Eosinophilic esophagitis (EoE) is characterized by eosinophilic infiltration of the esophagus and is a potential cause of dysphagia and food impaction, most commonly affecting young men. Esophageal manometry findings vary from normal motility to aperistalsis, simultaneous contractions, diffuse esophageal spasm, nutcracker esophagus or hypotonic lower esophageal sphincter (LES). It remains unclear whether esophageal dysmotility plays a significant role in the clinical symptoms of EoE.
AimOur aim is to review the pathogenesis, diagnosis, and effect of treatment on esophageal dysmotility in EoE.
MethodsA literature search utilizing the PubMed database was performed using keywords: eosinophilic esophagitis, esophageal dysmotility, motility, manometry, impedance planimetry, barium esophagogram, endoscopic ultrasound, and dysphagia.
ResultsFifteen studies, totaling 387 patients with eosinophilic esophagitis were identified as keeping in accordance with the aim of this study and included in this review. The occurrence of abnormal esophageal manometry was reported to be between 4 and 87% among patients with EoE. Esophageal motility studies have shown reduced distensibilty, abnormal peristalsis, and hypotonicity of the LES in patients with EoE, which may also mimic other esophageal motility disorders such as achalasia or nutcracker esophagus. Studies have shown conflicting results regarding the presence of esophageal dysmotility and symptoms with some reports suggesting a higher rate of food impaction, while others report no correlation between motor function and dysphagia.
ConclusionsMotility dysfunction of the esophagus in EoE has not been well reported in the literature and studies have reported conflicting evidence regarding the clinical significance of dysmotility seen in EoE. The correlation between esophageal dysmotility and symptoms of EoE remains unclear. Larger studies are needed to investigate the incidence of esophageal dysmotility, clinical implications, and effect of treatment on patients with EoE.
La esofagitis eosinofílica (EE) se caracteriza por la infiltración de eosinofilos en el esófago y es una causa potencial de disfagia e impactación alimentaria que en general afecta a jóvenes adultos. Los resultados obtenidos con la manometría esofágica son variados, y se ha observado motilidad normal y aperistaltis, contracciones simultáneas, esófago en cascanueces o esfínter esofágico inferior hipotónico. Aún no está claro si la dismotilidad esofágica desempeña un papel importante en los síntomas clínicos de la EE.
ObjetivoRevisar la patogenia, el diagnóstico y el efecto del tratamiento de la dismotilidad esofágica en la EE.
MétodosSe llevó a cabo una búsqueda de la bibliografía médica en PubMed utilizando los términos “esofagitis eosinofílica”, “dismotilidad esofágica”, “motilidad”, “manometría”, “impedancia planimétrica”, “esofagograma con contraste de bario”, “ultrasonido endoscópico” y “disfagia”.
ResultadosSe identificaron 15 estudios que se ajustaban al objetivo, que incluyeron a 387 pacientes con esofagitis eosinofílica,, y se incluyeron en esta revisión. La incidencia de manometría esofágica anormal reportada en los pacientes con EE fue del 4 al 87%. Estudios de motilidad esofágica han mostrado distensibilidad reducida, peristaltismo anormal e hipotonicidad del esfínter esofágico en pacientes con EE, que a la vez pueden mimetizar otros trastornos de motilidad esofágica, como la acalasia o el esófago en cascanueces. Los estudios han mostrado resultados contradictorios en relación con la presencia de dismotilidad esofágica y síntomas; así, hay reportes que sugieren tasas elevadas de impactación alimentaria mientras que otros no muestran ninguna relación directa entre la función motora y la disfagia.
ConclusionesLa disfunción de la motilidad esofágica en EE no se ha reportado en profundidad en la bibliografía y algunos estudios muestran evidencia contraria en cuanto a la importancia clínica de la dismotilidad observada en la EE. La correlación entre la dismotilidad esofágica y los síntomas de EE permanece aún poco clara. Se requieren estudios más amplios para investigar la incidencia de la dismotilidad esofágica, sus implicaciones clínicas y el efecto del tratamiento en pacientes con EE.
