Endoscopic retrograde cholangiopancreatography (ERCP) is associated with an acute inflammatory response and melatonin has a variety of immunomodulatory and antioxidant effects studied experimentally in pancreatobiliary pathology.
AimsThe aim of our study was to evaluate the effects of peri-procedural administration of melatonin on the inflammatory response and lipid peroxidation associated with ERCP.
MethodsIn this proof-of-concept clinical trial, 37 patients with a high probability of choledocholithiasis were randomized to receive peri-procedure (ERCP) melatonin or placebo. We measured the serum concentration of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), lipid peroxidation, amylase, and liver function tests 24h before and after the procedure.
ResultsWe found no pre-procedure or post-procedure differences between the melatonin group or the placebo group (P>.05) in the serum concentrations of TNF-alpha (melatonin: 153.8 vs. 149.4ng/m; placebo: 103.5 vs. 107.3ng/ml), IL-6 (melatonin: 131.8 vs. 133.3ng/ml; placebo: 177.8 vs. 197.8ng/ml), or VEGF (melatonin: 157.3 vs. 157.8pg/ml; placebo: 97.3 vs. 97.8pg/ml), or in relation to lipid peroxidation (melatonin: 39.2 vs. 72.3μg/ml; placebo: 66.4 vs. 90.5μg/ml). After ERCP, a significant decrease in the AST, ALT, and total bilirubin levels was found only in the melatonin group (P<.05). The administration of melatonin was safe and tolerable.
ConclusionsMelatonin is safe and tolerable in patients undergoing ERCP, but it does not appear to affect inflammatory cytokine concentrations or lipid peroxidation.
La colangiopancreatografía retrógrada endoscópica (CPRE) está asociada con la generación de respuesta inflamatoria aguda, la melatonina tiene una variedad de efectos inmunomoduladores y antioxidantes probados de manera experimental en patología pancreatobiliar.
ObjetivoEl objetivo del estudio fue evaluar los efectos de la administración periprocedimiento de melatonina sobre la respuesta inflamatoria y peroxidación lipídica asociada a la realización de CPRE.
MétodosEn este ensayo clínico se incluyó a 37 pacientes con riesgo alto de coledocolitiasis, se aleatorizaron para recibir melatonina o placebo periprocedimiento (CPRE). Se midió la concentración sérica de factor de necrosis tumoral alfa (FNT-alfa), interleucina-6 (IL-6), factor de crecimiento endotelial vascular (FCEV), peroxidación lipídica, amilasa y pruebas de funcionamiento hepático 24 h antes y después del procedimiento.
ResultadosNo encontramos diferencia entre las concentraciones séricas de FNT alfa (melatonina: 153.8 vs. 149.4ng/mL; placebo: 103.5 vs. 107.3ng/mL), IL-6 (melatonina: 131.8 vs. 133.3ng/mL; placebo: 177.8 vs. 197.8ng/mL) y FCEV (melatonina: 157.3 vs. 157.8pg/mL; placebo: 97.3 vs. 97.8pg/mL), o en peroxidación lipídica (melatonina: 39.2 vs. 72.3μg/mL; placebo: 66.4 vs. 90.5μg/mL) preprocedimiento vs. posprocedimiento en ninguno de los 2 grupos (p>0.05). Posterior a la CPRE, los niveles de aspartato aminotransferasa, alanina aminotransferasa y bilirrubina total disminuyeron significativamente solo en el grupo de melatonina (p<0.05). La administración de melatonina es segura y tolerada.
ConclusionesLa melatonina es segura y tolerada en pacientes que van a CPRE, pero no parece afectar las concentraciones de citocinas proinflamatorias o peroxidación lipídica en humanos.
