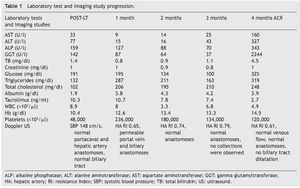
The patient is a 54-year-old woman who underwent a liver transplantation due to primary biliary cirrhosis with a Child-Turcotte-Pugh class C (10 points) and a MELD score of 16. An orthotopic liver transplantation (OLT) was performed on January 27, 2012, employing the piggy-back technique (sparing the vena cava). End-to-end (E-E) hepatic artery anastomosis was carried out, as well as E-E portacaval anastomosis, and an intraoperative biliary endoprosthesis was placed (it was removed on post-transplantation day 45 [post-OLT]). Basiliximab induction therapy and maintenance immunosuppressive therapy with prednisone, mycophenolate mofetil, and tacrolimus were carried out. Follow-up was weekly the first 3 months, after which it was every 2 weeks. At follow-up the patient had adequate blood levels of tacrolimus, using a generic brand of the drug (7.4-10.7 ng/ml). The mycophenolate mofetil dose was 1,000-1,500 mg every 12 h, according to tolerance and side effects. Prednisone was progressively reduced 2.5 mg per month and maintained at a dose of 10 mg per day.
On the fourth post-OLT month, the patient (without previously notifying the medical team) changed the generic brand of tacrolimus. At her next medical consultation, she presented with conjunctival icterus, cytolysis, and cholestasis (Table 1), and residual tacrolimus blood levels of 2.7 ng/ml (previously 7.4 ng/ml) with the same dose. Other causes of tacrolimus level reduction were ruled out (such as how the drug was administered, the intake of other medicine, gastrointestinal alterations, absorption problems, etc.). The patient was hospitalized and the medications used upon her admission were: mycophenolate 1,000 mg every 12 h, prednisone 10 mg a day, tacrolimus 3 mg every 12 h (the dose was doubled upon admission), amlodipine 5 mg a day, aspirin 100 mg every 24 h, metformin 500 mg every 8 h. Because the main causes of graft dysfunction are infection, vascular complications, and rejection, the necessary studies for the treatment approach were done. A Doppler ultrasound showed: normal arterial flow with a post-hepatic artery anastomosis resistance index of 0.61, normal portal and suprahepatic venous flow, and no dilatation of the biliary tract. A pp65 antigenemia assay was done to rule out CMV infection and was negative. Percutaneous biopsy was performed that showed changes consistent with acute cellular rejection (ACR) (Fig. 1). Upon admission, 3 boluses of 1 g of methylprednisolone were administered; there