Eosinophilic esophagitis (EoE) is a disease characterized by esophageal dysfunction and histologic evidence of eosinophilia and inflammation first described nearly 80 years ago.1 In 1977, Dobbins et al. described an association with atopy in a patient with a history of asthma and hay fever who presented with dysphagia and normal upper gastrointestinal imaging.2 The following year, Landres et al. reported a second patient with an allergy to trimethoprim/sulfamethoxazole and vigorous achalasia found to have marked eosinophilic infiltration into the submucosa of the esophagus.3 Currently, more than 200 cases have been reported with a recent increase in prevalence, which may be partially attributed to clinical awareness of the disease. EoE has been described in North American and European populations with prevalence varying from 0.4% in an open population to 6.5% in subjects with esophageal symptoms, though it is still considered somewhat more unusual in Latin American populations with a recent Mexican study finding a prevalence of 1.7% amongst patients with symptoms.4,5
EoE is likely triggered by an immune response to antigens presented by food ingested via the gastrointestinal tract or particles inhaled via the respiratory tract. The pathophysiologic mechanisms that drive esophageal dysmotility in patients with EoE are not completely understood, although several theories have been postulated. These theories are based on the premise that immune system activation leads to eosinophilic infiltration of the esophagus and activation of cytokine-mediated pathways, ultimately leading to remodeling and alterations to the epithelial and subepithelial tissue structure and the mechanical properties of the esophagus.6 The changes in structural properties of the esophagus are a result of the eosinophils, masts cells, and cytokines produced by the inflammatory, epithelial and stromal cells of the esophagus.7
Eosinophils are major effector cells of tissue fibrosis and remodeling in diseases such as asthma, scleroderma and other fibrotic disorders.8–10 Eosinophils release 4 cytotoxic granules that can cause cell death and tissue damage upon release: major basic protein, eosinophilic cationic protein, eosinophilic peroxidase and eosinophilic derived neurotoxin.11 Cell destruction and turnover is thought to cause similar destruction in the gastrointestinal tract to that in other organ systems, i.e. mucosal friability which results in painful mucosal tears and tissue remodeling which can lead to esophageal rings and strictures.7 Recent studies have found an association between eosinophilia and altered fibrogenesis and motility from biopsy specimens of the upper, middle and lower third of the esophagus, suggesting that eosinophils have the same effects on tissue fibrosis and remodeling in the esophagus as they do in other previously studied organs.12,13 However, it is important to consider that sampling techniques and imaging the deep layers of the esophagus have limited precision.14
The process of epithelial mesenchymal transformation (EMT), in which epithelial cells lose their characteristic properties and transform into mesenchymal cells has also been implicated in the pathogenesis of fibrosis seen in EoE.15,16 Using immunostaining for vimentin (an intermediate filament protein expressed by mesenchymal cells) and cytokeratins (proteins of keratin containing intermediate filaments expressed by epithelial cells) in esophageal mucosal biopsies, Kagalwalla et al. found evidence of EMT and reported a correlation between the degree of EMT and the fibrosis score.16 They also found a reduction in EMT after treatment with either steroid therapy or elemental and elimination diets.16
EoE affects males 3 times more commonly than females and the average age at presentation is 38 years.1 Clinically, EoE presents as upper gastrointestinal symptoms such as food impaction, dysphagia, or chest pain in adults and, less commonly, abdominal pain, gastroesophageal reflux disease (GERD), weight loss and diarrhea.1,17 Studies have shown that food impaction requiring endoscopic removal occurs in 33-54% of patients with EoE, and that up to 15% of all patients undergoing upper endoscopy for dysphagia have EoE.18–20 Although 33-70% of all patients with EOE present with dysphagia, the precise mechanism remains unclear.21 While presenting symptoms vary between patients, the dominant pathophysiologic features of the disease involve luminal stiffening and narrowing associated with esophageal wall thickening, fibrosis and stricture.22
Endoscopic findings in patients with EoE may include esophageal strictures, narrow caliber esophagus, linear furrows, white plaques or exudates, Schatzki ring or pallor or decreased vasculature.17 Studies have reported abnormal endoscopic findings in 33-95% of patients with EoE.19,23–25 Histologically, EoE presents with eosinophilic infiltration with ≥15 eosinophils/high-power field (hpf). However, other disorders causing esophageal eosinophilia such as GERD, infectious or drug-induced esophagitis and collagen-vascular disorders, must be exluded.1 Treatment options include dietary restrictions, medications such as proton pump inhibitors (PPI), swallowed corticosteroids, leukotriene receptor antagonists, and esophageal dilatation.26 Thus far, the data regarding the effect of EoE on esophageal motility is inconclusive. This review discusses the current data on the effect of EoE on esophageal motility (table 1).
