To estimate the number of patients that have access to treatment of hepatitis C with direct-acting antivirals in Argentina and evaluate the factors associated with the lack of access.
Materials and methodsA cross-sectional cohort study was conducted that included all the consecutive prescriptions of direct-acting antivirals issued at health centers that participated in the ECHOTM telemedicine project directed by the Hospital Italiano de Buenos Aires, within the time frame of January 2016 and February 2017.
ResultsA total of 143 treatment prescriptions were included and overall access was 70% (95% CI 62-77%). The only independent factor associated with a lack of treatment access was coverage by a public healthcare system (OR 4.98 [95% CI 2.05- 12.09]).
ConclusionsPatients with hepatitis C that were covered by a public healthcare system had a 4 times higher chance of not having access to treatment with direct-acting antivirals than patients covered by other healthcare systems (private insurance or the social welfare system).
Estimar la proporción de pacientes que acceden al tratamiento de la hepatitis C con antivirales de acción directa (AAD) en Argentina, y evaluar factores asociados a la falta de acceso.
MétodosEstudio de cohorte transversal que incluyó la totalidad de prescripciones consecutivas de AAD realizadas entre enero de 2016 y febrero de 2017 por centros de salud que participan en el proyecto de telemedicina ECHOTM dirigido por el Hospital Italiano de Buenos Aires.
ResultadosSe incluyeron 143 prescripciones de tratamiento. El acceso global fue del 70% (IC95%: 62-77%). Pertenecer al sistema de salud público fue el único factor independiente asociado con la falta de acceso al tratamiento [OR 4.98 (IC95% 2.05-12.09)].
ConclusiónLos pacientes con hepatitis C pertenecientes al sistema de salud público tienen 4 veces más chances de no acceder a tratamiento con AAD que los pacientes con dependencia de otros sistemas de salud (medicina privada u obras sociales).
Hepatitis C is one of the main causes of cirrhosis, hepatocellular carcinoma, and liver transplantation worldwide.1
With the recent introduction of direct-acting antivirals (DAAs), a reduction in the morbidity and mortality associated with hepatitis C infection has been reported.2 Due to their high cost, the treatment of patients with advanced fibrosis and other clinical situations associated with greater infection severity is prioritized. In addition, other factors, such as the healthcare system covering the patient, appear to be involved in treatment access. In the United States, patients with private medical insurance or Medicare had a 6.5 times greater chance of beginning treatment with a DAA, compared with patients covered by Medicaid (a federal and state program).3
In Argentina, as well as in other countries with intermediate and low resources, there is no information on the current barriers to antiviral treatment. The aim of the present study was to estimate the number of patients with hepatitis C that have access to treatment with DAAs in Argentina and identify the factors associated with the lack of treatment access.
Materials and methodsA cross-sectional cohort study that evaluated DAA prescriptions for hepatitis C that were issued to patients at public or private healthcare centers that participated in the ECHOTM telemedicine project directed by the Hospital Italiano de Buenos Aires.
The ECHOTM project is a distance medical education model that was developed through teleconferences between experts in chronic diseases and the professionals in charge of those patients to reduce the healthcare asymmetries between the large urban centers and their peripheral areas. The ECHOTM Project for the care of patients with hepatitis C has been directed by the Hospital Italiano de Buenos Aires since March 9, 2015, with the participation of more than 10 of the country's provinces.4
There are three healthcare subsystems in Argentina. The social welfare system (administered by unions, provincial governments, and the agency in charge of attention to retirees; 50% of the population), the private system (provides health insurance to volunteer members; 10% of the population), and the public healthcare system (40% of the population).
All the consecutive DAA prescriptions issued within the time frame of January 2016 to February 2017 that met the treatment access criteria of the guidelines of the Asociación Argentina para el Estudio de las Enfermedades del Hígado and the Health Department of Argentina were included. Those guidelines defined the following groups as having priority access to treatment: patients with advanced fibrosis (monoinfected patients with a METAVIR score of F3-F4; patients with HIV coinfection and a METAVIR score of F2-F4), liver transplantation recipients, and patients with severe extrahepatic manifestations associated with hepatitis C. Lack of access to treatment with a DAA was considered when treatment was not given within 60 days from its completed request. The factors associated with lack of treatment access were identified through a logistic regression model (STATA, StataCorp., version 14.2).
The study was conducted according to the Helsinki norms, with informed consent, and was approved by the research ethics committee of the Hospital Italiano de Buenos Aires.