Acute pancreatitis (AP) is a feared complication of endoscopic retrograde cholangiopancreatography (ERCP) that can occur in up to 30% of high-risk patients.1,2 Established risk factors for post-ERCP pancreatitis include young age, female sex, history of post-ERCP pancreatitis, sphincter of Oddi dysfunction, cannulation time > 10min, and passage of guidewire or contrast medium into the pancreatic duct.2,3 Sphincter spasm, edema, enzymatic injury, infection, or mechanical manipulation have been proposed as potential mechanisms responsible for post-ERCP pancreatitis.2,3 The development of an acute inflammatory state and cytokine activation could modulate these mechanisms and contribute to pancreatic injury. ERCP has been considered a useful model for studying the acute phase response in AP.3,4 After ERCP, elevations of amylase and inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6), are common and could predict the development of AP.4–6 Experimental studies have established that these cytokines, as well as a state of oxidative stress, contribute to the pathophysiology of AP.7,8
Clinical trials have demonstrated that pancreatic duct stent placement and pharmacologic therapy with nonsteroidal anti-inflammatory drugs can reduce the incidence of post-ERCP pancreatitis.9,10 Other agents have been studied as potential candidates for modulating the inflammatory response and oxidative stress associated with ERCP.10,11 However, the search for safe and effective alternatives is an active field of research.
Melatonin is a multifunctional endogenous indolamine produced in the pineal gland, classically associated with regulation of the circadian rhythm. Its administration in humans is safe and well tolerated.12 Clinical and experimental studies have shown that melatonin has potent anti-inflammatory and antioxidant effects, modulating TNF-alpha, IL-6, and the production of free radicals.13–15 Additionally, a large body of experimental evidence suggests that melatonin can reduce pancreatic injury, systemic inflammation, and oxidative stress in animal models of AP.16,17
The aim of the present study was to investigate the effects of melatonin on post-ERCP-induced hyperamylasemia and elevations in inflammatory and oxidative stress markers.
Materials and methodsPatients and proceduresThe study design was a randomized, double-blind, and placebo-controlled clinical trial. The study protocol was approved by the local ethics committee, and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. We included 37 consecutive patients admitted from March to July 2013 at the Hospital Universitario “Dr. José E. González” in Nuevo Leon, with suspicion of choledocholithiasis and scheduled to undergo ERCP.
Suspected choledocholithiasis was considered when patients presented with upper abdominal pain and clinical manifestations (e.g. fever, jaundice), a cholestatic pattern of liver injury, common bile duct (CBD) dilatation (> 6mm), or CBD stone visualization on transabdominal ultrasound (US). Study subjects were identified upon admission by gastroenterology residents, based on an assessment of clinical, radiologic, and biochemical variables.
Inclusion criteria were age above 18 years and a high probability of choledocholithiasis on admission, according to the American Society for Gastrointestinal Endoscopy 2010 criteria.18 Exclusion criteria included patients that had systemic inflammatory response syndrome (defined by the presence of 2 or more criteria: heart rate > 90 beats/min, core temperature < 36°C or > 38°C, a white blood cell count < 4000 or > 12000/mm3, a respiratory rate > 20min), acute pancreatitis or unresolved pancreatitis, cholangitis, a history of any rheumatologic or inflammatory disease, patients that were pregnant or breast-feeding, patients with a history of sensitivity to melatonin, or those with a history of being intubated and on ventilator support.
ERCPs were performed by an experienced gastroenterologist blinded to patient allocation. The indication for ERCP in all patients was a high probability of choledocholithiasis. The development of pancreatitis was diagnosed and graded following published consensus criteria.9 ERCP risk score for post-ERCP pancreatitis was obtained using a validated tool published by Friedland et al.19 For all procedures, under midazolam induction and propofol sedation, a Pentax EPK-1000 high-resolution video processor (NJ, USA) and a Pentax ED-3430 TK duodenoscope (NJ, USA) with a triple lumen sphincterotome Wilson Cook Medical Inc., Winston Salem NC, USA were used. Sphincterotomy was performed in all patients.
Blood samples from eligible patients undergoing ERCP were collected 24h before (baseline) and 24h after the procedure. Serum samples were stored at −70°C until analyzed. Melatonin (10mg) was administered sublingually in 14 patients in five doses (each 8h apart), starting 10h before the procedure, up to 24h after the ERCP (total dose 50mg). Another group of 16 patients received matching placebo and served as controls.