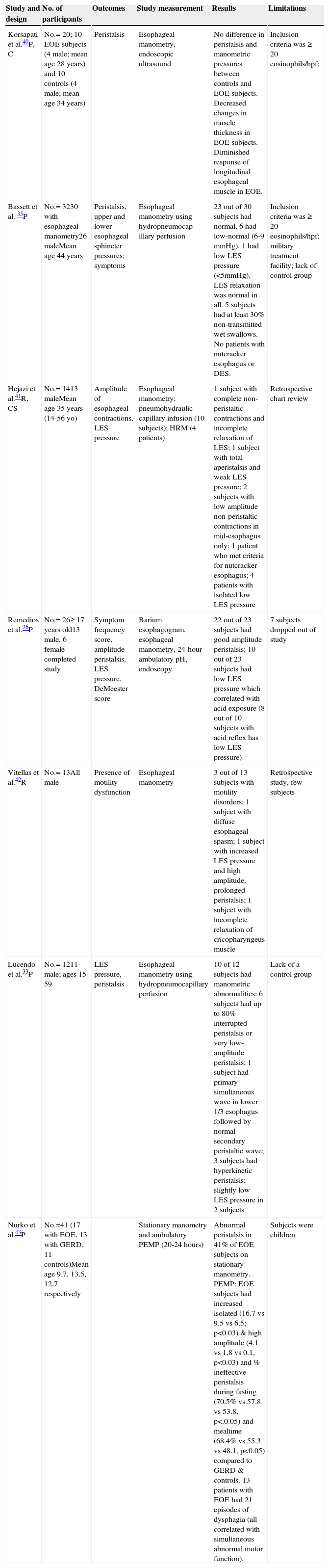
Studies of esophageal motility in eosinophilic esophagus.
| Study and design | No. of participants | Outcomes | Study measurement | Results | Limitations |
|---|---|---|---|---|---|
| Korsapati et al.40P, C | No.= 20; 10 EOE subjects (4 male; mean age 28 years) and 10 controls (4 male; mean age 34 years) | Peristalsis | Esophageal manometry, endoscopic ultrasound | No difference in peristalsis and manometric pressures between controls and EOE subjects. Decreased changes in muscle thickness in EOE subjects. Diminished response of longitudinal esophageal muscle in EOE. | Inclusion criteria was ≥ 20 eosinophils/hpf; |
| Bassett et al. 35P | No.= 3230 with esophageal manometry26 maleMean age 44 years | Peristalsis, upper and lower esophageal sphincter pressures; symptoms | Esophageal manometry using hydropneumocap-illary perfusion | 23 out of 30 subjects had normal, 6 had low-normal (6-9 mmHg), 1 had low LES pressure (<5mmHg). LES relaxation was normal in all. 5 subjects had at least 30% non-transmitted wet swallows. No patients with nutcracker esophagus or DES. | Inclusion criteria was ≥ 20 eosinophils/hpf; military treatment facility; lack of control group |
| Hejazi et al.41R, CS | No.= 1413 maleMean age 35 years (14-56 yo) | Amplitude of esophageal contractions, LES pressure | Esophageal manometry; pneumohydraulic capillary infusion (10 subjects); HRM (4 patients) | 1 subject with complete non-peristaltic contractions and incomplete relaxation of LES; 1 subject with total aperistalsis and weak LES pressure; 2 subjects with low amplitude non-peristaltic contractions in mid-esophagus only; 1 patient who met criteria for nutcracker esophagus; 4 patients with isolated low LES pressure | Retrospective chart review |
| Remedios et al.26P | No.= 26≥ 17 years old13 male, 6 female completed study | Symptom frequency score, amplitude peristalsis, LES pressure. DeMeester score | Barium esophagogram, esophageal manometry, 24-hour ambulatory pH, endoscopy | 22 out of 23 subjects had good amplitude peristalsis; 10 out of 23 subjects had low LES pressure which correlated with acid exposure (8 out of 10 subjects with acid reflex has low LES pressure) | 7 subjects dropped out of study |
| Vitellas et al.42R | No.= 13All male | Presence of motility dysfunction | Esophageal manometry | 3 out of 13 subjects with motility disorders: 1 subject with diffuse esophageal spasm; 1 subject with increased LES pressure and high amplitude, prolonged peristalsis; 1 subject with incomplete relaxation of cricopharyngeus muscle | Retrospective study, few subjects |
| Lucendo et al.33P | No.= 1211 male; ages 15-59 | LES pressure, peristalsis | Esophageal manometry using hydropneumocapillary perfusion | 10 of 12 subjects had manometric abnormalities: 6 subjects had up to 80% interrupted peristalsis or very low-amplitude peristalsis; 1 subject had primary simultaneous wave in lower 1/3 esophagus followed by normal secondary peristaltic wave; 3 subjects had hyperkinetic peristalsis; slightly low LES pressure in 2 subjects | Lack of a control group |