was progressive improvement in the liver function tests (LFTs) and upon release they were: ALT 79 U/l, AST 42 U/l, alkaline phosphatase 292 U/l, GGT 1,283 U/l, total bilirubin 2.3 mg/dl, and albumin 3.8 g/dl. The patient was released with immunosuppressive treatment: PrografTM (tacrolimus) 10 mg every 12 h with residual blood levels of 22 ng/ml, mycophenolate mofetil 1,500 mg every 12 h and prednisone 20 mg per day. Her hospital stay was one week.
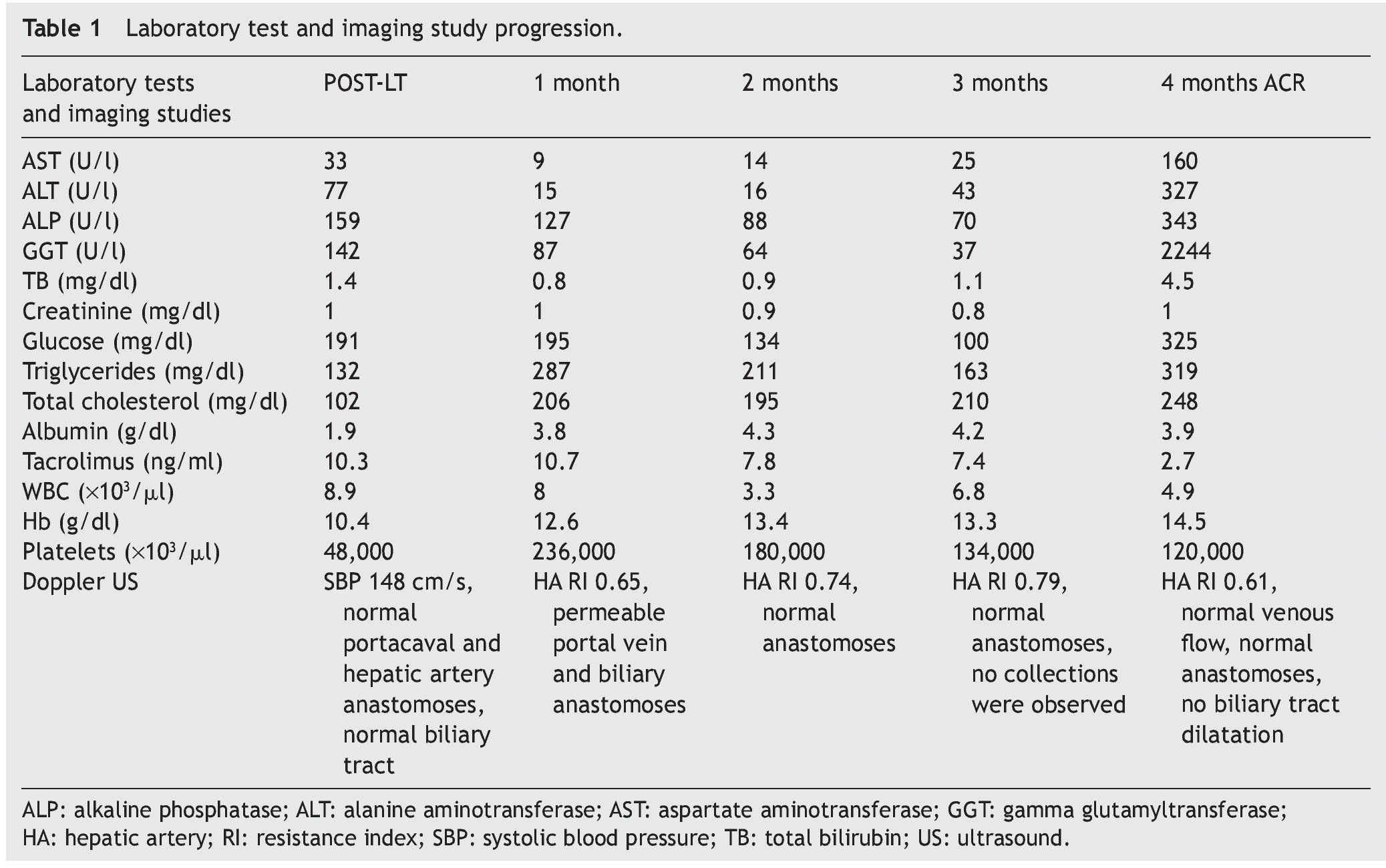
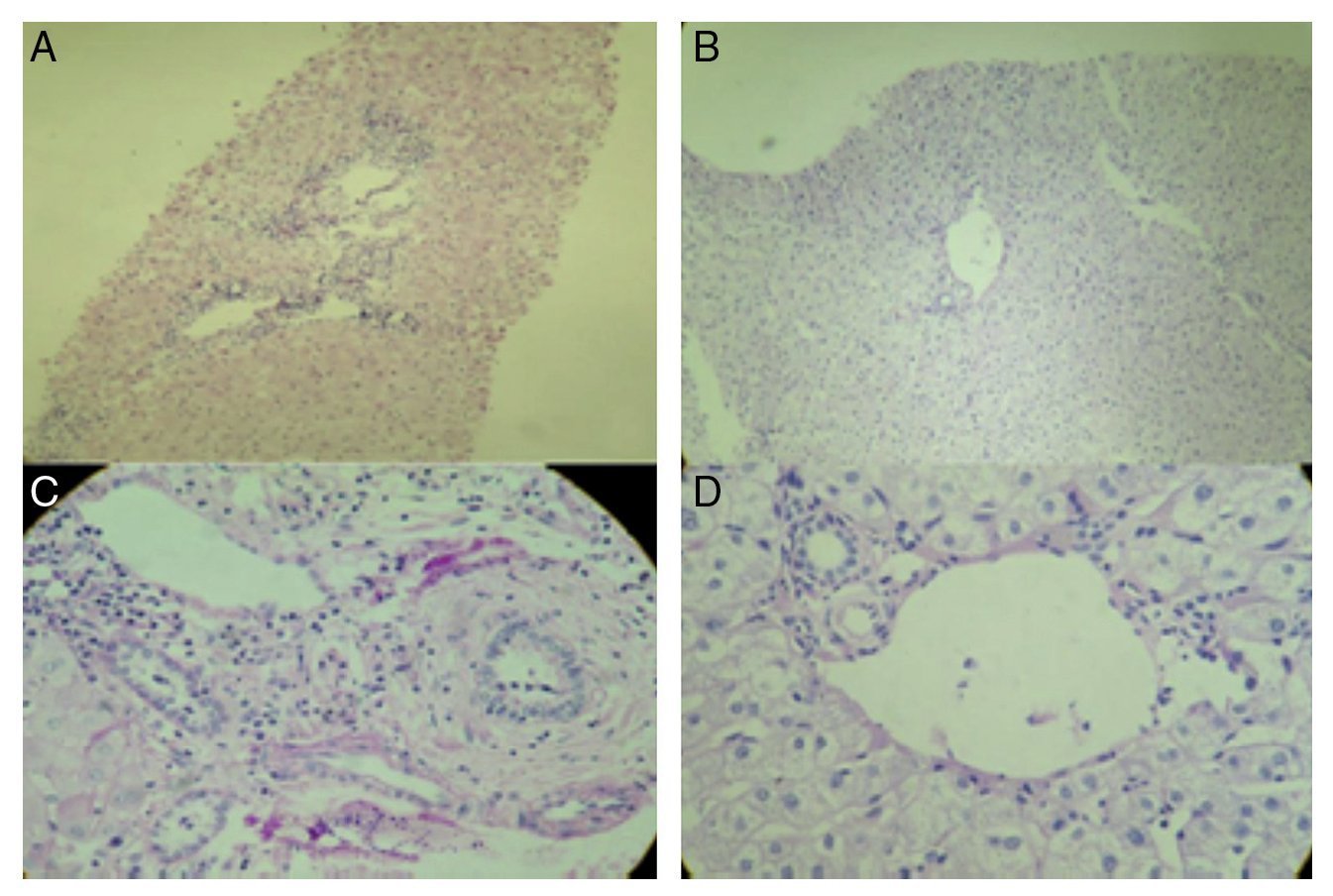
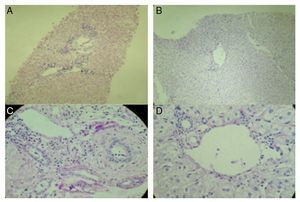
Figure 1. A) Hematoxylin and eosin stain of the percutaneous liver biopsy of the patient showing acute cellular rejection without ductopenia. The analyzed sample has 6 portal spaces, all of which have inflammatory infiltrate made up of lymphocytes, plasma cells, eosinophils, and histiocytes. Ductopenia is not observed. There is endotheliitis. Two of the centrolobular veins have inflammation of the intimal and luminal layers and perivenular necrosis. The Banff score is 7 (moderate rejection). B) Annual control PAS stain in the liver transplantation patient. Normal hepatocytes are observed, the portal spaces do not present with lesions or vasculitis, there is no decrease of cholangioles, and no rejection data. Biopsy is within the normal range, without alterations. C) PAS stain: patient with ACR. D) PAS stain: normal liver parenchyma (annual control liver biopsy of the liver transplantation patient).
Four months after the first ACR episode the patient presented with cytolysis and cholestasis (AST 152 U/l, ALT 223 U/l, alkaline phosphatase 186 U/l, GGT 494 U/l). The patient refused a new liver biopsy. A Doppler ultrasound was done and was normal. Antinuclear antibodies (ANA), IgG, and anti-smooth muscle antibodies were ordered; the ANA test was positive at a dilution of 1:320 with a homogeneous pattern and the IgG was 1.5 N (these studies were normal pre-OLT) and so it was decided to treat the patient for de novo autoimmune hepatitis; her LFTs are currently normal.
The introduction of generic immunosuppressive (IS) medication was approved by the Food and Drug Administration (FDA) in August 2009.1,2 In the twenty-first century, tacrolimus is regarded as the immunosuppressant of choice. The patented name of tacrolimus (PrografTM, Astellas Pharma) lost its patent protection in April 2008 and on August 10, 2009, the FDA approved the first generic tacrolimus.2 In order to be bioequivalent, the generic drug must contain the same