Laboratory examinationsLiver function tests (LFTs), amylase, TNF-a, IL-6, vascular endothelial growth factor (VEGF), and lipid peroxidation were measured in the blood samples of all the patients. LFTs and amylase were measured using standard biochemical methods. Serum TNF-alpha, IL-6, and VEGF levels were measured by enzyme-linked immunosorbent assay according to the manufacturer's instructions (Pre-ProTech Mexico; ELISA ASYS Hitech GmbH Expert Plus UV G020151). Lipid peroxidation was measured using a colorimetric assay kit (TBARS method, Cayman Chemicals, Ann Arbor, MI), which expresses lipid peroxidation as malondialdehyde (MDA) concentrations.
Asymptomatic hyperamylasemia was defined as a post-procedure value above the upper normal limit (UNL) of our laboratory (100 IU) if the pre-procedure value was normal, or as any increase if the pre-procedure value was above the UNL, and if there were no symptoms of AP.
Statistical methodsThe primary end point was to assess the effects of melatonin on post-ERCP-induced hyperamylasemia and elevations in inflammatory and oxidative stress markers. The secondary outcome was to determine the occurrence of post-ERCP complications and the effect on liver function enzymes between groups.
A restricted randomization procedure was used to control the probability of obtaining an allocation sequence with an undesirable sample size imbalance in the intervention groups. Blocking was used, utilizing a one-to-one ratio until a total of 40 individuals was reached, to ensure that the groups were the same size. An outside individual not involved in the care of the trial patients and not at the study site was responsible for the allocation and preparation of the medication and its containers. Sequentially numbered, sealed, opaque containers were used to transport the drugs. All of the containers were