Point-of-care ultrasound (POCUS) refers to the use of ultrasound imaging through pocket-sized sonographic devices at the patient’s bedside, to make a diagnosis or direct a procedure and immediately answer a clinical question. Its goal is to broaden the physical examination, not to replace conventional ultrasound studies. POCUS has evolved as a complement to physical examination and has been adopted by different medical specialties, including hepatology. A narrative synthesis of the evidence on the applications of POCUS in hepatology was carried out, describing its usefulness in the diagnosis of cirrhosis of the liver, metabolic dysfunction-associated steatotic liver disease (MASLD), decompensated cirrhosis, and portal hypertension. The review also encompasses more recent applications in the hemodynamic evaluation of the critically ill patient with cirrhosis of the liver, patients with other liver diseases, as well as in the ultrasound guidance of procedures.
POCUS could make up part of the daily clinical practice of gastroenterologists and hepatologists, simplifying the initial evaluation of patients and optimizing clinical management. Its accessibility, ease of use, and low adverse event profile make POCUS a useful tool for the properly trained physician in the adequate clinical setting. The aim of this review was to describe the available evidence on the usefulness of POCUS in the daily clinical practice of gastroenterologists and hepatologists.
La ecografía en el punto de atención (POCUS) se refiere a la utilización del ultrasonido (US) mediante dispositivos ultrasonográficos de bolsillo, al pie de la cama del paciente, con el objetivo de establecer un diagnóstico o dirigir un procedimiento y responder a una cuestión clínica de forma inmediata, su finalidad es ampliar la exploración física, no sustituir la evaluación ultrasonográfica convencional. POCUS ha evolucionado como un complemento del examen físico siendo adoptado por distintas especialidades médicas, incluyendo la hepatología. Se elaboró una síntesis de evidencia narrativa sobre las aplicaciones de POCUS en hepatología, describiendo la utilidad de POCUS en el diagnóstico de cirrosis hepática, enfermedad hepática esteatósica asociada a disfunción metabólica (MASLD, por sus siglas en inglés), cirrosis descompensada y el diagnóstico de hipertensión portal, así como las más recientes aplicaciones de POCUS en la evaluación hemodinámica del paciente con cirrosis hepática en estado crítico, otras enfermedades hepáticas y guía ultrasonográfica de procedimientos.
POCUS podría formar parte de la práctica clínica diaria de gastroenterólogos y hepatólogos, simplificando la evaluación inicial de los pacientes y optimizando el manejo clínico. Su accesibilidad, facilidad de uso y bajo perfil de efectos adversos la hacen una herramienta útil para el médico propiamente entrenado en el escenario clínico adecuado, por lo que el objetivo de esta revisión fue describir la evidencia que existe sobre la utilidad de POCUS en la práctica clínica diaria de gastroenterólogos y hepatólogos.
Understanding the current context of modern medicine would be difficult without the innovations and controversies in the practice of medicine of the past. The tools utilized in the conventional physical examination of the patient have experienced their curve of usefulness and validity. Obviously, auscultation with a Pinard stethoscope had to come first, paving the way for auscultation with modern stethoscopes and Doppler ultrasound for fetal auscultation. Thus, the technology in clinical strategies advances as needs change, in a type of “trial and error” that perhaps will never produce the perfect tool for examining the patient. With respect to technology, more is not necessarily better. The advent of new tools and advances is accompanied by discrepancies in their acceptance. To adopt or reject their implementation, we should ask ourselves: Am I rejecting it because I haven’t learned how to use it? Am I rejecting it because I’m unfamiliar with it? Am I rejecting it because I don’t think it’s useful? Am I accepting it just because it’s new?1–4
