scaled dose of 225 mg of pregabalin BID for 12 weeks was given to 85 patients (86% women) with IBS, according to Rome III criteria, with at least 3 pain attacks per month, including IBS-D (n = 37, 44%), IBS-M (n = 29, 35%), and IBS-C (n = 18, 21%). The evaluation criterion was a weekly questionnaire employing the intestinal symptom pain scale at weeks 9 and 12. The pregabalin group had lower pain scores at weeks 9 and 12 (during the last 4 weeks of the study), compared with placebo (25 vs 42, p = 0.008), as well as a lower score for intestinal symptom severity (26 vs 42, p = 0.009). In addition, there were differences in the scores for diarrhea and bloating (p = 0.049 and 0.016, respectively) and no differences in constipation between groups.
In a systematic review and meta-analysis on the role of neuromodulators in the treatment of pain in patients with IBS, 13 studies, with a total of 629 participants, were included. Six trials evaluated amitriptyline, 4 evaluated the α2δ ligands (pregabalin n = 3 and gabapentin n = 1), and 3 evaluated duloxetine.191 In the studies that evaluated the α2δ ligands (pregabalin n = 129 patients; gabapentin n = 43 subjects), in which 47% of the patients had IBS-D and 21% had IBS-C, there was no consistent improvement in abdominal pain. Only one of the 4 studies reported pain improvement in the active group, whereas 2 studies reported improvement in some of the pain scales, and another study reported no differences in pain improvement between active treatment and placebo. The results related to IBS severity and quality of life were reported in only one of the 4 studies, with improvement in severity but not in quality of life.
Adverse eventsIn general, pregabalin is well-tolerated and associated with mild-to-moderate dose-dependent events that are generally transitory. Dizziness and somnolence are the most frequent, followed by xerostomia, ataxia, headache, peripheral edema, blurry vision, weight gain, concentration difficulty, euphoria, or attention deficit. Cases of constipation, nausea and vomiting, myoclonia, asterixis, and gynecomastia have also been reported. Pregabalin should be gradually removed to minimize the possibility of an increase in the frequency of convulsions in patients with epilepsy. If pregabalin is suspended, the dose should be gradually reduced for a minimum of one week.
Availability, recommended dose, and treatment durationIn Mexico, pregabalin (Table 8) is available in capsules of 25, 50, 75, 150, and 300 mg and in a bottled solution of 105 ml (2 g pregabalin/100 ml). The dose should be titrated to prevent adverse effects, starting with 75 mg BID for 3 days, then 150 mg BID for 3 days, 225 mg BID for 10 weeks, and gradually decreasing doses during week 12 (150 mg BID for 3 days, then 75 mg BID for 3 days). Therapeutic response takes place at 8 weeks.
QuetiapineMechanism of actionQuetiapine is an atypical antipsychotic agent that has dopamine D2, 5-HT2A, H1, α1, α2, and M receptor antagonist properties and is a partial 5-HT1A serotonergic receptor agonist. It is also a NE reuptake inhibitor, which explains its analgesic effect. Through M and H1 antagonism and 5-HT1A agonism, quetiapine can reduce intestinal contraction, and in turn, abdominal pain, as well as diarrhea due to its M antagonist effect. The indirect mechanisms of quetiapine include its antidepressive, analgesic, anxiolytic, and sedative effects.192–194
IndicationsQuetiapine is indicated as complementary treatment when monotherapy is insufficient in severe abdominal pain that is refractory to other neuromodulators in IBS-D and in patients with chronic pain with fibromyalgia, insomnia, and severe anxiety and depression disorders.
Clinical evidenceAt present there are no CCTs with adequate statistical power for determining quetiapine efficacy in IBS. Only one retrospective study has been conducted, in which 21 patients with severe gastrointestinal symptoms were evaluated. They had persisted with anxiety disorder, insomnia, or refractory abdominal pain, or developed intolerable adverse events to different neuromodulators, and received the addition of quetiapine at a dose of 25 to 100 mg/day.195 Doses were adjusted according to clinical response or adverse effects. Mean treatment duration was 90 days (range: 1-330 days). Only 11 patients continued the treatment, given that 10 interrupted it due to lack of response or adverse effects. Six of the 11 patients reported overall symptom improvement and 9 were satisfied with the treatment results. Case reports were conducted, in which a dose of prolonged-release quetiapine of 100 mg/day combined with 300 mg/day of venlafaxine produced rapid, notable improvement of abdominal pain and reduced stool frequency. There was also rapid and complete remission of IBS-D symptoms at 2 weeks, and of major depression disorder at 2 months.190 The benefit of adding quetiapine at a dose of 50-300 mg/day in placebo-controlled clinical trials in patients with fibromyalgia, as well as in patients with sleep disorders, has been reported.196,197
Adverse eventsThe most common adverse events are sedation, fatigue, somnolence (that decreases in 1 to 2 weeks of its use), xerostomia, dyspepsia, extrapyramidal symptoms, constipation, metabolic syndrome (weight gain, hyperglycemia, hyperlipidemia), headache, and in less than 1% of cases, altered liver function tests, pancreatitis, and QT interval prolongation (dose-dependent).
