Colorectal cancer is frequent in the developed countries, with a cancer-specific mortality rate of 33%. Different biomarkers are associated with overall survival and the prediction of monoclonal treatment effectiveness. The presence of mutations in the K-ras oncogene alters the response to target therapy with cetuximab and could be an independent prognostic factor.
AimsTo analyze the difference in survival between patients with mutated K-ras and those with K-ras wild-type status.
MethodsThirty-one clinical records were retrospectively analyzed of patients presenting with colorectal cancer that underwent K-ras sequencing through real-time polymerase chain reaction within the time frame of 2009 to 2012 at the Hospital de Alta Especialidad de Veracruz of the Instituto para la Salud y Seguridad Social de los Trabajadores del Estado (HAEV-ISSSTE). Survival analysis for patients with and without K-ras mutation was performed using the Kaplan Meier method. Contrast of covariates was performed using logarithmic transformations.
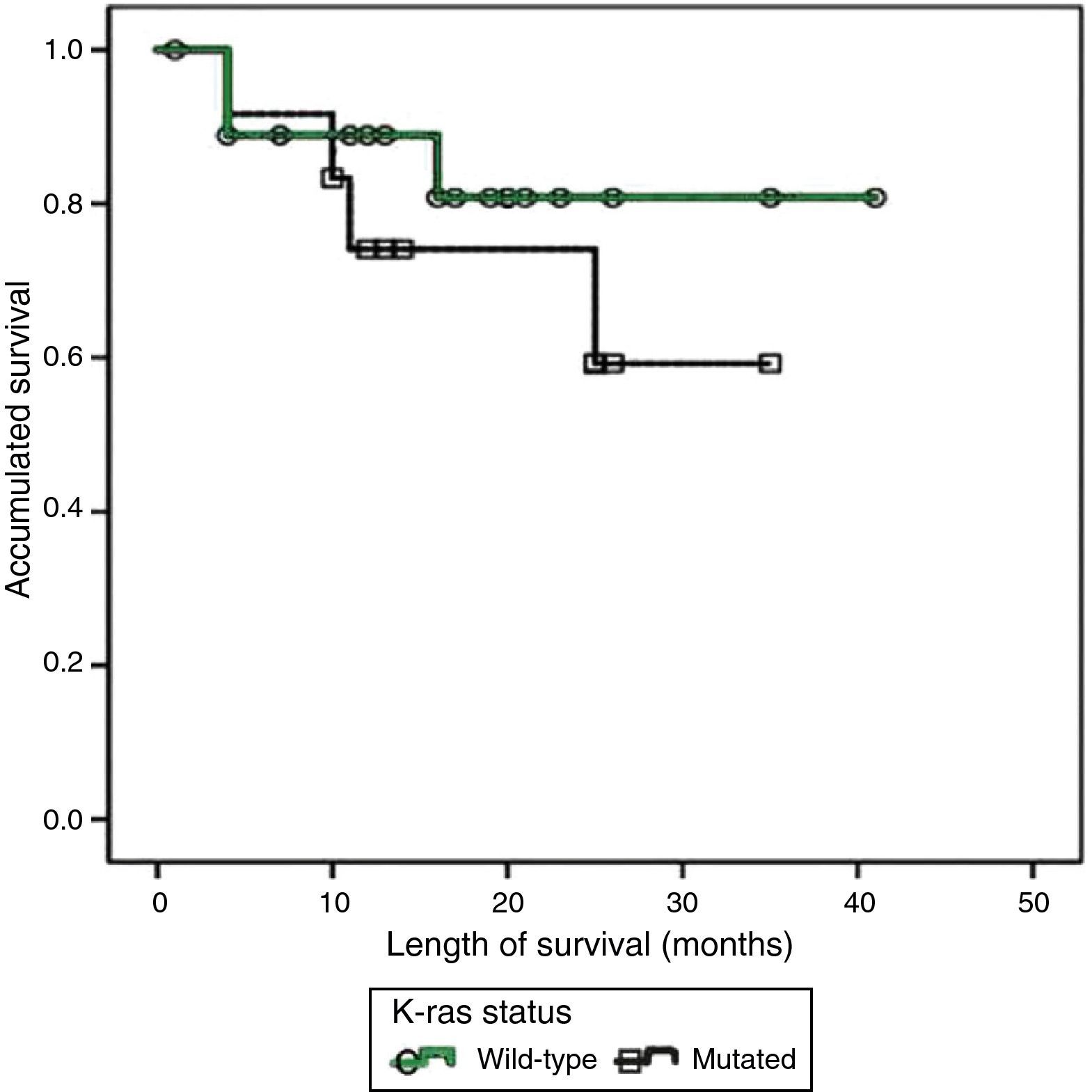
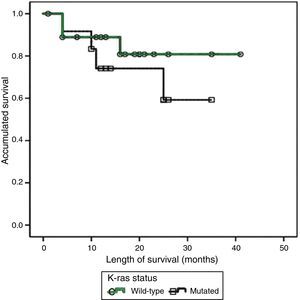
ResultsNo statistically significant difference was found in relation to survival in the patients with mutated K-ras vs. those with K-ras wild-type (P=.416), nor were significant differences found when analyzing the covariants and survival in the patients with mutated K-ras: ECOG scale (P=.221); age (less than, equal to or greater than 65years, P=.441); clinical stage according to the AJCC (P=.057), and primary lesion site (P=.614).
ConclusionsNo relation was found between the K-ras oncogene mutation and reduced survival, in contrast to what has been established in the international medical literature. Further studies that include both a larger number of patients and those receiving monoclonal treatment, need to be conducted. There were only 5 patients in the present study that received cetuximab, resulting in a misleading analysis.