If the answer is “yes” to any of those questions, most likely we have chosen to miss the opportunity to validate a method involved in the diagnostic process. Our actions in medicine should not be guided by preference alone. Preferring a hard copy of a journal over a digital version is not the same thing as preferring to utilize a plain chest x-ray over a tomography scan at a center at which both tools are available; but it would be much less appropriate to try and replace the diagnostic method with only pulmonary ultrasound (US) because it is new and available. The added value inherent in US would be the overall examination of the hemodynamic status, the pumping function of the heart, and the pulmonary and abdominal status, among others, directly related to the central motivation behind the design and implementation of diagnostic tools: the benefit to the patient.2,5,6 Therein lies the importance of the words “point of care”. The term does not refer to a dichotomous diagnostic test but rather a means for providing the patient with a comprehensive instrumented evaluation aimed at decision-making, just as physical examination has done throughout history.2
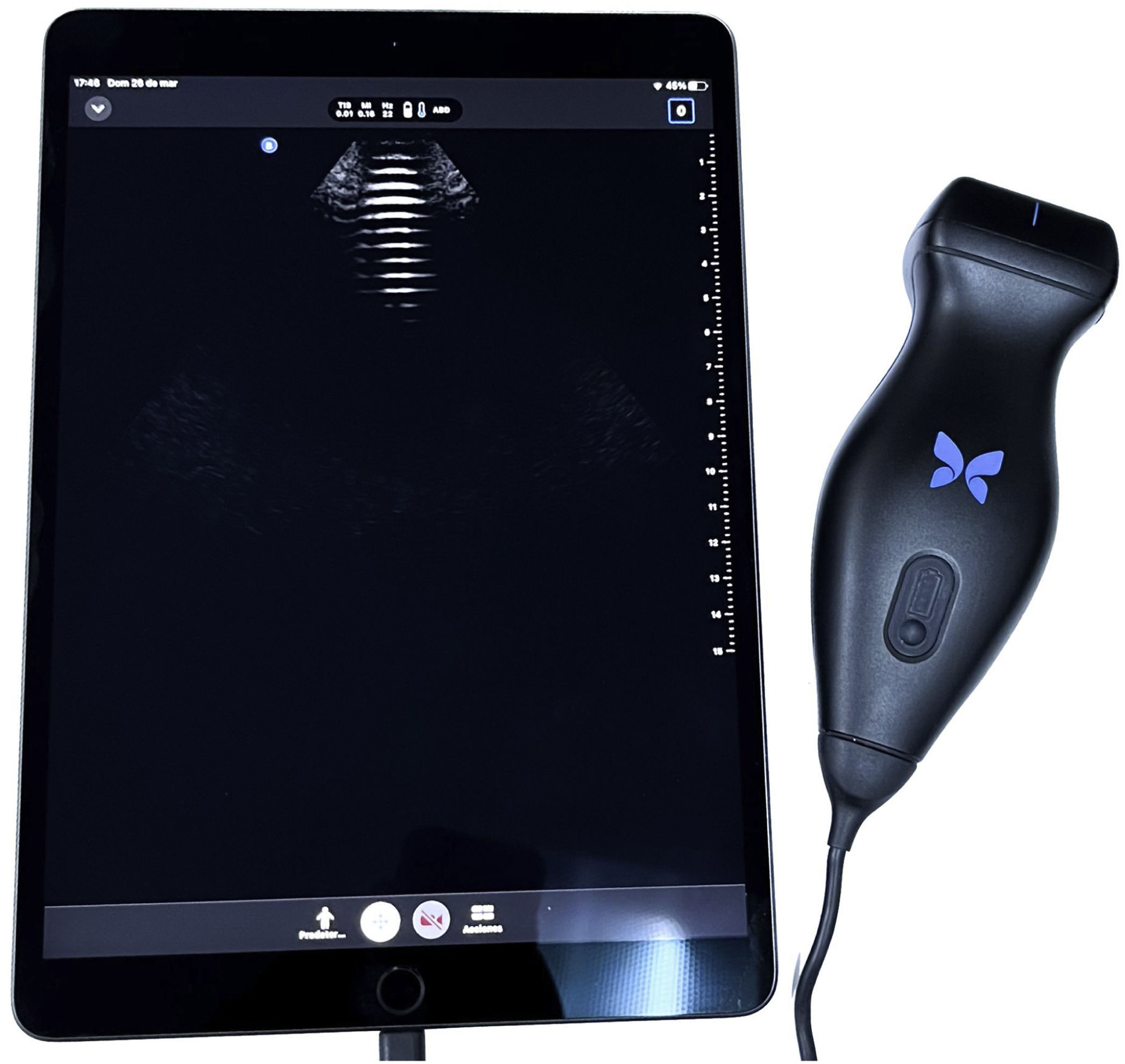
Point-of-care ultrasound (POCUS) generally utilizes US with pocket-sized sonographic devices (Fig. 1) at the patient’s bedside, to make a diagnosis or direct a procedure and immediately answer a clinical question. Its goal is to broaden the scope of physical examination, not to replace it or make US a systematic modality, as employed by radiologists. POCUS has evolved as a complement to physical examination and has been adopted by different medical specialties, including hepatology.1–4
MethodologyA narrative synthesis of evidence was carried out according to the following steps. First step: topics focusing on POCUS in hepatology were selected. Second step: two coauthors (DKTC and JAVRV) evaluated the information, synthesizing and codifying it into different topics. Third step: after codifying the information, specific questions were formulated and a systematic review was carried out, utilizing the DynaMed, Google Scholar and PubMed databases. The hierarchy of the pre-evaluated evidence pyramid was employed to obtain the information, starting with evidence summaries (clinical practice guidelines, UpToDate, and Dynamed), followed by evidence syntheses/synopses (systematic reviews), and lastly, original studies (randomized controlled trials and observational studies). The information search was carried out in Spanish and English. The following keywords were used: “cirrhosis”, “decompensated cirrhosis”, “compensated chronic liver disease”, “steatosis”, “nonalcoholic fatty liver disease”, “portal hypertension”, “POCUS”, “liver ultrasound”, “pocket-sized ultrasound”, “bedside ultrasound”, with articles dating from 1992 to 2023. The results were sent to the team of coauthors, who, utilizing the standardized format, extracted the relevant information for its inclusion in the present narrative synthesis of evidence. The aim of this narrative review was to describe the available evidence on the usefulness of POCUS in the daily clinical practice of gastroenterologists and hepatologists. The most relevant topics are described below.
POCUS in compensated chronic liver diseaseDiagnosis of cirrhosis of the liverLiver biopsy is the gold standard for diagnosing cirrhosis of the liver, but it is an invasive test, which hinders its use in daily clinical practice. Liver US is a safe, inexpensive, and easy-to-use tool, with 55–80% sensitivity and 86% specificity for diagnosing cirrhosis.7,8 B-mode US enables the evaluation of parameters associated with chronic liver disease, such as the size of the liver and spleen, atrophy of the right lobe of the liver, hypertrophy of the caudate lobe, rounded liver edge, granular liver parenchyma, heterogeneity of the parenchyma, and nodular liver surface.9,10
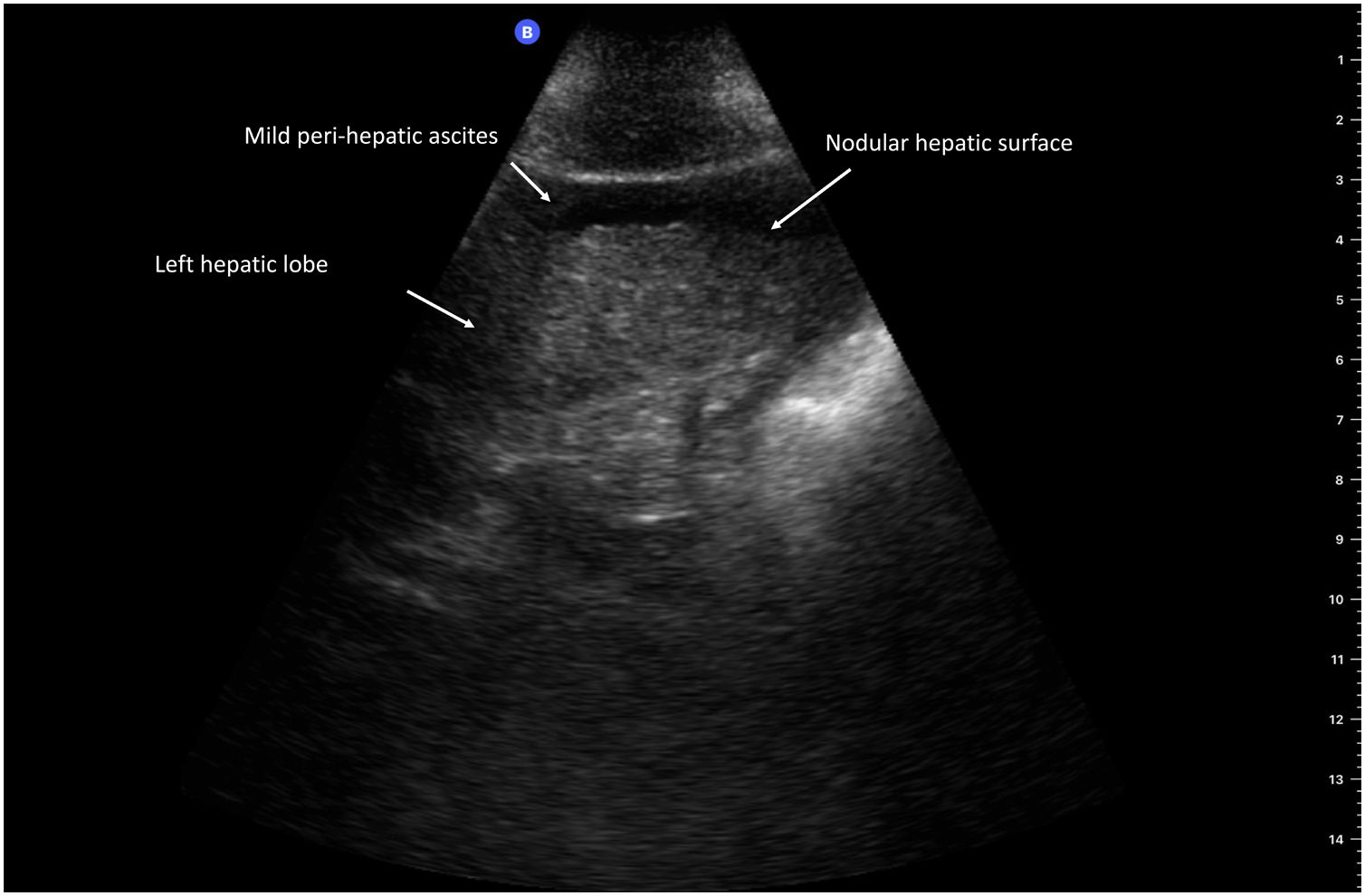
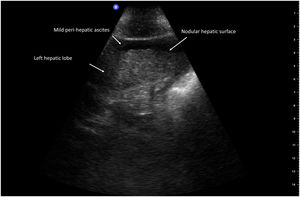
POCUS has been shown to be useful for diagnosing cirrhosis of the liver by identifying a nodular liver surface (Fig. 2), which is the most accurate sonographic sign for its diagnosis, utilizing a pocket-sized device with a 5–12 MHz probe. It has a reported 87.5% sensitivity and 76.8% specificity, as well as adequate interobserver agreement (Cohen kappa coefficient: 0.87), suggesting that POCUS can be used as a first-line tool for identifying patients requiring more specialized techniques, consequently shortening the time it takes to make clinical decisions.11