| Nurko et al.43P | No.=41 (17 with EOE, 13 with GERD, 11 controls)Mean age 9.7, 13.5, 12.7 respectively | Stationary manometry and ambulatory PEMP (20-24 hours) | Abnormal peristalsis in 41% of EOE subjects on stationary manometry. PEMP: EOE subjects had increased isolated (16.7 vs 9.5 vs 6.5; p<0.03) & high amplitude (4.1 vs 1.8 vs 0.1, p<0.03) and % ineffective peristalsis during fasting (70.5% vs 57.8 vs 53.8, p<.0.05) and mealtime (68.4% vs 55.3 vs 48.1, p<0.05) compared to GERD & controls. 13 patients with EOE had 21 episodes of dysphagia (all correlated with simultaneous abnormal motor function). | Subjects were children |
C: controlled; CS: case series; DES: diffuse esophageal spasm; EOE: eosinophilic esophagitis; hpf: high-power field; HRM: high-resolution manometry; LES: lower esophageal sphincter; P: prospective; PEMP: prolonged esophageal manometry and pH-metry; R: retrospective
The PubMed database was searched using the following keywords: “eosinophilic esophagitis”, “esophageal dysmotility”, “motility”, “manometry”, “impedance planimetry”, “barium esophagogram”, “endoscopic ultrasound”, and “dysphagia”. There was no time limit and the words “AND” and “OR” were used as logical combining terms, enabling us to limit the information. The original and review articles resulting from the search were selected in accordance with the main aim of this study. Two reviewers screened abstracts from the literature search and included relevant studies of patients with diagnosed EoE undergoing all types of motility evaluation.
ResultsA number of alterations in the biomechanical properties of the esophagus have been implicated in the motility dysfunction described in patients with EoE in the absence of true structural abnormalities. These mechanisms include abnormal peristalsis, abnormal duration and strength of contraction in both the circular and longitudinal muscles of the esophagus, and changes to the dispensability of the esophageal wall. The changes to these biomechanical properties are evaluated using a variety of techniques, as described below.
High resolution impedance planimetryTwo prior studies have evaluated esophageal distensibility as measured by high resolution impedance planimetry and investigated a correlation between clinical symptoms and dysmotility in patients with EoE.27–29 Kwiatek et al. included 35 patients with EoE and previously documented symptoms of dysphagia, food impaction, chest pain or heartburn.29 These were compared with 15 control subjects. Patients were evaluated during endoscopy using the EndoFLIP® probe (Crospon, Ireland), which is comprised of a compliant cylindrical bag with 16 impedance planimetry segments. The bag was distended in a stepwise fashion with intra-bag pressures and intra-luminal geometry measurements taken in the distal esophagus. Authors found that esophageal distensibility (calculated as cross-sectional area [CSA] vs pressure) was substantially reduced in EoE patients compared with controls (with median CSA 267 vs 438mm2, p < 0.01).