El cáncer de colon es frecuente en los países desarrollados, con una mortalidad específica del 33%. Diversos biomarcadores son asociados a la sobrevida global de los pacientes o predicen el efecto del tratamiento monoclonal. La presencia de mutaciones en el oncogén K-ras afecta la respuesta a terapia blanco con cetuximab, y podría mostrar valor pronóstico de manera independiente.
ObjetivosAnalizar la diferencia en la supervivencia de pacientes K-ras mutados y en estado nativo.
MétodosSe analizaron retrospectivamente 31 expedientes clínicos de pacientes con cáncer de colon con secuenciación de K-ras utilizando reacción en cadena de polimerasa en tiempo real realizados desde el año 2009 y hasta 2012 en el Hospital de Alta Especialidad de Veracruz del Instituto para la Salud y Seguridad Social de los Trabajadores del Estado (HAEV-ISSSTE). Se analizó la supervivencia entre los pacientes con mutación y aquellos que no la tienen usando el método de Kaplan-Meier; las covariables se contrastaron usando transformación logarítmica.
ResultadosNo se halló una diferencia significativa en la sobrevida de los pacientes en los que se encontró K-ras mutado versus K-ras nativo (p=0.416). Al analizar covariables y supervivencia de los pacientes K-ras mutados, tampoco se obtuvieron diferencias significativas: escala ECOG (p=0.221); edad (<menor a=""> o <igual o="" mayor="" a=""> 65 </igual></menor>años, p=0.441); estadio clínico según AJCC (p=0.057); sitio de lesión primaria, p=0.614.
ConclusionesNo se encontró relación entre la mutación del oncogén K-ras y disminución en la sobrevida, a diferencia de lo establecido en la literatura. Es importante realizar estudios con un mayor número de pacientes y que se incluya el tratamiento monoclonal, que, en el presente, fueron solo 5 y su análisis es inverosímil.