Metabolic dysfunction-associated steatotic liver diseaseMetabolic dysfunction-associated steatotic liver disease (MASLD),12 formally known as nonalcoholic fatty liver disease (NAFLD), is the most common chronic liver disease, with a 25% prevalence worldwide.13
MASLD is currently described as a multisystemic disease, with extrahepatic manifestations and a close relation to metabolic syndrome (MetS); MASLD is considered the hepatic manifestation of MetS. The prevalence of MASLD increases as the components of MetS increase, such as abdominal obesity, dyslipidemia, high blood pressure, and type 2 diabetes mellitus (DM2).14–16
New knowledge about the pathogeny of the disease and its association with MetS and its components has resulted in the need to modify the diagnostic criteria. Demonstrating liver steatosis through imaging studies, serum biomarkers, or liver biopsy, added to the presence of overweight or obesity, DM2, or signs of metabolic dysfunction with at least two metabolic risk criteria (waist circumference ≥ 102/88 cm in men/women, blood pressure ≥ 130/85 mmHg, triglycerides ≥ 150 mg/dl, HDL cholesterol < 40/50 mg/dl in men/women, prediabetes, HOMA > 2.5 or C-reactive protein > 2 mg/l), is sufficient for diagnosing MASLD.17
Current guidelines recommend liver US as the first-line test for diagnosing steatosis. It is highly accurate for detecting moderate-to-severe steatosis but is not very reliable when steatosis is below 20%; and it is suboptimum in individuals with a BMI > 40 kg/m2, showing a 49% decrease in sensitivity and a 75% decrease in specificity.17,18 When the extension of steatosis is ≥20–30%, US achieves a sensitivity of 84.8% and a specificity of 93.6%, with an area under the receiver operating characteristic (AUROC) curve of 0.93 (0.91−0.95), similar to other imaging studies, such as tomography and magnetic resonance imaging, albeit with an elevated interoperator and interobserver variability.19
Liver steatosis produces an increase in the echogenicity of liver tissue, with a “bright liver” aspect. The image produced by B-mode US enables the severity of hepatic infiltration to be subjectively calculated into 4 grades: absence of steatosis (grade 0): normal echogenicity of the liver, compared with the kidney; mild (grade 1): diffuse increase in liver echogenicity, with adequate visualization of the diaphragm and portal vein wall; moderate (grade 2): moderate increase in liver echogenicity, with a slightly impaired appearance of the portal vein wall and the diaphragm; and severe (grade 3): a marked increase in liver echogenicity, with the absence of visualization of the diaphragm, the portal vein wall, and the posterior part of the right lobe of the liver.20
The usefulness of POCUS in the diagnosis of liver steatosis has been evaluated in different studies. Riley et al. trained a group of gastroenterologists (with no previous US training) so they could identify a series of sonographic characteristics of liver steatosis utilizing POCUS. They employed a Sonosite (Bothell, WA, USA) portable echograph, with an abdominal curved array probe and a bandwidth of 5−2 MHz, reporting an elevated sensitivity and specificity of 80 and 99%, respectively, as well as adequate interobserver agreement (kappa 0.76).21 Stock et al. described the diagnostic value and duration of the POCUS evaluation, with a pocket-sized US device at a frequency of 2–4 MHz, compared with a high-end instrument with a curved array probe and a frequency of 2–6 MHz (Sonoline Antares). The pocket-sized instrument detected 32 (84%) of the 38 pathologic liver findings, identifying all cases of liver steatosis (n = 20). However, the diagnostic criteria utilized were not described. Examination duration was shorter with the pocket-sized device (25.0 vs 29.4 min) (p < 0.001) because more time was needed to move and prepare the high-end equipment, but the actual duration of the US examination itself was shorter with the top-of-the-line instrument.22 Miles et al. evaluated the diagnostic yield for the specific detection of liver steatosis, using pocket-sized US (probe with a frequency of 5−1 MHz), compared with conventional US (GE Healthcare LOGIQ E9 with a curvilinear probe). The POCUS evaluations