A 16-year-old boy had a past medical history of primary hypogonadism, due to bilateral anorchia. He presented with gallstones located in the gallbladder and a mild dilatation of the intrahepatic biliary tree. The histology study reported cholesterol gallstones. The patient had been treated with testosterone replacement therapy since infancy. We suggest a possible correlation between testosterone replacement therapy and the presence of cholesterol gallstones.
Tratamos a un niño de 16 años de edad con hipogonadismo primario debido a la anorquia bilateral, que presentó cálculos biliares en la vesícula biliar con una leve dilatación del árbol biliar intrahepático. La histología de los cálculos biliares confirmó la naturaleza de colesterol de los mismos. El niño fue tratado desde la infancia con terapia de reemplazo de testosterona. Sugerimos una posible correlación entre la terapia de reemplazo de testosterona y la presencia de cálculos biliares de colesterol.
Primary hypogonadism due to bilateral anorchia is a rare condition characterized by the complete absence of testicular tissue in an individual with a normal karyotype and phenotype. Its etiology is not completely understood, and various hypotheses have been proposed: an alteration in gonadal vascularization, an endocrinologic disorder, or a possible genetic etiology.1,2
Clinical data on those patients are very limited. Virilization is the primary aim of therapy and it usually involves injectable testosterone ester or transdermal testosterone application.3
The aim of the present article was to report the case of a 16-year-old boy with primary hypogonadism, due to bilateral anorchia, who developed cholelithiasis, which in our opinion was caused by his replacement therapy. He underwent treatment with injectable ester testosterone enanthate, since infancy, and the current monthly dose of the intramuscular injection was 200mg. The patient arrived at our hospital to undergo clinical and laboratory follow-up after a 3-month history of right upper abdominal pain. Upon physical examination, his height was 1.73 m and weight was 76.7kg. Abdomen was normal, blood pressure was 126/71mmHg, and heart rate was 68 bpm. Laboratory data showed normal testosterone (2.54 ng/ml), reduced luteinizing hormone (0.10 U/l) and follicle-stimulating hormone (0.4 U/l), and a mild elevation of serum gamma glutamyl transferase (345 U/l), aspartate aminotransferase (72 U/l), alanine aminotransferase (255 U/l), and direct bilirubin (1.6mg/dl). Plasma cholesterol levels (low-density lipoprotein [LDL] and high-density lipoprotein [HDL]) were normal.
Abdominal ultrasound revealed normal liver structure, but gallstones located in the gallbladder with mild dilatation of the intrahepatic biliary tree. An MRI study confirmed the mild dilatation of the intrahepatic biliary tree, multiple gallstones (one measured 2.8cm and the others less than 1cm) in the gallbladder, and common hepatic duct stricture. None of the gallstones had a signal on the fat sat sequence, corroborating cholesterol gallstones.
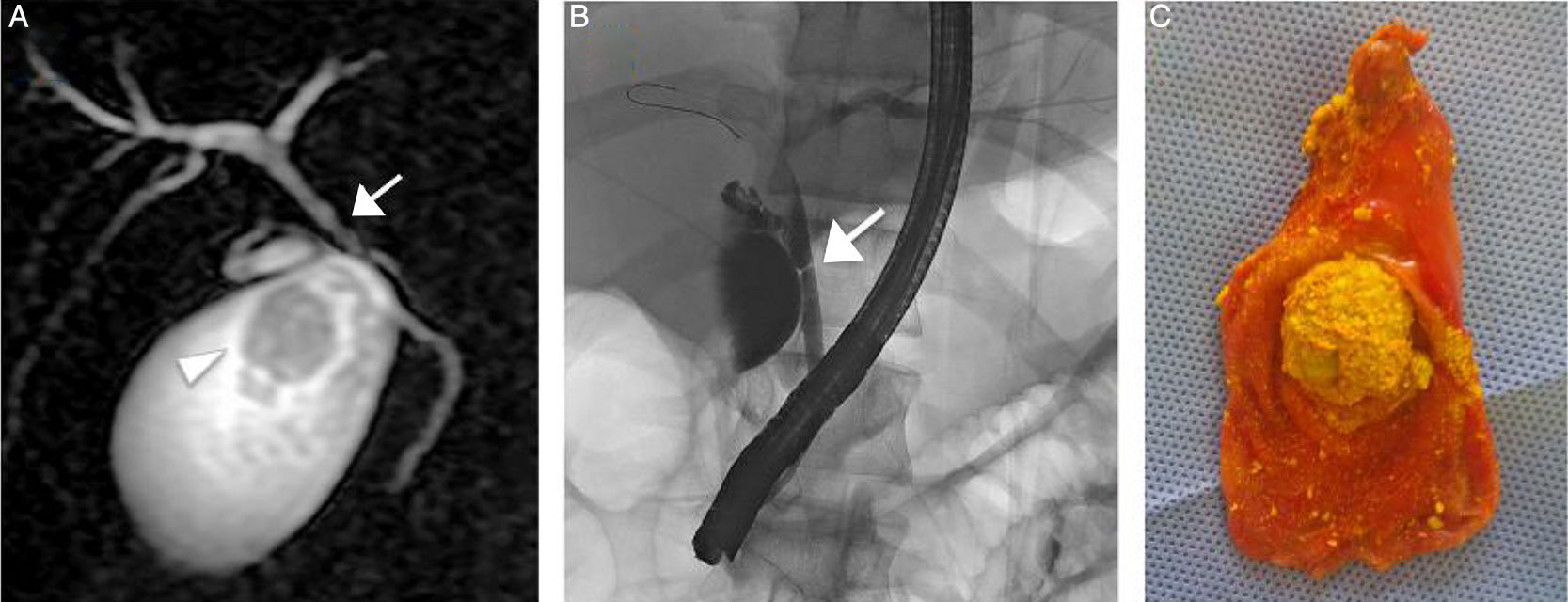
The patient underwent endoscopic retrograde cholangiopancreatography, confirming the common hepatic duct stricture, with mild dilatation of the intrahepatic biliary tree. Consequently, he was treated with bilioplasty (6 x 40mm, COOK, Bloomington, Indiana, USA), with the placement of a 10-Fr biliary endoprosthesis (COOK, Bloomington, Indiana, USA). Seventy-two hours after the endoscopic retrograde cholangiopancreatography, the patient underwent laparoscopic cholecystectomy, corroborating mild dilatation of the gallbladder with multiple gallstones. The histologic study reported gallstones with a mean cholesterol content of 83% and a higher biliary saturation index (143). Figure 1 shows the MRI (A), endoscopic retrograde cholangiopancreatography (B), and laparoscopic cholecystectomy (C).