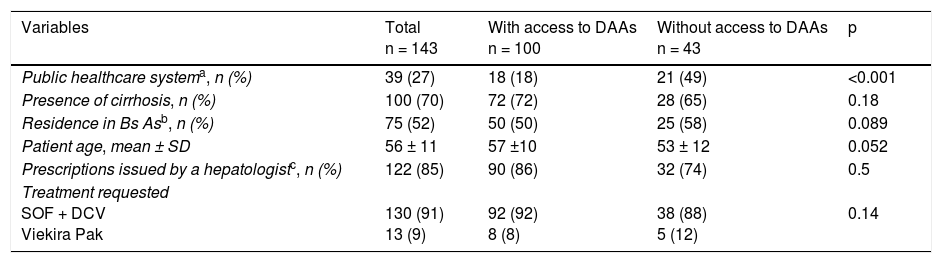
ResultsA total of 143 prescriptions issued at 13 healthcare centers in 8 Argentinian provinces within the study time frame were included. Of the 143 prescriptions, 100 (70%) corresponded to patients with cirrhosis and 39 (27%) to patients covered by the public healthcare system (Table 1).
Characteristics of the patients that were prescribed direct-acting antivirals for the treatment of hepatitis C (January 2016-February 2017). Bivariate analysis.
| Variables | Total n = 143 | With access to DAAs n = 100 | Without access to DAAs n = 43 | p |
|---|---|---|---|---|
| Public healthcare systema, n (%) | 39 (27) | 18 (18) | 21 (49) | <0.001 |
| Presence of cirrhosis, n (%) | 100 (70) | 72 (72) | 28 (65) | 0.18 |
| Residence in Bs Asb, n (%) | 75 (52) | 50 (50) | 25 (58) | 0.089 |
| Patient age, mean ± SD | 56 ± 11 | 57 ±10 | 53 ± 12 | 0.052 |
| Prescriptions issued by a hepatologistc, n (%) | 122 (85) | 90 (86) | 32 (74) | 0.5 |
| Treatment requested SOF + DCV Viekira Pak | 130 (91) 13 (9) | 92 (92) 8 (8) | 38 (88) 5 (12) | 0.14 |
Bs As: Buenos Aires; DAAs: direct-acting antivirals; DCV: daclatasvir; SOF: sofosbuvir; Viekira Pak: ombitasvir, paritaprevir + ritonavir + dasabuvir.
The prescriptions issued to patients covered by the public healthcare system were compared with those of the patients covered by private insurers or by the social welfare system.
Of the 143 prescriptions evaluated, 100 of the patients had access to treatment, signifying an overall access of 70% (95% CI: 62-77%). Access for patients covered by the public health system was 46% (95% CI: 30-63%), and it was 78% (95% CI: 70-86%) for the patients covered by private insurers and the social welfare system.
Table 1 shows the differences between patients with and without access to treatment. In the multivariate analysis, belonging to the public health system, as opposed to the other healthcare systems (private medicine or the social welfare system), was the only independent factor associated with the lack of treatment access (OR: 4.98 [IC 95% CI: 2.05-12.09]), adjusted by the demographic and clinical variables of the patients (age and cirrhosis) and by the region of the healthcare center that requested the treatment.
DiscussionThe main findings of the present study were that overall access to treatment with DAAs was 70% and that the patients with hepatitis C that depended on the public health system to receive the antiviral treatment had a 4 times greater chance of not having access to treatment with a DAA than the patients that depended on the social welfare system or private medical services.
The number of patients with no access to treatment reported in our study is greater than the 8-16% described in the United States in one of the few studies evaluating that situation.3,5
The disparate access to treatment according to patient health coverage has been reported before. For example, in the United States, patients on Medicaid have less treatment access.3,5
Another previously identified barrier to access is the limited availability of specialists dedicated to the treatment of hepatitis C in less densely populated areas.6 In our study, patient place of residence was not a determining factor, but it must be kept in mind that the present analysis was carried out within the framework of the ECHOTM telemedicine program, which guides and advises professionals living far from big cities on the integrated treatment and management of patients with hepatitis C.
ConclusionGiven the clear benefits and elevated effectiveness of DAAs, the barriers, especially those associated with healthcare financing, should be eliminated as soon as possible to offer patients equal access to hepatitis C treatment.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank everyone involved in the ECHO project, especially Dr. Sanjeev Arora and Erika Harding for their continuous support.
Please cite this article as: Marciano S, Haddad L, Borzi SM, D’Amico C, Gaite LA, Aubone MV, et al. Evaluación del acceso a antivirales para el tratamiento de la hepatitis C en un país con recursos limitados. Revista de Gastroenterología de México. 2018;83:208–211.