were performed by an internist certified in POCUS and a radiologist with 40 years of experience. The POCUS probe was placed on the right mid-axillary line at the level of the xiphoid process and adjusted for intercostal space visualization. Images of the hepatorenal interface were obtained in 2D B-mode. Fat infiltration was considered if the liver echogenicity was increased, compared with the right renal cortex, for POCUS, and the subjective classification of steatosis was utilized (4 grades from 0 to 3) for conventional US. Of the 100 patients included, 40% had fat infiltration in both evaluations, and POCUS sensitivity and specificity were 91 and 88%, respectively. There were discordant results in 11 patients and so a second revision was carried out. Two of the patients presented with steatosis, as had been shown in the evaluation with POCUS, suggesting that the discrepancy between the two tests is infrequent. Those patients were older, compared with the rest of the population (61 ± 9 vs 53 ± 15 years, p < 0.05), but sex and BMI were similarly distributed.23 In a recent study, Sourianarayanane and McCullough demonstrated that the histologic NAFLD activity score was correlated with the ultrasound fatty liver index (USFLI) of the POCUS examination (r = 0.59). A USFLI ≥ 6 is diagnostic for metabolic dysfunction-associated steatohepatitis (MASH), with 81% sensitivity, and MASH is ruled out with a USFLI ≤ 3, with 100% sensitivity.24
These findings suggest that the evaluation of MASLD through POCUS could be useful as a screening method for determining the presence or absence of steatosis. However, the precise quantification of the extension of steatosis and the evaluation of the grade of fibrosis, which is a determining factor in the prognosis of the disease, are beyond the scope of POCUS. Thus, other methods, whether estimating the grade of fibrosis through noninvasive scores, such as FIB-4, or elastography methods, such as FibroScan® (Echosens, Paris, France), should be employed. Table 1 describes the findings suggestive of MASLD in B-mode US and in POCUS.
Diagnosis of MASLD through B-mode US and POCUS.
| B-mode US | POCUS |
|---|---|
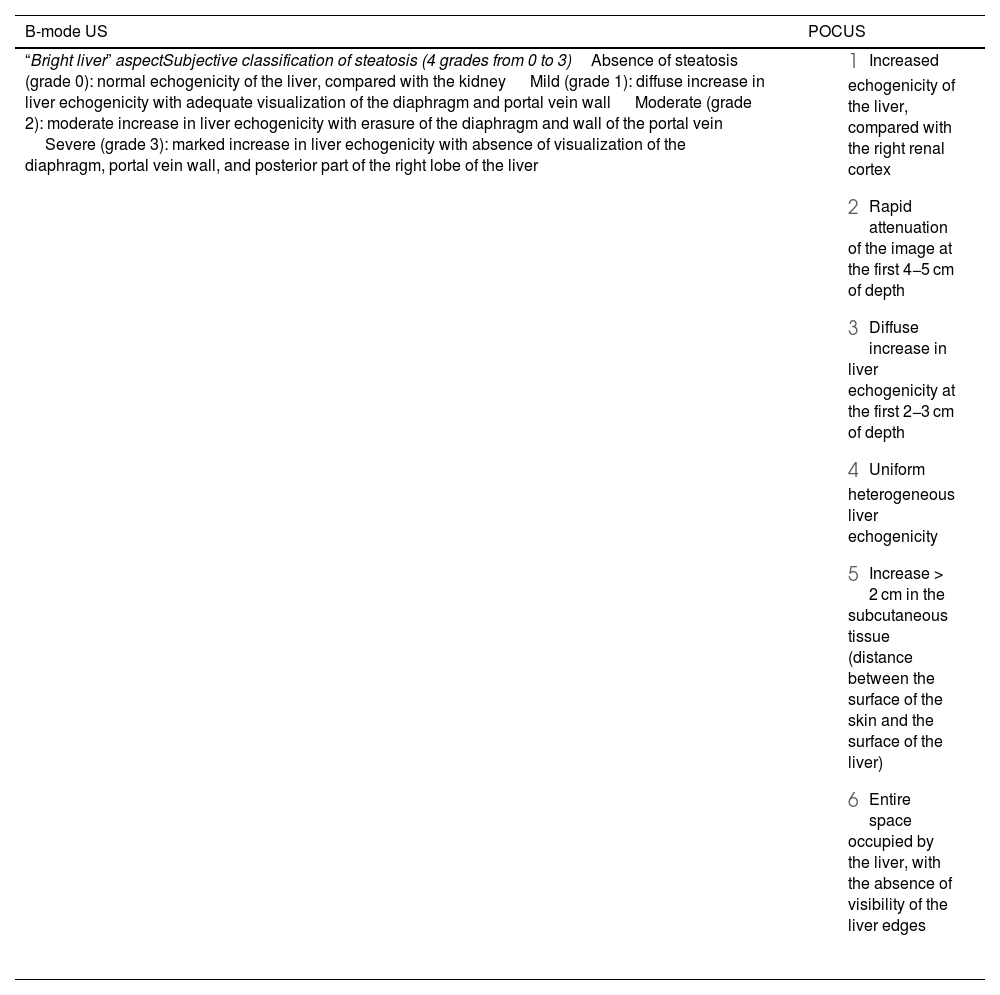
| “Bright liver” aspectSubjective classification of steatosis (4 grades from 0 to 3)Absence of steatosis (grade 0): normal echogenicity of the liver, compared with the kidney Mild (grade 1): diffuse increase in liver echogenicity with adequate visualization of the diaphragm and portal vein wall Moderate (grade 2): moderate increase in liver echogenicity with erasure of the diaphragm and wall of the portal vein Severe (grade 3): marked increase in liver echogenicity with absence of visualization of the diaphragm, portal vein wall, and posterior part of the right lobe of the liver |
|
With the increase in the prevalence of MASLD, the evaluation of steatosis through POCUS could make up part of the daily clinical practice of gastroenterologists and hepatologists, simplifying the initial evaluation of patients and limiting the number of visits. Even though POCUS cannot replace liver biopsy in monitoring the progression of simple steatosis to steatohepatitis or elastography in evaluating the grade of fibrosis, its accessibility, ease of use, and low adverse event profile make it a useful tool for the properly trained physician in the appropriate clinical setting.25
POCUS in decompensated chronic liver diseaseDetection of portal hypertensionPortal hypertension (PH) is the main consequence of cirrhosis of the liver and is a determining factor in the outcome of the disease.26 The increase in portal pressure ≥10 mmHg, also known as clinically significant portal hypertension (CSPH), predicts the development of clinical decompensation (ascites, variceal bleeding, or hepatic encephalopathy).27,28
The gold standard for evaluating portal pressure is measuring the hepatic venous pressure gradient (HVPG), which is the gradient between the pressure of the occluded hepatic sinusoidal capillary network pressure and the free suprahepatic venous pressure.29 HVPG measurement is unsuitable for daily clinical practice because it is an invasive technique, is costly, and is not widely available.9,29 Therefore, the use of noninvasive tools for diagnosing PH, such as liver US, plays an important role.