tamper-proof, equal in weight, and similar in appearance. A nurse was in charge of administering the medication. The medication consisted of 5-mg white pills of similar size, shape, texture, and flavor. Patients participating in the trial, care providers, and those assessing outcomes were blinded to the intervention assignment. No changes were made to the methods after trial commencement.
Statistical analysisAll statistical analyses were assessed using the SPSS program (SPSS version 17.0; SPSS Inc., Chicago, IL). Statistical comparisons between the groups were made using the Student's t test (when variables were normally distributed), the Mann-Whitney U test (for non-normally distributed variables), or the Wilcoxon's test (two related samples), as required. Other categorical variables (reported as percentage frequency of occurrence) such as sex, hyperamylasemia, and comorbidities were compared using the X2 test. All parameters were then expressed as mean values ± standard deviation or median and interquartile range (IQR), as appropriate. A p value < 0.05 was considered statistically significant.
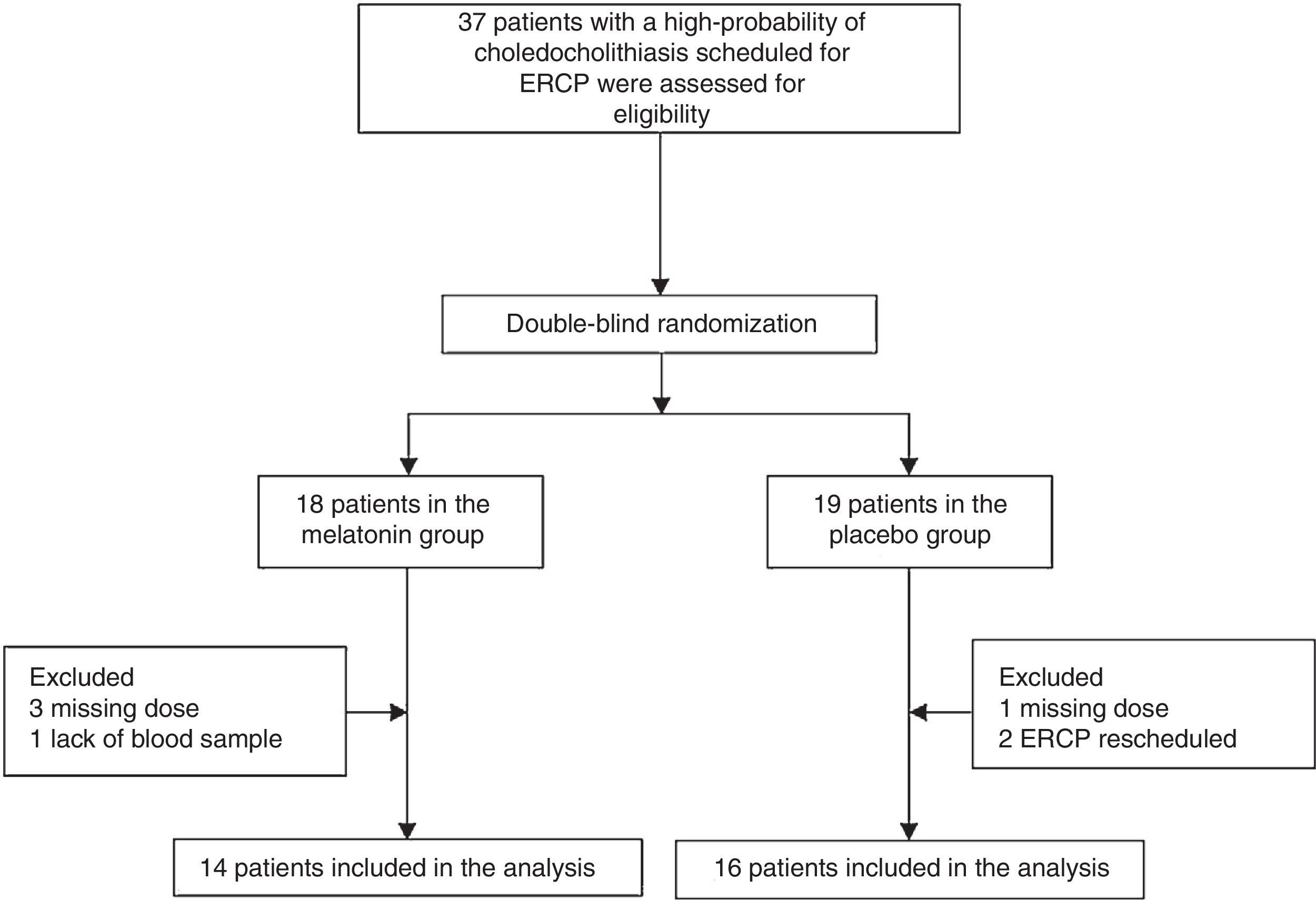
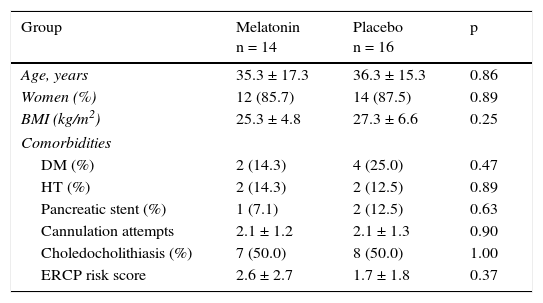
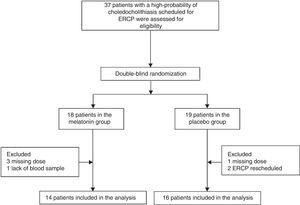
ResultsPatientsFrom March 2013 to July 2013, 37 patients with a high probability of choledocholithiasis and scheduled for ERCP were assessed for eligibility (fig. 1). Seven patients were excluded, and the remaining 30 eligible patients with a high probability of choledocholithiasis were included. The melatonin group consisted of 14 patients with a mean age of 35.3 ± 17.3 years and 12 (85.7%) of them were women. The placebo group was made up of 16 patients with a mean age of 36.3 ± 15.3 years and 14 (87.5%) of them were women. The rest of the baseline demographic characteristics were comparable between the two groups (table 1).