It has been stated that one million persons worldwide develop colorectal cancer each year, resulting in a cancer-specific mortality rate in the developed countries that nears 33%, only behind the mortality rate for lung cancer, which is 13% in Mexico. In 2002, 108,064 new cases were reported in Mexico, representing a 50% increase in incidence over a period of 25 years.1–3 The etiology of this neoplasia is complex and includes a relation between environmental and genetic factors. The Kirsten Rat Sarcoma Viral Homolog (K-ras) oncogene encodes for a guanosine triphosphate (GTP)-binding protein (GTPase) that can mutate at very early stages of tumor development; it has been reported that 35 to 42% of adenomas possess a mutation in the K-ras gene, which promotes hyperplastic growth of the colonic epithelium, aberrant morphology in the crypt foci, tumor growth, progression, and local invasion and the formation of metastases.4,5 There are various techniques for detecting these mutations and direct sequencing of a PCR product is the method of choice, resulting in the so-called mutated K-ras and the mutated codon, or if there is no mutation, it is known as the wild-type K-ras.6–11
In relation to colorectal cancer management, initial treatment should be surgical with the intention to cure; the role of adjuvant therapy is limited by the modest increase in survival, and is considered for patients with clinical, histologic, and molecular high-risk recurrence factors.12 The addition of biologic therapy is on the rise because of its benefits over conventional cytotoxic therapy, but due to its high cost it is reserved for a specific group of patients. A broad review of the subject 13 concluded that K-ras status is an independent outcome factor, given that patients presenting with wild-type K-ras live longer and benefit more from monoclonal treatment; clinical trials such as CRYSTAL14 and OPUS15 found that these patients had greater treatment response, progression-free survival, and overall survival than those patients with mutated K-ras in groups undergoing conventional chemotherapy and chemotherapy plus monoclonal therapy. The evidence in recent studies, with respect to mutational status, tumor stage, and survival rate in patients under different treatment regimens showed that the detected mutations (especially at codon 12) conferred a worse outcome, as did sudden and aggressive liver metastases. The drug cetuximab is a monoclonal antibody that inhibits the epidermal growth factor receptor (EGFR) and is currently the biologic therapy of choice for colorectal cancer. Its use is reserved for wild-type K-ras patients only and is associated with other drugs such as capecitabine and oxaliplatin (XELOX regimen) or folinic acid and oxaliplatin (FOLFOX regimen).16,17 It is important to know the clinical differences between the mutated and non-mutated groups in the local population, given that their study in the Mexican population has been very limited and its relevance to clinical decision-making has been pointed out and reported in publications in other countries. The primary aim of the present study was to confirm whether there was a difference in survival related to the finding of mutations and if it was modified by other variables, in the same manner as has been described in the abovementioned international literature.
MethodsA retrospective, comparative, and analytic low-risk study was conducted in accordance with the 2008 Declaration of Helsinki and the Health Research Regulation of the General Health Law. Access to the medical records of our patients was authorized by the general administration office of the Hospital de Alta Especialidad de Veracruz that belongs to the Instituto para la Salud y Seguridad Social de los Trabajadores del Estado (HAEV-ISSSTE) and by the Bioethics Committee of the Universidad Veracruzana. Signed statements of informed consent for the study were not required given that the patients were not operated on or harmed in any way. The identity of each patient was protected through the use of numerical codes. We analyzed the data of the clinical case records of adult patients within the HAEV-ISSSTE health system using the following inclusion criteria: histopathologic diagnosis of colorectal cancer, K-ras sequencing through PCR (mutated or wild-type K-ras), length of time of survival in months, acceptance of medical and surgical treatment in the coadjuvant modality, no clinical suspicion of familial colorectal cancer, and the inclusion of age (under 65 years or 65 years and older), sex, clinical stage (I to IV according to the AJCC), and ECOG scale (0 to 5). The study period encompassed 3 years, from January 1, 2009, the date in which K-ras sequencing was begun in the HAEV-ISSSTE, to December 31, 2012, taking into account all the sequencings carried out during that period. The patients that abandoned treatment or follow-up by the oncology service, whose recurrence through imaging studies or whose death were not corroborated and/or whose clinical records did not mention all the inclusion criteria, were excluded from the study. The descriptive data were: the number of mutations and their distribution in the abovementioned co-variables, the relation of the mutations to the deaths, and the presence of metastasis; these were analyzed through the Pearson chi-square test using the Epi-Info 7.1.3 software. The survival rate in months was compared between the patients presenting with wild-type K-ras or mutated K-ras statuses using the Kaplan-Meier method and the data was represented through graphs. The Log-Rank method was used to compare the differences in the survival results between the wild-type K-ras and the mutated K-ras and their distribution in the co-variables.