B-mode US enables the size of the spleen to be determined. Splenomegaly (1–2 standard deviations above the average) determined through the splenic volume index has been described as a predictor of PH.30 In addition, the combination of parameters, such as splenic elasticity, the diameter of the spleen, and platelet count, can be utilized to identify patients with CSPH.31 The application of POCUS, utilizing a pocket-sized device, has been shown to identify the presence of splenomegaly with a specificity >90% and a sensitivity >70%, along with a high level of agreement between conventional US and POCUS (kappa >0.6).32
In patients with PH, the umbilical vein is frequently found to be recanalized. It is the most specific sonographic sign for PH and can be observed as an enlarged vein at the level of the falciform ligament.33
The hemodynamics of the portal venous system can be evaluated using Doppler US, determining the parameters of blood flow and volume of the PV, the mean and peak PV velocity (PVV), the PV congestion index, and the resistance indices of the hepatic and splenic arteries.34 The SHV waveform can also be analyzed through pulsed wave Doppler US, which in healthy individuals has a triphasic pattern with 2 negative waves and one positive wave, whereas patients with PH show a biphasic or monophasic wave pattern, with 76% sensitivity and 82% specificity.9
The normal diameter of the PV is ≤13 mm in normal respiration, with a hepatopetal flow (toward the liver) and mean flow velocity of 15–18 cm/s. When there is an increase in portal pressure, the diameter of the PV increases (>13 mm), the direction of the flow can be inverted (hepatofugal) and the mean PVV is reduced due to the increase in intrahepatic vascular resistance,10 considering <15 cm/s the best cutoff point for detecting PH.35 On the other hand, due to the presence of portosystemic shunts, some patients with cirrhosis of the liver can present with normal or elevated PVV values;9 a significant correlation between the HVPG and the PVV has been demonstrated.36 Even though the new devices utilized in POCUS have the Doppler tool, the determination of PV flow velocities and the resistance indices are beyond the scope of POCUS.
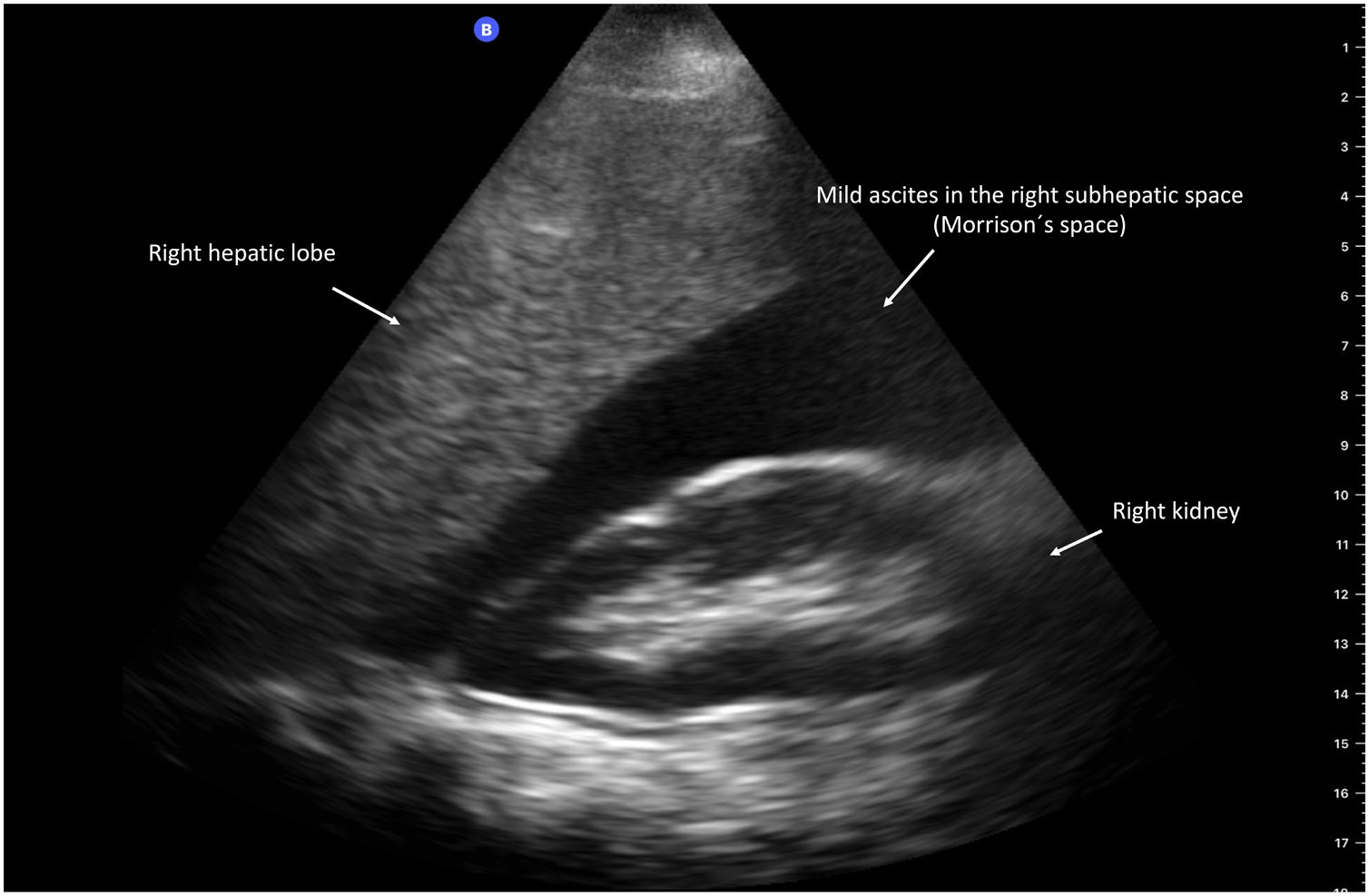
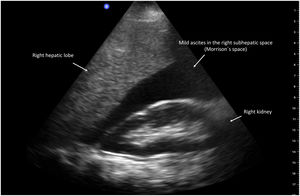
Ascites is the most frequent clinical decompensation in patients with cirrhosis of the liver. It is classified in 3 grades, according to the amount of fluid in the abdominal cavity: grade 1 or mild (sonographic), grade 2 or moderate (clinical), and grade 3 or severe (large or gross ascites with marked abdominal distension).37 POCUS is a reliable tool for evaluating ascites. Different studies have shown a good correlation between US with a pocket-sized device and conventional US with high-end equipment,4,38 reporting 95.8% sensitivity and 81.8% specificity for diagnosing ascites (Fig. 3).39
Hemodynamic evaluation of the critically ill patientBasic hemodynamic monitoring of the critically ill patient includes carrying out a complete clinical history with a directed physical examination and vital sign evaluation (heart rate, mean arterial pressure, respiratory rate, temperature, capillary oxygen saturation, and urinary output quantification). However, new evidence suggests that this initial approach may be insufficient for the adequate evaluation of the hemodynamic status of critically ill patients.40 Hemodynamic status evaluation has evolved thanks to the implementation of sonographic indicators of fluid status. POCUS can evaluate the heart, abdominal veins, and lungs (“pump-pipes-leaks” focus), obtaining information about the hemodynamic system and optimizing clinical management.41
Lung US can identify the presence of fluid in the pulmonary interstitium (extravascular lung water) through the observation of B lines suggestive of diffuse pulmonary edema that are equivalent to the Kerley B lines observed in chest x-rays.41 This technique can differentiate between pneumonia, pleural effusion, interstitial edema, and collections, as well as determine the causes of ventilation weaning failures, such as stroke, atelectasis, and pneumothorax.42,43 The diagnostic yield of POCUS for detecting pulmonary congestion is superior to physical examination, making the diagnosis even before the appearance of symptoms.41,42
Cardiac POCUS is limited to the evaluation of left and right ventricular function and the presence of pericardial stroke.41 Cardiac POCUS has been shown to be useful in the hemodynamic evaluation of critically ill patients with liver cirrhosis. In situations of hypotension, POCUS can show alterations in the movement of the left ventricle that reflect the need for volume expansion with crystalloids or albumin to increase the pre-charge or decrease in systolic function requiring the use of inotropes.44