Demographic characteristics.
| Group | Melatonin n = 14 | Placebo n = 16 | p |
|---|---|---|---|
| Age, years | 35.3 ± 17.3 | 36.3 ± 15.3 | 0.86 |
| Women (%) | 12 (85.7) | 14 (87.5) | 0.89 |
| BMI (kg/m2) | 25.3 ± 4.8 | 27.3 ± 6.6 | 0.25 |
| Comorbidities | |||
| DM (%) | 2 (14.3) | 4 (25.0) | 0.47 |
| HT (%) | 2 (14.3) | 2 (12.5) | 0.89 |
| Pancreatic stent (%) | 1 (7.1) | 2 (12.5) | 0.63 |
| Cannulation attempts | 2.1 ± 1.2 | 2.1 ± 1.3 | 0.90 |
| Choledocholithiasis (%) | 7 (50.0) | 8 (50.0) | 1.00 |
| ERCP risk score | 2.6 ± 2.7 | 1.7 ± 1.8 | 0.37 |
Data are shown in absolute values (%) and means ± standard deviation.
BMI: body mass index; DM: diabetes mellitus; ERCP: endoscopic retrograde cholangiopancreatography; HT: hypertension.
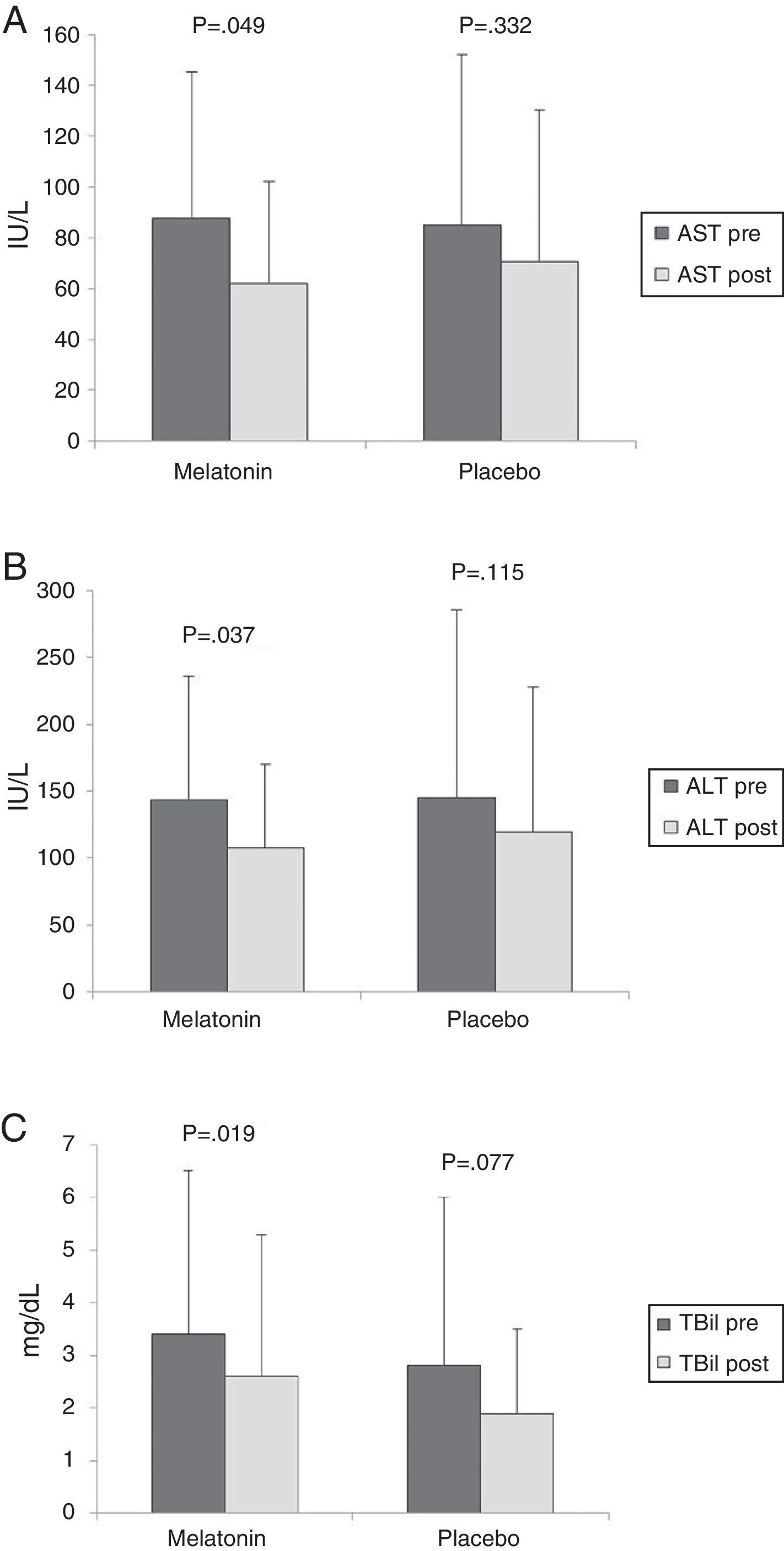
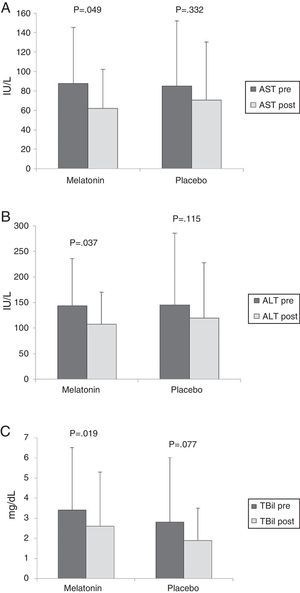
There was no significant difference between the melatonin and placebo groups in the pre-procedure values of AST (87.5 ± 57.8 vs. 85.2 ± 66.9 IU/L, p = 0.924), ALT (143.4 ± 91.9 vs. 144.8 ± 140.8 IU/L, p = 0.976), or total bilirubin (3.4 ± 3.1 vs. 2.8 ± 3.1mg/dL, p = 0.591). After 24h, a significant decrease in the AST, ALT, and total bilirubin values was found only in the melatonin group (fig. 2). However, there was no difference between groups in the post-ERCP values of AST, ALT, or total bilirubin (p > 0.05).