Nicoderme et al. evaluated 70 patients with EoE who underwent esophageal biopsy and high resolution impedance planimetry.27 The investigators utilized a functional lumen imaging probe and documented the occurrence of food impaction, requirement for dilation and symptom severity over an average 9-month period. Ten controls underwent endoscopy with biopsy and the EndoFLIP® protocol for comparison. Biopsies were obtained from the distal and mid-proximal esophagus and distensibility was measured 3cm proximal to the esophago-gastric junction. The authors concluded that patients with prior food impactions or who had undergone previous dilation had significantly lower distensibility plateau (DP) values (DP < 225mm2) compared with those who had only solid food dysphagia.
Interestingly, both studies reported that the severity of mucosal eosinophilia, quantified by the number of eosinophils/hpf did not correlate with the risk of food impaction, severity of dysphagia or the degree of esophageal distensibility. This finding remained true even in patients who had been treated with PPIs; repeat biopsies revealed fewer than 15 eosinophils/hpf.27
While these studies suggest high resolution impedance planimetry is an effective tool to evaluate esophageal distensibility, the lack of correlation between the distensibility curve profile and eosinophilic density on biopsy or treatment with PPI with a resulting reduction in eosinophil count calls into question the clinical applicability of this tool. Most likely, there are additional mechanisms by which eosinophils lead to dysmotility that have not been identified. If the presence of poor esophageal distensibility is a marker for higher disease severity, as suggested by the authors, further studies are required in order to stratify findings in patients with EoE who present with less severe symptoms in order to make a larger statement on clinical applicability of impedance planimetry. These studies also indicate that eosinophil reduction on histopathology alone does not correlate with clinical resolution.
Barium esophagogramLee et al. performed a barium esophagogram in 11 patients with EoE before and after 6 weeks of topical corticosteroid therapy to determine the minimum and maximum esophageal diameters.30 When compared with controls, patients with EoE had a statistically significant reduction in baseline maximum esophageal diameters (19mm in EOE vs 24mm in controls, p = 0.004). Median increases with corticosteroid therapy were not statistically significant, except in those who had an abnormal baseline diameter and there was no correlation with clinical symptoms as documented by the Mayo Dysphagia Questionnaire. The diameter measurements were reproducible, but normal in approximately 50% of patients with EoE.30 Results suggest that those with narrowing may have improvement in structural changes with steroids; however this may not correlate with clinical outcomes.
Given that only approximately 50% of patients with EoE have abnormal baseline esophageal diameters and statistically significant changes in diameter had no correlation to clinical outcomes, there is currently no role for barium esophagogram in evaluating patients with eosinophilic esophagitis.
Esophageal manometryStudies using esophageal manometry to evaluate patients with EoE have produced inconsistent results including normal peristalsis, aperistalsis, and ineffective peristalsis secondary to simultaneous contractions and high amplitude esophageal body contractions, diffuse esophageal spasms, tertiary contractions, achalasia, nutcracker esophagus, and high amplitude contractions in the lower esophagus. In a meta-analysis of 77 patients with EoE, Furuta et al. reported abnormal esophageal manometry in 53% of patients with 30 patients having inadequate peristalsis.1
To date, there have been at least 25 case reports or published series documenting the results of esophageal manometry in patients with EoE. In 1993 Attwood et al. reported the outcomes of 12 patients with EoE undergoing esophageal manometry.31 Subjects with a high concentration of intraepithelial esophageal eosinophils on biopsy but without anatomic abnormality were included. Results revealed nonspecific motility disturbances in 10 of the 12 patients (83%), including diffuse esophageal spasm (DES), nutcracker esophagus, mean amplitude contractions less than 2.5 percentile of normal and short duration contraction. All patients had normal lower esophageal sphincter (LES) pressure and function.31
Lucendo et al. performed endoscopies in patients with EoE before and after corticosteroid treatment, documenting lower esophageal sphincter dysfunction and distal esophageal dysfunction through manometry in 73 and 57% of cases, respectively.32 The same authors also conducted a case study of 12 patients diagnosed with EoE who presented with dysphagia or food impaction.33 Seven patients (58%) had abnormalities on manometry. Three patients had hyperkinetic peristaltic waves in the distal third of the esophagus. One patient had alteration in the motor dynamics with 80% of deglutatory complexes formed by a simultaneous first wave in the lower two thirds of the esophagus followed by a second wave with normal duration and amplitude. The LES tone was normal in 10 patients and reduced in 2 cases. In 7 of 9 patients with dysmotility, the motor disorder improved with treatment with steroid lavage (using fluticasone propionate).33
Monnerat et al. reported abnormal esophageal manometry in 5 of 20 patients (25%) with EoE.34 Motility abnormalities, specifically low amplitude peristaltic contractions and/or non-transmitted contractions (n = 3), hypertensive (n = 1) and hypotensive (n = 1) LESs were present in 40% (2/5) of patients with dysphagia for solid foods and 36% (4/11) of patients with recurrent food impaction.