ResultsA total of 63 K-ras sequencings were carried out through the real-time PCR technique at the HAEV-ISSSTE during the abovementioned study period. A total of 31 cases were enrolled; the other 32 were excluded due to incomplete medical records. A total of 61.3% (n=19) of the patients received an initial regimen with XELOX and 38.7% (n=12) with FOLFOX; 38.7% of the patients also received radiotherapy. Only 5 of the total number of patients received cetuximab as adjuvant therapy. Table 1 shows the clinical stage distribution and the ECOG functional evaluation.
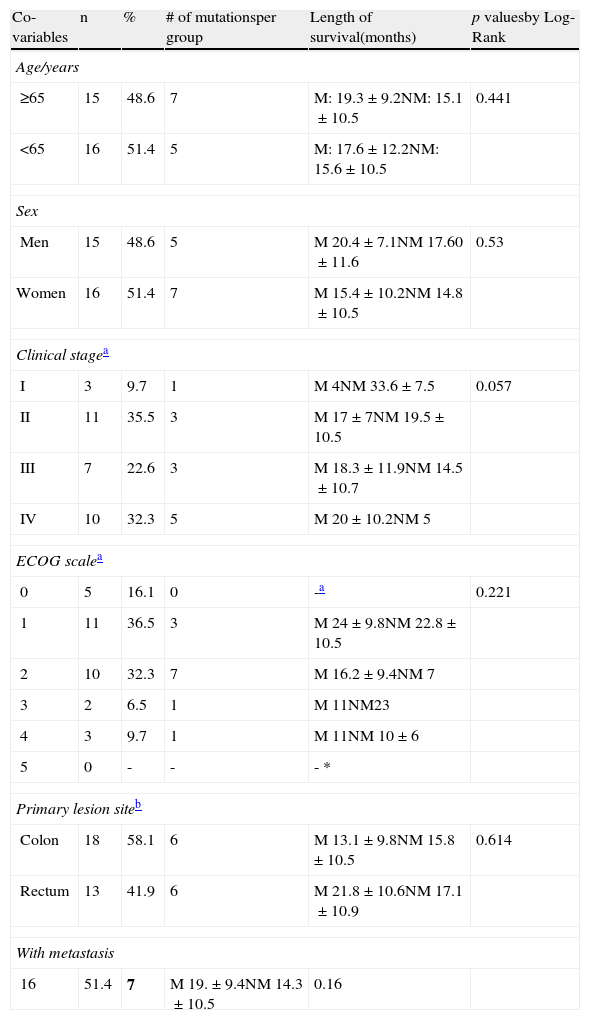
Distribution of the population characteristics, number of mutations, and their comparison in relation to survival.
| Co-variables | n | % | # of mutationsper group | Length of survival(months) | p valuesby Log-Rank |
| Age/years | |||||
| ≥65 | 15 | 48.6 | 7 | M: 19.3±9.2NM: 15.1±10.5 | 0.441 |
| <65 | 16 | 51.4 | 5 | M: 17.6±12.2NM: 15.6±10.5 | |
| Sex | |||||
| Men | 15 | 48.6 | 5 | M 20.4±7.1NM 17.60±11.6 | 0.53 |
| Women | 16 | 51.4 | 7 | M 15.4±10.2NM 14.8±10.5 | |
| Clinical stagea | |||||
| I | 3 | 9.7 | 1 | M 4NM 33.6±7.5 | 0.057 |
| II | 11 | 35.5 | 3 | M 17±7NM 19.5±10.5 | |
| III | 7 | 22.6 | 3 | M 18.3±11.9NM 14.5±10.7 | |
| IV | 10 | 32.3 | 5 | M 20±10.2NM 5 | |
| ECOG scalea | |||||
| 0 | 5 | 16.1 | 0 | -a | 0.221 |
| 1 | 11 | 36.5 | 3 | M 24±9.8NM 22.8±10.5 | |
| 2 | 10 | 32.3 | 7 | M 16.2±9.4NM 7 | |
| 3 | 2 | 6.5 | 1 | M 11NM23 | |
| 4 | 3 | 9.7 | 1 | M 11NM 10±6 | |
| 5 | 0 | - | - | - * | |
| Primary lesion siteb | |||||
| Colon | 18 | 58.1 | 6 | M 13.1±9.8NM 15.8±10.5 | 0.614 |
| Rectum | 13 | 41.9 | 6 | M 21.8±10.6NM 17.1±10.9 | |
| With metastasis | |||||
| 16 | 51.4 | 7 | M 19.±9.4NM 14.3±10.5 | 0.16 | |
M: mutated K-ras; NM: wild-type K-ras.