Availability, recommended dose, and treatment durationIn Mexico, immediate-release quetiapine in tablets of 25, 100, and 300 mg and prolonged-release quetiapine at doses of 50, 150, 200, 300, and 400 mg are available (Table 8). An initial dose of 25-100 mg every 24 h before going to bed, for 3 months, is indicated and then titrated according to treatment response and patient tolerance.
SulpirideMechanisms of actionSulpiride is an atypical antipsychotic agent with central and peripheral dopamine D2 antagonistic properties, with a prokinetic effect at the gastric level; it also reduces postprandial motility (gastro-colic reflex) in the sigmoid colon. Levosulpiride is the (–) enantiomer of the R (+) sulpiride that has shown greater central antidopaminergic activity, with good evidence in FD and fewer adverse effects than sulpiride.198
IndicationsThere is not sufficient evidence for recommending sulpiride for the treatment of IBS. It is used in the treatment of psychotic disorders, including schizophrenia and anxiety disorders. It has the potential for use as concomitant therapy to reduce pain but at present there is no formal evidence in the treatment of IBS. There is evidence on levosulpiride in the management of dyspepsia/gastroparesis symptoms and it could be used in cases of overlap of those entities.199
Clinical evidenceIn a study on 12 patients with IBS, the postprandial motor response of the colon was analyzed. In 6 cases, sulpiride 100 mg IM was administered, which significantly reduced the gastrocolic reflex that is increased in some patients with IBS.198 Sulpiride efficacy has currently only been reported in a Russian CCT conducted on 40 patients with IBS that were randomized into 2 groups: one received a sulpiride dose of 200-450 mg/day for 6 weeks and the other received standard medical treatment. Eighty-five percent of the patients stated having improvement in abdominal pain and stool consistency, as well as on anxiety and depression scales.200
Adverse eventsAlbeit infrequently, dizziness, somnolence, headache, extrapyramidal effects, late dyskinesia, hyperprolactinemia, constipation, gynecomastia, and xerostomia have been reported.201
Availability, recommended dose, treatment durationSulpiride is available in Mexico in tablets of 50 and 200 mg, and levosulpiride in tablets of 25 mg.
Trifluoperazine/isopropamideIn Mexico, the combination of an antipsychotic agent (trifluoperazine) and an anticholinergic (isopropamide) has been used in certain cases for the treatment of psychosomatic disorders associated with digestive manifestations.
Mechanisms of actionTrifluoperazine is an antipsychotic medication that belongs to the phenothiazine class. It is mainly used in the treatment of psychiatric disorders, such as schizophrenia, as well as for treating severe anxiety symptoms. It functions by blocking certain dopamine receptors in the brain, which aids in reducing symptoms, such as delirium, hallucinations, and agitation.202 Isopropamide is an anticholinergic compound that is primarily used for treating gastrointestinal disorders, such as ulcers and abdominal pain. It acts by reducing gastric acid production and decreasing muscle tone of the gastrointestinal tract, which helps alleviate the symptoms associated with those conditions.203
IndicationsEven though the use of this combination is not initially recommended for the treatment of IBS, its mild sedative and antispasmodic effects, together with its action against nausea, make it a viable option as second-line treatment in IBS cases, in which anxiety-associated pain persists. In addition, due to its gastric acid antisecretory effect, it could be considered in cases that also present with FD, with a predominance of epigastric pain. According to its health record in Mexico, it could also be effective in managing aerophagia.
Clinical evidenceThere is scant information on this drug combination and data are from studies conducted in the 1960s. At that time, indications were for managing symptoms related to peptic acid disease or for conditions previously described as “gastric neurosis”, “irritable colon”, or “gastrointestinal irritability”. At present, there are no studies involving current diagnostic criteria for IBS. However, trifluoperazine/isopropamide is a viable option as second or third-line treatment, according to the abovementioned indications.