In contrast, Remedios et al. described a case series of 26 patients with EoE, all of whom had a history of dysphagia and 11 of whom presented acutely with food bolus obstruction26. Esophageal manometry demonstrated good quality peristalsis in 22 of 23 patients. They reported reduced LES pressure in 10 patients, however, this finding correlated with GERD and acid exposure.
Bassett et al. performed a prospective study and found a prevalence of non-specific esophageal motility disorder, defined as ≥ 30% non-transmitted wet swallows.35 However, results showed there was no difference in reported symptoms such as swallowing difficulty, heartburn, and chest pain in those with non-specific esophageal motility disorder and those without. They also found no difference in eosinophil count on mucosal biopsy between the two groups.
In comparison, Nurko et al. performed prolonged esophageal manometry (20-24hours) and found 13 of 17 EoE patients experienced 21 episodes of dysphagia and all of these episodes corresponded to abnormal esophageal motility (90% with non-peristaltic contractions, 90% with isolated and repetitive contractions, amplitude > 180 mmHg in 70%, abnormal peaked contractions in 41%, reflux events < 5 min in 29.5%, and reflux events > 5 min in 7%).36
Van Rhijn et al. compared esophageal high resolution manometry measurements between 31 adults with EoE, 31 age and sex-matched GERD controls, and 31 healthy controls.37 These authors found a higher frequency of failed (12 vs 6%) and weak (27 vs 15%; p < 0.001) contractions among EoE subjects than in healthy controls but a similar occurrence to that of patients with GERD (failed 14%, weak 27%). Importantly, there was no difference in related symptoms (dysphagia, heartburn, limited food intake) or history of food impaction between EoE patients with normal and abnormal motility. The prevalence of abnormal motility was higher with longer disease duration (36%, 0-5 years vs 83%, ≥ 16 years; p < 0.05).37
In a similar study, Roman et al. performed high resolution manometry on 48 patients with EoE, 48 patients with GERD, and 50 asymptomatic controls.38 They found a higher rate of motility disorders among patients with EoE compared with controls but similar in prevalence and presentation to patients with GERD. Both GERD and EoE subjects demonstrated similar rates of weak and failed peristalsis. However, EoE patients were more likely than patients with GERD or controls (33% vs 12% vs 0%) to have abnormal bolus pressurization patterns during swallowing; specifically, patients with EoE were found to have early pan-esophageal pressurization (17% of patients with EoE vs 2% of patients with GERD).
This finding is supported by Martin et al., who evaluated 21 patients with EoE and compared them to 21 controls who had symptoms or GERD and/or dysphagia but < 5 eosinophils on biopsy.39 These authors found that the most frequent esophageal motor abnormality measured by high resolution manometry was a pan-esophageal pressurization (48%), with a statistically significant difference in the prevalence of pressurization abnormality in patients with EoE vs those without (10 [48%] vs 0 [0%], p < 0.05).