Twelve (38.7%) patients presented with one or various mutations in the K-ras oncogene sequencing, all of which were located at codon 12; 4 patients had additional mutations at codon 13. Metastasis was documented in 7 (58.3%) cases and there were 4 (33.3%) deaths. Nineteen (61.3%) patients presented with wild-type K-ras, 9 (42.1%) of whom progressed to metastatic disease and 3 (15.7%) of whom died. The results of an initial analysis of the K-ras mutations with the presence of metastasis (p=0.82) or deaths (p=0.48) were not statistically significant. The length of time of survival in general had a mean value of 16.77±9.90 months. Mean survival in the wild-type K-ras patients was 16.31±10.50 months; in contrast the patients with mutated K-ras had a mean survival of 17.5±9.27 months. The length of time of survival and the K-ras status were compared using the Kaplan-Meier method and there was no statistical significance (p=0.416) (fig. 1). The co-variables were contrasted using the Log-Rank method and the numerical results are summarized in Table 1; none of the results were significant. Only 5 patients were in treatment with cetuximab, all of them with wild-type K-ras, and they had a mean survival of 10±3 months and a mean recurrence-free time of 16.6±10.2 months. Anecdotically, one patient received treatment with cetuximab, despite presenting with mutated K-ras, and had one of the longest survival periods of this report (26 months).
DiscussionThe percentages of the mutational findings and distribution characteristics by age, sex, and primary lesion site of the present study population were very similar to those reported in the literature. The RASCAL II study,18 and other recent smaller studies, concluded that the presence of K-ras mutations were associated with a lower survival rate, compared with wild-type K-ras patients, and their risk ratios with greater statistical weight were reported in more advanced clinical stages (Dukes C); it was also reported that the mutations at codon 12 conferred aggressive biology.19 However, studies conducted with a similar methodology, but a notably lower number of patients,11,20 in which the K-ras sequencing was carried out with the method of choice and in a local population, did not find any difference in survival between the mutated and wild-type groups, nor was it influenced by the co-variables. Carrying out sequencing on all the patients with colorectal cancer is a very important part of directing treatment; it has been consistently demonstrated that the mutations in exon 1 of this oncogene make treatment with cetuximab less effective, modifying survival.21 In the present study, the number of patients treated with cetuximab was not large enough for a useful statistical test to be performed. Suffice it to say that survival was very poor (10±3 months), as well as anecdotal in one case in which the K-ras mutation was corroborated in a second sequencing, and survival was above the mean (26 months). The abovementioned is a problem for researchers in the so-called emerging countries because, even in referral centers, K-ras sequencing is not carried out routinely and monoclonal treatment is not given to all the patients with wild-type K-ras, due to the high cost of both.
ConclusionsIn the present pilot study, no relation was found between the presence of mutated K-ras and progression to metastasis or association with a greater proportion of deaths, in contrast to what has been reported in the medical literature. Further studies with a larger number of patients receiving monoclonal treatment need to be conducted in order to extrapolate the published information to the Mexican population.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the administrative personnel of the HAEV-ISSSTE for their collaboration in facilitating the development of the present study, as well as Dr. José María Remes-Troche and Dr. Sara Aquino-Pérez for their critique of the manuscript.
Please cite this article as: Cabrera-Mendoza F, Gainza-Lagunes S, Castañeda-Andrade I, Castro-Zárate A. Relevancia clínica del oncogén K-ras en cáncer de colon, experiencia en una población mexicana. Revista de Gastroenterología de México. 2014;79:166–170.