quantity of active substance, the same administration route, and the same dose; there should be no significant difference in the rate (maximum concentration) and the concentration of the area under the curve from the reference drug. Generic medications must have a 90% confidence interval and the bioequivalence should be between 80 and 125%.3 In Mexico the NOM-177-SSA1-1998 guideline establishes the criteria and requisites that must be met when the tests for demonstrating the interchangeability of generic medications are carried out,4 and there are presently 4 brands of oral generic tacrolimus5 (approved by the COFEPRIS).
Factors such as age, race, sex, diet, and metabolism and enteric transport alterations, as well as the polymorphisms of the enzymes that catabolize the IS drugs, can be the source of inter-individual variability.6 There are different recommendation guidelines for the use of generic drugs in transplantation7; however, the bioequivalence of some of them is still controversial.2,8,9 In a study conducted in Mexico, 3 different brands of generic tacrolimus were compared with the innovative substance (PrografTM). It was found that they were not bioequivalent to PrografTM.9 Nevertheless, even though they do not have the same bioequivalence, other studies have shown them to be effective and safe for use.10
Generic medications can be a useful and efficacious option in the treatment of transplantation patients. However, not all generic medication presentations have demonstrated bioequivalence and bioavailability. Moreover, there can be important differences among the different brands of generic IS drugs. Individual dose adjustment is crucial when using generic IS medications, not only for preventing acute rejection, but also for prolonging patient and graft survival.
Financial disclosure
No financial support was received in relation to this study.
Conflict of interests
The authors declare that there is no conflict of interest.
Acknowledgments
Navarro Reynoso Francisco Pascual, M.D., García-Covarrubias Luis M.D., Luque Hernández Alejandro, M.D., Cicero-Lebrija Alejandra M.D., Hinojosa Heredia Héctor, M.D., Fernández Ángel Diana, M.D., Silva de Navarro Carolina, Espinosa Escobar Carolina, T.S.
* Corresponding author at:
Hospital General de México "Dr. Eduardo Liceaga" OD, México, DF.
Dr. Balmis 148 Colonia Doctores.
Código Postal 06726. Delegación Cuauhtémoc. Mexico City.
Tel.: +27 89 2000 extensión 1253.
E-mail addresses:jacquiemex2@yahoo.com.mx, jacquiemexfr@hotmail.com (J. Córdova).