Inferior vena cava (IVC) evaluation through POCUS can determine fluid status, estimating the right atrial pressure (RAP), measuring the height of the point of collapse (analogous to the highest venous pulsation point when the internal jugular vein [IJV] pressure is inspected).42 The IVC collapses during inspiration due to negative intrathoracic pressure; a diameter ≤2.1 cm and collapse >50% indicate normal RAP (3 mmHg), whereas a diameter >2.1 cm and an inspiratory collapse < 50% indicate elevated RAP (≥15 mmHg).41 IJV POCUS is subject to errors due to the angle of inappropriate head elevation, the involuntary application of excessive pressure of the probe, and limited access to the neck due to catheters, tracheostomy collars, or orthopedic devices.42 IVC diameter and the IVC collapsibility index (IVCCI) have been shown to be reliable markers for estimating the fluid status of patients with cirrhosis of the liver and spontaneous breathing.45 However, a recent study showed that the evaluation by POCUS of the collapsibility index of the transverse area of the internal jugular vein at 30º had a better correlation with central venous pressure (r = –0.56, p = 0.001), compared with IVC diameter.46
IVC and/or IJV ultrasound are useful for estimating RAP, but the effects of elevated RAP in the organs should be measured through Doppler US of the abdominal veins, typically the SHV, PV, and renal vessels. This technique is known as venous excess ultrasound or VExUS.42
The evaluation of fluid status through VExUS can be difficult in patients with liver cirrhosis due to the characteristic hemodynamic changes in PH, such as the absence of flow pulsatility of the PV in cases of severe congestion and increased pulsatility not associated with RAP or the presence of a biphasic or monophasic pattern in the SHV. In addition, the diameter of the IVC can be falsely reduced in cases of tense ascites, despite excess intravascular volume,9,41,47 signifying that evaluations through POCUS and VExUS must be interpreted within the clinical context of the patient.41
POCUS in the administration of albuminGiven the consequences of iatrogenic fluid overload, empiric albumin administration has been a subject of attention, and findings suggest a high incidence of pulmonary complications.48,49 Because POCUS evaluates hemodynamic status more accurately than physical examination and conventional imaging studies,47,50 its routine use in patients at high risk for hypervolemia would be prudent.50
The diagnosis of type 1 hepatorenal syndrome (HRS-1) involves the absence of response to albumin administration for 48 h,51 but no objective evaluation parameter of fluid status is utilized. Velez et al. demonstrated that IVC and IVCCI measured through POCUS made it possible to evaluate the intravascular volume status in patients with cirrhosis and acute kidney injury that had received intravenous albumin due to the clinical suspicion of HRS-1. Only 36% (n = 19) of the patients were euvolemic, suggesting that the standard albumin dose could be insufficient for re-establishing volume, or contrastingly, could lead to fluid overload in some patients. Treatment modification with albumin administration at a dose of 1 g/kg/day, in patients with signs of volume depletion, and furosemide administration at a dose of 80–160 mg/8−12 h, in patients categorized as fluid-overloaded in the POCUS evaluation, resulted in early improvement of kidney function in 23% of the cases.52
Therefore, the combination of hemodynamic parameters evaluated by POCUS enables pulmonary and cardiac problem detection in patients with liver disease, fluid resuscitation dose quantification, opportune vasopressor commencement, dialysis dose quantification, and albumin or blood component safety determination in patients with volume overload.43,53
POCUS in other liver diseasesPOCUS in liver transplantationLiver transplantation (LT) is the definitive treatment for terminal liver diseases and acute liver failure. Currently, improvement in immunosuppression therapy and the perfecting of surgical techniques have resulted in an increase in the one, 5 and 10-year survival rates of up to 85, 73, and 62%, respectively. However, postoperative complications lead to a high risk for morbidity and mortality in the recipient and vary from 7 to 30%. Grey scale US and the color Doppler technique offer early identification of alterations in arterial, venous, and biliary anastomoses, minimizing complications and preventing graft loss.54
Hepatic artery thrombosis is the most dreaded postoperative vascular complication of LT, given that it can affect the biliary tract and produce graft loss; the resulting damage is directly proportional to the time of evolution. On the other hand, hepatic artery stricture progresses slowly and insidiously, conditioning biliary damage due to ischemia and the formation of collateral circulation. Another less frequent complication is the formation of a pseudoaneurysm, and even less frequently, hypoperfusion due to arterial steal. The initial clinical manifestations of thrombosis and stricture of the hepatic artery are specific, thus, vigilance through color Doppler US in the postoperative and follow-up periods is crucial for the timely diagnosis and therapeutic approach.55,56
Complications of venous anastomoses are rare. Their graft function repercussions and clinical manifestations vary; they can be asymptomatic or present with clinical characteristics typical of PH, according to the area and extension of the lesion. Both complications show highly suggestive images on the color Doppler test, added to the identification of ascites due to the increase in portal pressure.57