Adverse eventsConstipation, xerostomia, blurry vision, restlessness, or insomnia, and in a few cases, urinary retention, have been reported. Even though extremely rare at low doses, persistent late dyskinesia can appear in some patients receiving prolonged treatment or even after having suspended therapy. The risk appears to be greater in advanced-age patients, especially women, or with high doses of the drug combination. The adverse events in some patients appear to be irreversible.
Availability, recommended dose, and treatment durationA single presentation in tablets containing 1 mg of trifluoperazine dihydrochloride and 5 mg of isopropamide iodide is available in Mexico. The recommended dose is one tablet every 12 h, according to tolerance. If there is no important sedative effect with the nighttime dose, a morning dose can be administered. There is no specific treatment duration, but the combination can be tried for at least 2-4 weeks.
MesalazineMesalazine, also known as mesalamine, is a derivative of 5-aminosalicylic acid (5-ASA) and is an anti-inflammatory medication primarily used for treating inflammatory bowel diseases, such as ulcerative colitis and Crohn’s disease. Some studies suggest that, in a subset of patients with IBS, especially those with IBS-D and IBS-PI, there can be low-grade inflammation that contributes to symptoms.204
Mechanisms of actionEven though it is not fully understood, mesalazine’s mechanism of action is based on the activation of nuclear receptors (specifically the peroxisome proliferator-activated receptor-gamma), which in turn, downregulates inflammation and reduces inflammatory cytokine release.205 In addition, mesalazine and sulfasalazine (a combination of the sulfonamide antibiotic with 5-ASA) can also downregulate mast cell function in humans and rodents, an important characteristic of immune system activation in IBS.206,207
IndicationsEven though it had traditionally been considered that there was not sufficient evidence for recommending 5-ASA use in IBS, or that it was a controversial measure, more recent evidence suggests that mesalazine could be moderately efficacious for improving overall IBS-D symptoms.208 Likewise, in IBS-PI, mesalazine (in particular the prolonged-release formulations) could be efficacious.
Clinical evidenceA recently published systematic review included 8 CCTs and 820 patients. Of those patients, 432 were treated with mesalamine, which was shown to be more efficacious than placebo for overall IBS symptoms, with a RR of 0.86 (95% CI 0.79-0.95) and a NNT of 10.208 Nevertheless, there were no significant benefits in abdominal pain reduction, bowel habit, or stool frequency. In the subgroup analysis, mesalamine was efficacious only in patients with IBS-D. There was no significant increase in the incidence of adverse events with mesalamine, compared with placebo (RR 1.20; 95% CI 0.89-1.63). In conclusion, although mesalamine can be modestly efficacious for overall IBS symptoms, the quality of evidence is low, and better-designed clinical trials need to be carried out.
Regarding IBS-PI, a recently published study on the efficacy of mesalamine was conducted on 61 patients with that IBS subtype.209 The patients were randomized to receive 2.4 g of prolonged-release mesalamine or placebo, daily, for 8 weeks. Mesalamine was more efficacious than placebo for lowering overall intestinal symptom and quality-of-life scores.
Adverse eventsIn clinical practice, the most commonly reported gastrointestinal complaints due to mesalazine are abdominal pain, diarrhea, nausea, and flatulence. Headache is also a notably common adverse effect. With respect to more serious events, albeit rare, mesalazine can induce chronic or acute interstitial nephritis, which can progress to kidney failure. Likewise, hypersensitivity reactions that can include Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported but are also rare. Liver toxicity is also a concern, particularly in patients with underlying liver dysfunction, given that mesalazine can elevate liver enzymes, and in rare cases, cause cholestatic hepatitis.
Availability, recommended dose, and treatment durationIn Mexico, mesalazine is available in different presentations: as tablets, capsules, prolonged-release tablets, and prolonged-release granules. Use of the prolonged-release or enteric-coated presentations is the preferred recommendation. Available doses include 500 mg, 1 g, and 1.2 g. Treatment duration based on evidence is 8 weeks and the evidence-based recommended dose is 2.4 g a day, preferably using prolonged-release tablets.
Recommendation summaryTable 9 and Fig. 1 summarize the general recommendations and availability, with respect to all the therapeutic classes evaluated.