Pre- ERCP (dark bars) and post-ERCP (light bars) AST values (A), ALT values (B), and TBil values (C) in the melatonin and placebo groups
Results are expressed as means, with error bars representing the standard deviation.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; TBil: total bilirubin.
Seven (43.7%) patients in the placebo group and 6 (42.8%) in the melatonin group developed hyperamylasemia as previously defined, p = 0.960. A significant increase in the median (IQR) values of amylase levels after ERCP was observed in both the melatonin (pre-CPRE 54.0 [23.7] vs. post-CPRE 87.0 [64.5], p = 0.004) and the placebo (pre-CPRE 101.0 [75.0] vs. post-CPRE 107.0 [125.0], p = 0.008) groups. However, no difference between the groups in pre-ERCP or post-ERCP amylase levels was recorded (p > 0.05).
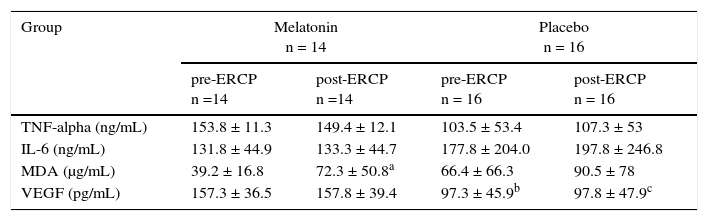
Cytokines and lipid peroxidationThe complete results of TNF-alpha, IL-6, VEGF, and lipid peroxidation (MDA) are shown in Table 2. There was no difference between groups in the pre-procedure or post-procedure serum concentrations of any of the markers studied, except for the VEGF values, which were significantly higher in the melatonin group compared with the placebo group. Only a trend towards increased MDA (p = 0.09) was found when comparing pre-procedure and post-procedure levels in the melatonin group.