A) MRI study showing the 2.8cm gallstones (arrowhead) and confirming the common hepatic duct stricture (arrow). B) Endoscopic retrograde cholangiopancreatography confirming the common hepatic duct stricture (arrow) with mild dilatation of the intrahepatic biliary tree. C) Gallbladder with multiple gallstones (note the largest one measuring 2.8cm) after laparoscopic cholecystectomy.
The biliary endoprosthesis was endoscopically removed at the follow-up at one month. No abdominal symptoms were demonstrated in the clinical observation, abdominal symptom assessment, laboratory data, and abdominal ultrasound at the follow-up at 36 months. Serum liver enzyme levels were normal, and the liver and biliary tree had regular aspects.
Risk factors for gallstones in children include hemolytic disease, obesity, prematurity, sepsis, total parenteral nutrition, chronic liver disease, inflammatory bowel diseases, diuretic use, and ceftriaxone use, all of which were ruled out, clinically and through laboratory and imaging data, as causes of the gallstones in our patient.
It is well-known that testosterone plays an important role in male sexual differentiation, as well as being a central modulator of metabolic processes. To date, limited data are available regarding testosterone's effects on the modulation of hepatic cholesterol homeostasis-related proteins.4–5
Cholesterol homeostasis is controlled by coordinated changes in the expression of multiple genes involved in cholesterol biosynthesis, uptake, and efflux. The transhepatic transport of cholesterol from plasma lipoproteins into the bile is critical for overall cholesterol homeostasis and its alterations may lead to cholesterol gallstone formation.6
Several studies have been conducted on animals in relation to the effects of androgens on lipid metabolism. Tyagi et al. and Kline et al. demonstrated that LDL cholesterol levels increased significantly in animals treated with testosterone enanthate and its active metabolite inhibited gallbladder motility through nongenomic actions.7,8
Concerning plasma cholesterol uptake in the liver, the low-density lipoprotein receptor (LDLr) is responsible for removing LDL cholesterol from the blood. A recent study demonstrated that testosterone deficiency causes severe hypercholesterolemia and altered hepatic LDLr expression, with reduced LDL-cholesterol clearance. That could be reversed by testosterone replacement therapy, with increased cholesterol uptake by the liver and its biliary concentration.5
There are also several studies in the literature conducted on prostate cancer cell lines demonstrating that androgens cause a marked and coordinated upregulation of the expression of several lipogenic genes.9 In particular, Heemers et al. demonstrated androgen-induced changes in lipogenic gene expression in androgen-responsive tissues outside of the genital tract.10
In addition, our patient had an elevated biliary saturation index and mean cholesterol content in the gallstones, according to the literature.
All these data support the hypothesis that testosterone plays an important role in cholesterol metabolism. In fact, testosterone increases cholesterol uptake by the liver through the regulation of LDLr expression, most likely leading to augmented biliary concentration and reduced gallbladder motility.5,8
Considering all the above data, we support the hypothesis that androgen therapy improves cholesterol production and uptake by the liver, with an augmented risk for gallstones. Further studies providing more data are needed to support our hypothesis.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Squarza S, Rossi UG, Torcia P, Cariati M. Asociación de colelitiasis y terapia de reemplazo de testosterona en un paciente con hipogonadismo primario. Revista de Gastroenterología de México. 2018;83:205–207.