Summary of recommendations, indications, and availability of drugs utilized in Mexico for IBS management
| Therapeutic class | Recommendation/indication | Availability |
|---|---|---|
| Antispasmodics | ||
| Alone | Recommended as first-line treatment in any IBS subtype when pain is the predominant symptom | Adequate. Marketed by numerous pharmaceutical companies |
| Combined with dimethicone | Recommended as first-line treatment in any IBS subtype for managing pain and bloating | Adequate. Marketed by numerous pharmaceutical companies |
| Combined with dimethicone + alpha-galactosidase | Recommended as first-line treatment in any IBS subtype for managing pain and bloating associated with the intake of highly fermentable foods | Exclusive. A single presentation marketed by only one pharmaceutical company |
| Osmotic laxatives | ||
| Polyethylene glycol | Recommended in IBS-C management because stool consistency and the number of bowel movements are improved. It has no effect on pain | Adequate. Marketed by numerous pharmaceutical companies |
| Lactulose | Not recommended because it can worsen symptoms, such as bloating. | Adequate. Marketed by numerous pharmaceutical companies. |
| Antidiarrheals | ||
| Loperamide, lidamidine | Recommended as first-line treatment in patients with IBS-D because stool consistency is improved and bowel movement frequency is reduced | Adequate for loperamide. Marketed by numerous pharmaceutical companies |
| Exclusive for lidamidine. Marketed by only one pharmaceutical company | ||
| Cholestyramine | Can be used in patients with IBS-D when ileal bile acid malabsorption is suspected | Limited due to its regularly scarce indication as a hypolipidemic agent, but it is marketed by several pharmaceutical companies |
| Serotoninergic agents | ||
| 5-HT3 antagonists | ||
| Ondansetron | Recommended in patients with IBS-D. It improves stool consistency and bowel movement frequency, as well as abdominal pain | Adequate. Marketed by numerous pharmaceutical companies |
| 5-HT4 agonists | ||
| Prucalopride | Although approved for CD, it can be used in patients with IBS-C because there is evidence of improvement in pain, abdominal discomfort, and subjective bloating | Exclusive. Marketed by only one pharmaceutical company |
| Mosapride | Can be used in IBS-C. Scant evidence | Exclusive. Marketed by only one pharmaceutical company |
| Secretagogues | ||
| Linaclotide | Recommended for IBS-C management. It improves stool consistency, bowel movement frequency, abdominal pain, and bloating. It can be used as first-line treatment. | Exclusive. Marketed by only one pharmaceutical company |
| Lubiprostone | Recommended for IBS-C management. It improves stool consistency, bowel movement frequency, and abdominal pain | Currently unavailable |
| Nonabsorbable antibiotics | ||
| Rifaximin-alpha | Recommended for IBS-D and/or IBS-M management. It improves other symptoms, such as bloating, flatulence, and bowel urgency. It can be used as first-line treatment and repeated as needed, if there is improvement with its use. If there is no response to initial treatment, its repetition is not recommended | Exclusive. Marketed by only one pharmaceutical company. Although there are other forms of rifaximin that are marketed by numerous pharmaceutical companies, they do not correspond to the alpha polymorph |
| Probiotics | Recommended as adjuvant therapy for the “overall” management of symptoms and abdominal pain. They can improve other symptoms, such as bloating and flatulence | Exclusive, for the 2 strains with sufficient evidence, given that each one is marketed by a single pharmaceutical company, respectively |
| Herbal therapies | ||
| STW-5 | Recommended for reducing abdominal pain and overall IBS symptoms | Exclusive. Marketed by only one pharmaceutical company |
| Peppermint | Recommended for reducing abdominal pain and overall IBS symptoms | Adequate. Marketed by numerous pharmaceutical companies |
| Neuromodulators | ||
| Selective serotonin reuptake inhibitors | Recommended as second-line therapy in patients with anxiety, hypervigilance, concomitant depression, and maladaptive cognition, as long as pain and diarrhea are not predominant symptoms (IBS-C) | Adequate. Marketed by numerous pharmaceutical companies |
| Tricyclic antidepressants | Recommended as second-line therapy for abdominal pain and diarrhea management in patients with IBS-D | Exclusive. Marketed by only one pharmaceutical company |
| Tetracyclic antidepressants | Can be considered concomitant therapy when the patient presents with anxiety and overlapping symptoms with functional dyspepsia. There is limited evidence on its potential use in IBS-D | Adequate. Marketed by numerous pharmaceutical companies |
| Serotonin and norepinephrine reuptake inhibitors | Recommended as second-line therapy for abdominal pain management, particularly in patients with IBS-C. They can be started as first-line therapy in patients with concomitant anxiety and depression | Adequate. Marketed by numerous pharmaceutical companies |