Cytokines and lipid peroxidation.
| Group | Melatonin n = 14 | Placebo n = 16 | ||
|---|---|---|---|---|
| pre-ERCP n =14 | post-ERCP n =14 | pre-ERCP n = 16 | post-ERCP n = 16 | |
| TNF-alpha (ng/mL) | 153.8 ± 11.3 | 149.4 ± 12.1 | 103.5 ± 53.4 | 107.3 ± 53 |
| IL-6 (ng/mL) | 131.8 ± 44.9 | 133.3 ± 44.7 | 177.8 ± 204.0 | 197.8 ± 246.8 |
| MDA (μg/mL) | 39.2 ± 16.8 | 72.3 ± 50.8a | 66.4 ± 66.3 | 90.5 ± 78 |
| VEGF (pg/mL) | 157.3 ± 36.5 | 157.8 ± 39.4 | 97.3 ± 45.9b | 97.8 ± 47.9c |
Data are shown in means ± standard deviation.
ERCP: endoscopic retrograde cholangiopancreatography; IL: interleukin; MDA: malondialdehyde; TNF: tumor necrosis factor; VEGF: vascular endothelial growth factor.
One patient in the placebo group developed post-ERCP pancreatitis. No major or minor adverse effects related to melatonin or placebo were recorded in this study.
DiscussionTo the best of our knowledge, this is the first prospective, randomized, double-blind, and placebo-controlled trial evaluating the effects of melatonin on pancreatic inflammation in humans after ERCP. We did not find any differences in the changes of inflammatory mediators at 24h after ERCP with the use of melatonin. Only one patient developed post-ERCP pancreatitis (in the placebo group), but our study was neither designed nor powered to find an effect on this variable. Serum AST, ALT, and bilirubin levels were significantly reduced at 24h in the melatonin group and not in the placebo group, which could suggest a hepatoprotective effect. However, the difference was small and is of uncertain clinical significance.
During AP, an imbalance between pro-inflammatory and anti-inflammatory mediators, cytokine release, and increased oxidative stress participate as effectors of local and systemic injury.8,20 These inflammatory alterations, as well as different degrees of hyperamylasemia, are also associated with ERCP and its related complications. In a study of 70 patients undergoing ERCP, early (within 24h) serum elevations of IL-6 and C-reactive protein occurred in those patients that developed post-ERCP pancreatitis.4 Another study of 45 patients undergoing ERCP showed similar results, with early elevations of IL-6 and TNF-alpha occurring in patients with complications.6
We studied a homogeneous group of low-risk patients according to the Friedland score,19 under the assumption that even in asymptomatic cases, stimulation of the inflammatory cascade during ERCP could be the initial signal responsible for the development of complications such as pancreatitis. This paradigm could be used to study both the acute phase response and relevant therapeutic interventions. In experimental models that could partially mimic some of the alterations associated with ERCP, bile duct ligation induced cholestatic liver injury and increased oxidative stress, and these changes were ameliorated by melatonin.21 However, in our study we did not observe relevant changes in the serum concentrations of IL-6 or TNF-alpha after ERCP in either the treatment or the placebo groups. We found lower pre-procedure and post-procedure levels of VEGF in the placebo group, compared with the melatonin group, but no changes within the groups that could be attributed to ERCP or to an effect of melatonin. Serum VEGF is known to be increased in experimental models of acute pancreatitis in rats,22 and melatonin could increase VEGF expression in a rat model of gastric ulcer healing.23 However, melatonin has been shown to reduce VEGF expression in a variety of human cells, including pancreatic and liver cancer cells, making a melatonin-dependent effect unlikely in our study.24,25 Inadequate dosing and a single observation period (24h post-procedure) could also account for our negative results.