Bile duct leak, the formation of biloma, anastomotic stricture, and non-anastomotic stricture are the most frequent biliary complications. Other bile duct complications are choledocholithiasis and biliary sludge, which are associated with denervation during the surgical procedure. Grey scale US enables the identification of intrahepatic and extrahepatic bile duct dilation and stricture, as well as dilation zones, providing a basis for performing further studies, such as cholangioresonance, for complete characterization and treatment selection.58,59
Grey scale US and evaluation with color Doppler in LT patients is an easy, accurate, and rapid method for the early detection of vascular structure and bile duct alterations, enabling the selection of patients that require further tests for achieving timely treatment, thus improving survival by preventing graft injury and loss.58
Liver abscessLiver abscess is an infectious liver lesion of bacterial (Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus or Streptococcus species) or amebic (Entamoeba histolytica) etiology. The classic symptom triad is fever, jaundice, and right upper quadrant sensitivity. It presents in from only 7–43% of cases.60 US is the first-line diagnostic tool, whose sensitivity ranges from 67 to 86%. POCUS has been reported as useful in making the differential diagnosis for abdominal emergencies seen at the emergency room, including liver abscess, thus optimizing clinical management.60,61 Blomquist et al. described the clinical case of a 92-year-old woman with sepsis, in whom POCUS evaluation prior to abdominal tomography facilitated the diagnosis of liver abscess and the early start of antibiotic therapy.62
Ultrasound-guided procedures: paracentesisAscites is the pathologic accumulation of fluid in the peritoneal cavity. Cirrhosis of the liver is the main cause of ascites (80% of cases) and annually presents in 10% of patients with compensated liver cirrhosis.37
Abdominal paracentesis consists of extracting ascitic fluid by inserting a needle into the peritoneal cavity, and it is generally a bedside procedure. It can be diagnostic or therapeutic and is performed with the “blind” venipuncture technique or is US-guided.63 There are few absolute contraindications for carrying out paracentesis, such as reduced intravascular coagulation. Relative contraindications include pregnancy, prolonged coagulation times, thrombocytopenia, adhesions, intestinal obstruction, distended bladder, infection or hematoma at the puncture site, and poor patient cooperation. In general, the complications of paracentesis are rare, such as persistent ascitic fluid leakage, puncture site infection, or abdominal wall hematoma. More severe complications are bleeding, with an estimated incidence below 0.2%, and puncture of other organs or the inferior epigastric artery.64
Imaging-guided diagnostic and therapeutic procedures are the cornerstone of contemporary clinical practice, given that they reduce morbidity and improve safety and efficacy. The availability of portable devices enables the use of POCUS to guide procedures performed by the treating physician at the time of clinical evaluation.1 US can diagnose ascites, identify the puncture site prior to the procedure, and evaluate needle insertion in real time.63
US-guided paracentesis has been described as safer and more efficacious than blind paracentesis.65 Bard and Lafortune reported the presence of gas-filled intestinal segments between the abdominal wall and the fluid in the expected tract of the needle in 6 out of 8 patients in whom fluid had been detected at the usual blind puncture site (flanks).66 In a retrospective analysis of 1297 abdominal paracenteses, of which 723 (56%) were US-guided and 574 (44%) were blind procedures, a lower incidence of adverse events was reported in the US-guided interventions (1.4% vs 4.5%, p = 0.01), including post-paracentesis infection (1.41% vs 2.44%, p = 0.01), hematoma (0.0% vs 0.87%, p = 0.01), and seroma (0.14 vs 1.05 %, p = 0.03).67 The recent study conducted by Rodrigues et al. concurs with those findings. Using POCUS, a change in needle insertion ≥ 5 cm from the conventional anatomic puncture site was made in 69% of the 45 procedures performed, and the average depth of fluid was greater at the POCUS site, compared with the conventional puncture site (5.4 ± 2.8 cm vs 3.0 ± 2.5 cm, p < 0.005).68 In a retrospective study of 72 cases of ascites evaluated by POCUS, 30% were not candidates for paracentesis, upon showing less than 1 cm of fluid in the peritoneal cavity.39 Lastly, the use of Power Doppler, now available in the majority of POCUS devices, has been shown to improve visualization of the abdominal vasculature and reduce the risk for vascular puncture and possible subsequent bleeding.69
The use of US for guiding procedures requires competence on the part of the clinician for performing the procedure. In general, 25–50 evaluations are needed to ensure competence in the majority of sonographic diagnostic procedures and 10 for guiding the therapeutic procedures with US.1 The following are recommendations for performing POCUS-guided paracentesis:65
- -
Utilize a 3 MHz curvilinear probe for the initial evaluation and a 10 MHz linear probe to confirm the puncture site.