| Atypical antidepressants and others | ||
| Trazodone | Can be used when depression and/or sleep disorders coexist | Exclusive. Marketed by only one pharmaceutical company. |
| Pregabalin | Recommended as second-line therapy for treating abdominal pain and bloating in patients with IBS-D and IBS-M. It can also be used in IBS patients with comorbidities, such as fibromyalgia and abdominal wall pain | Adequate. Marketed by numerous pharmaceutical companies. |
| Quetiapine | Can be used as a complement when monotherapy results are insufficient regarding severe abdominal pain that is refractory to other neuromodulators in IBS-D, as well as in patients with chronic pain with fibromyalgia, insomnia, and severe anxiety and depression disorders. | Adequate. Marketed by numerous pharmaceutical companies. |
| Sulpiride, levosulpiride | Sulpiride can be used as concomitant therapy for reducing pain, but formal evidence is currently insufficient regarding treatment in IBS | Sulpiride: Adequate. Marketed by numerous pharmaceutical companies |
| Levosulpiride can be used in cases of overlap with dyspepsia/gastroparesis | Levosulpiride: Exclusive, marketed by only one pharmaceutical company | |
| Trifluoperazine/isopropamide | Could be used as second-line treatment in cases of IBS with the persistence of anxiety-associated pain. Its use could also be considered in cases that also present with FD with predominant epigastric pain. According to its health record in Mexico, it could also be effective in aerophagia management | Exclusive, marketed by only one pharmaceutical company |
| Mesalazine | Could be useful for improving overall IBS-D symptoms. In IBS-PI, mesalazine (particularly the prolonged-action formulations) could be efficacious. | Adequate. Marketed by numerous pharmaceutical companies |
CD: Crohn’s disease; FD: functional dyspepsia; IBS: irritable bowel syndrome; IBS-C: constipation-predominant IBS; IBS-D: diarrhea-predominant IBS; IBS-M: mixed IBS; IBS-PI: post-infectious IBS.
Even though there are different types of rifaximin, the fact that the alpha polymorph is the type with adequate evidence is emphasized.
Pharmacologic management of irritable bowel syndrome in Mexico.
* Probiotics are considered adjuvant therapy.
** It can be used as first-line treatment and repeated as needed, if there is improvement with its use. If there is no response to initial treatment, its repetition is not recommended.
*** In cases with moderate-to-intense symptoms, linaclotide could be considered as first-line therapy because of its antinociceptive effect.
The position statement of the Asociación Mexicana de Gastroenterología (AMG) on the management of IBS in Mexico is a very relevant document, based on scientific evidence, for guiding healthcare professionals in their decision-making in clinical practice based on scientific evidence, as well as providing information on local treatment availability. This position statement comprehensively addresses the therapeutic recommendations according to the different medication classes, based on their efficacy, safety, and availability in the Mexican clinical context.
Financial disclosureThe Alfasigma and Carnot laboratories provided the financial support regarding logistics, travel expenses, and the face-to-face meeting for all the experts that formulated this document. No participant received fees for the development of these guidelines. These recommendations are endorsed by the AMG.
Conflict of interestJ.M. Remes-Troche is an adviser and advisory council member for Asofarma, Carnot, PRO.MED.CS Praha a.s., and Pisa. He is a speaker for Asofarma, Abbot, Carnot, Chinoin, Ferrer, Johnson and Johnson, Medix, and Medtronic.
E. Coss-Adame has been a speaker for Asofarma, Alfa-Sigma, Megalabs, Astra-Zeneca, Carnot, Medtronic, Abbott, Chinoin, and Grunenthal.
M. Schmulson is an advisory council member of Daewoong South Korea, Gemelli Biotech Inc. USA, Moksha 8 Mexico, and PRO.MED.CS Praha a.s. He has been a speaker for Alfa Sigma Mexico, Armstrong Mexico, Carnot, Daewoong South Koprea, Ferrer Mexico/Central America, Medix Mexico, Megalabs Ecuador, Tecnofarma Colombia/Bolivia, and Medicamenta-Tecnofarma Ecuador. He has provided educational materials for Carnot and Moksha 8.
K. García-Zermeño has been a speaker for Carnot, Ferrer, Megalabs, and M8.
M.A. Valdovinos has been a speaker for Carnot, Megalabs, M8, and Bayer.
M. Amieva Balmori has been a speaker for Carnot, AstraZeneca, Asofarma, and Alfa-sigma.
E.C. Morel Cerda has been a speaker for AstraZeneca and Megalabs.
A.S. Villar Chávez has been a speaker for Carnot, Asofarma, Alfasigma, and Schwabe.
L.R. Valdovinos García has been a speaker for Carnot, AstraZeneca, Asofarma, and Chinoin.
O. Gómez-Escudero has been a speaker for Carnot, Chinoin, and Asofarma.
M. Icaza-Chávez and R. Carmona declare they have no conflict of interest.
A. López-Colombo has been a speaker for Chinoin, M8, Europharma, and PRO.MED.CS Praha a.s.