The only variables that appeared to be affected by melatonin in our study were ALT, AST, and total bilirubin, each of which had a reduction in their post-procedure levels. Although we speculate that the post-ERCP decreases in these parameters could indicate resolution of cholestasis-induced hepatocellular injury, this finding is of unknown clinical significance. However, melatonin receptors exist in the liver and melatonin administration has been shown to reduce cholestasis-induced hepatocellular injury, as well as transaminase and bilirubin elevations, in experimental models of bile duct ligation.26,27 Sequential measurement studies or inclusion of high-risk patients would be needed to confirm a biochemical benefit, if there were one.
Melatonin at therapeutic and physiologic doses acting as a free radical scavenger reduces every step in lipid peroxidation and promotes the production of endogenous antioxidants. It also reduces production of TNF-alpha, IL-6, prostaglandins, and nitric oxide (NO).28 Toxicity studies have shown that exogenous melatonin is safe and tolerable, with no discernible toxic or dose-limiting effects.12 Clinical studies have been published that explore melatonin's immunomodulatory properties. Melatonin could reduce circulating levels of NO and pro-inflammatory cytokines in septic neonates, changes that were accompanied by improved outcomes.13 In adult patients that underwent aortic surgery, perioperative melatonin was associated with reduced levels of oxidative stress.14 In our study, we observed no peri-anesthetic side effects or excessive drowsiness, and we concluded that melatonin administration was safe and tolerable, in accordance with other human studies that have used similar doses.29
Our negative results are surprising in light of the available indirect evidence. A physiologic role for melatonin in pancreatic function has been proposed. Melatonin receptors exist in pancreatic tissue and melatonin can modulate pancreatic secretion and blood flow.17 In experimental models, the protective effects of melatonin are enhanced at night, corresponding to the circadian rhythm and higher endogenous levels of melatonin.30 Additionally, a recent study showed that low endogenous melatonin levels in patients with AP correlated with increased disease severity.31 There are also studies that suggest that melatonin might be pancreatoprotective. Qi et al. were the first to show that melatonin could reduce tissue injury in an experimental model of cerulein-induced AP in rats.16 The increase in endogenous levels of melatonin led to reduction of cerulein-induced AP severity, as well as reductions in TNF-alpha.32 Melatonin supplementation has been shown to induce reductions in tissue injury, inflammatory markers, and multiorgan failure in other animal models of AP, such as L-arginine administration,33 pancreatic ischemia-reperfusion injury,34 pancreatic duct ligation,35 and taurocholate administration.36 Reductions in free radical production, lipid peroxidation, and mitochondrial injury and increases in endogenous antioxidants, such as glutathione, have also been demonstrated in models of AP after melatonin administration.37 Other studies on AP have also shown modulation of TNF-alpha and IL-1 production by melatonin.38 Melatonin appears to be pancreatoprotective through the modulation of multiple inflammatory pathways. However, we did not find evidence that melatonin could modulate inflammatory cytokine production or lipid peroxidation in the context of ERCP.
Limitations in our study design could well explain this discrepancy, such as single dose, small sample size, single measurement of biochemical variables, and biochemical end-points (instead of clinical endpoints, such as post-ERCP pancreatitis). Melatonin does appear to be safe and tolerable (no reported adverse events or excessive drowsiness) in patients undergoing ERCP, but we did not find a significant effect of melatonin on inflammatory cytokine concentrations or lipid peroxidation at the dose studied. The apparently beneficial effect on transaminases and bilirubin are of unknown significance. Large clinical trials using different doses and periods of evaluation, as well as different (high-risk) populations, are warranted to determine if melatonin can reduce the incidence of post-ERCP pancreatitis or AP severity.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that the procedures followed conformed to the ethical standards of the responsible committee on human experimentation and were in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Hernández-Velázquez B, Camara-Lemarroy CR, González-González JA, García-Compean D, Monreal-Robles R, Cordero-Pérez P, et al. Efectos de la melatonina en la respuesta inflamatoria aguda asociada con la colangiopancreatografía retrógrada endoscópica: un ensayo clínico aleatorizado, doble ciego y controlado con placebo. Revista de Gastroenterología de México. 2016;82:141–148.