- -
Locate the largest fluid pocket (≥3 cm)
- -
Identify critical vascular structures, such as the inferior epigastric vein and artery (through B-mode or color Doppler US imaging)
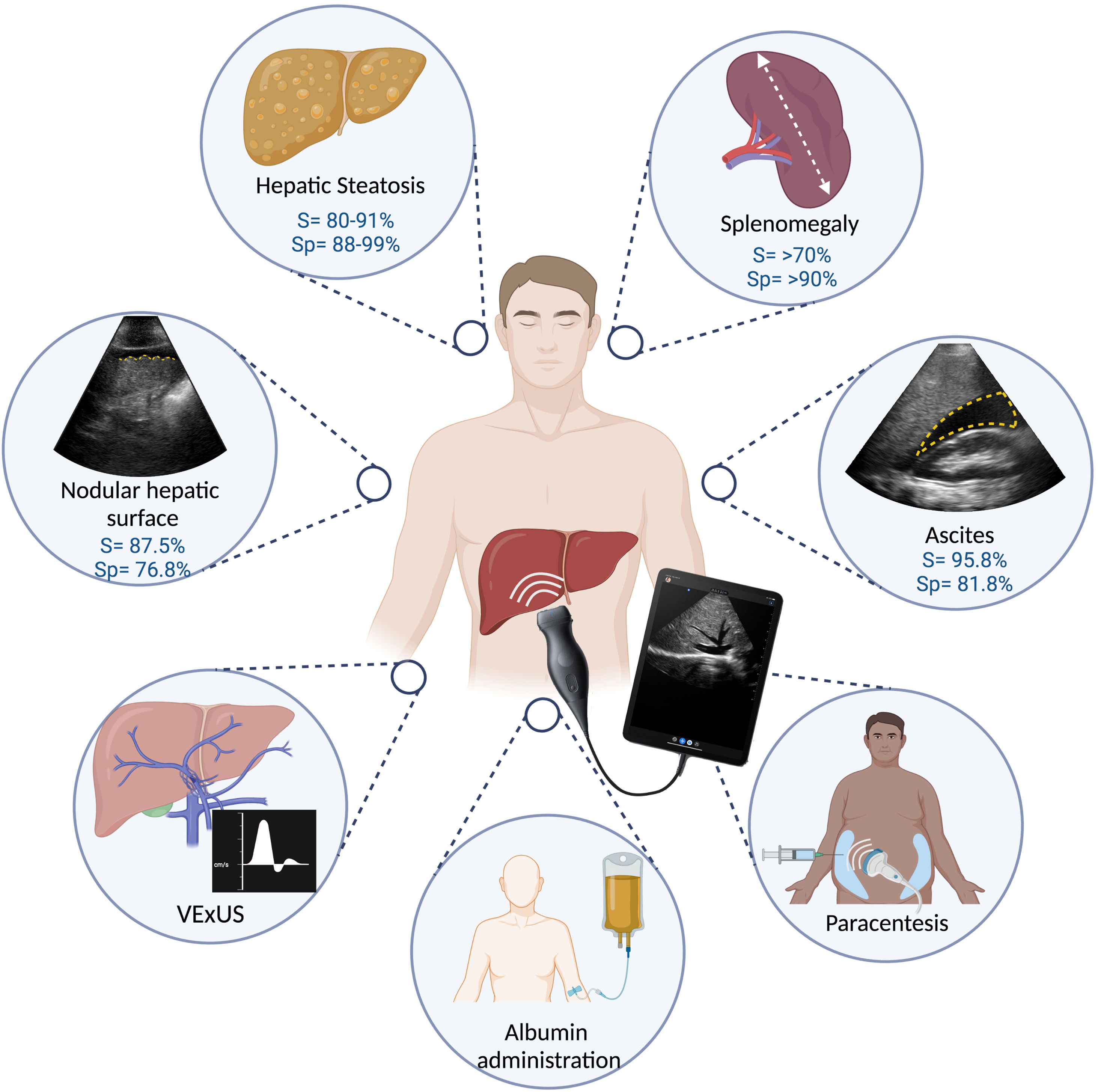
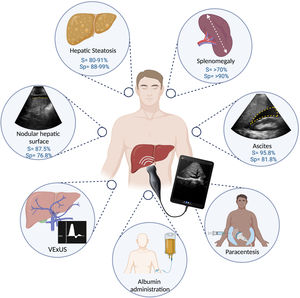
The impact of POCUS in medicine goes hand in hand with its existence and availability, without yet being able to surpass the definitive diagnostic tools of each medical condition. It enables decisions to be made based on habitual criteria: history, symptoms, and signs (today clinical and laboratory). Anyone who discredits the value of POCUS would also have to discredit giving immediate treatment when an exacerbation of asthma is suspected, after evidence of dyspnea and pulmonary wheezing is identified during auscultation with the conventional stethoscope. Its importance in the standard of care of patients involves offering them the best attention, through having adequate resources. Concretely, in adequate hands, the implementation of POCUS has shown its usefulness in the decision-making process, which directly or indirectly has an impact on the items considered markers in quality of care, such as: days of hospital stay, significant changes in the diagnosis or main treatment, and the addition of relevant diagnoses.2,5,6 The COVID-19 pandemic has shown that, in rapid decision-making settings, the availability of bedside tools can optimize the diagnostic process.70 It also coincides with the uptick in enthusiasm for learning to use POCUS, with the potential benefits at the individual and healthcare system levels. POCUS makes up part of the daily clinical practice of diverse specialties and has shown its usefulness in different clinical settings in hepatology (Fig. 4), requiring basic training for evaluating the liver surface, steatosis, splenomegaly, and procedures such as diagnostic paracentesis and the more specialized fluid status evaluation (VExUS), as offered by the “Alliance for Physician Certification & Advancement”.11,21,23,32,39 The acquisition of pocket-sized US equipment requires an initial investment that varies, depending on the quality of the equipment. Because it is a relatively new tool, more comparative studies are needed to evaluate the performance of POCUS, in the field of hepatology, including the evaluation of the hemodynamic status in the critically ill patient with cirrhosis of the liver, as well as albumin administration.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Velarde-Ruiz Velasco JA, Tapia Calderón DK, Llop Herrera E, Castro Narro G, García Jiménez ES, Cerda Reyes E, et al. Más allá de la exploración física convencional en hepatología: POCUS. Rev Gastroenterol Mex. 2023;88:381–391.