Results from studies using esophageal manometry to evaluate potential motility disorders in EoE have been widely variable. In the absence of an obvious anatomic abnormality, manometry can be helpful in identifying an additional population of patients who have failed or weak peristalsis, LES dysfunction or abnormal esophageal pressurization. However, review of the literature suggests there is an additional population of patients whose motility disorder will be missed.
Endoscopic ultrasoundEsophageal manometry primarily measures the circular muscular function of the esophagus. Given that not all motility disorders are discovered by this method, endoscopic ultrasound has emerged as a way to evaluate the longitudinal muscle.
Korsapati et al. correlated findings of manometry and esophageal ultrasound, demonstrating that patients with EoE had changes in the function of the longitudinal muscle.40 In the absence of abnormal circular muscle function witnessed on manometry, a decrease in longitudinal contraction amplitude and duration as well as dissociation between circular and longitudinal muscle contractions was observed.40 In addition, the authors used edrophonium to inhibit acetylcholine breakdown to increase the level of acetylcholine at the neuromuscular junction. Control patients were able to increase circular and longitudinal muscle contractions, whereas patients with EoE were not, even in those with normal baseline circular muscle function. While the sample size was ultimately too small to draw clinically significant conclusions, this study raised questions about an additional potential mechanism for muscular motor dysfunction.
The use of endoscopic ultrasound has led to a proposed mechanism that would help account for patients with symptoms of dysphagia and food impaction where other tools such as conventional manometry have failed to identify an etiology. Larger studies need to be done to further evaluate the relationship between this proposed physiologic mechanism and its relation to the pathophysiology of EoE so that correlations can be drawn in relation to the clinical symptoms of the disease.
Effect of treatment on esophageal motility abnormalitiesSeveral treatment options are available with varying efficacy for the management of EoE. However, limited data is available on the effect of these treatments on esophageal dysmotility. Lucendo et al. reported an increase in the percentage of normal peristaltic waves (p = 0.018) and a decrease in the percentage of non-transmitted waves in 7 patients following 3 months of treatment with swallowed fluticasone propionate therapy (500μg twice daily).32 Remedios et al. included 19 patients who completed 4 weeks of treatment with swallowed fluticasone propionate.26 All patients reported dramatic improvements in symptoms, with complete resolution in 11 of the 19 (58%) patients. Biopsy results showed a significant decrease in eosinophilic infiltration. Unfortunately, motility measurements were only made prior to treatment and it is unclear if an improvement in motility may have contributed to symptom improvement.
ConclusionsEoE is an esophageal disorder with increasing incidence and recognition. It may present with dysphagia or food impaction and typically affects younger men. Studies have shown that EoE is likely a multifactorial disease triggered by allergens that stimulate an immune response leading to eosinophilic infiltration of the esophagus. EoE has been associated with varying degrees of esophageal dysmotility and structural abnormalities from fibrosis and tissue remodeling that may contribute to symptoms. Motility dysfunction of the esophagus in EoE has not been well reported in the literature and studies have reported conflicting evidence regarding the clinical significance of dysmotility seen in EOE. High resolution impedance planimetry testing has shown reduced distensibility, or compliance, that has been associated with the occurrence of food impaction.27 Esophageal manometry studies in EoE patients have been few and included small patient cohorts or case reports. The occurrence of abnormal esophageal manometry was reported to be between 4% and 87% among patients with EoE. Dysmotility may present as abnormal peristalsis or hypotensive LES. Reports have demonstrated that EoE may mimic other esophageal motility disorders such as achalasia or nutcracker esophagus, thus making the diagnosis more difficult. EoE has also been shown to have a similar rate of motility dysfunction, particularly weak or absent peristalsis, to that of patients with GERD.38 The inconsistencies on esophageal manometry studies may be explained by disease activity at the time of the study or disease duration, with some studies indicating a higher frequency of dysmotility associated with longer disease duration. Additionally, it remains unclear if a correlation exists between esophageal dysmotility and symptoms of EoE. Larger studies are needed to investigate the incidence of esophageal dysmotility, clinical implications, and effect of treatment on patients with EoE.
Financial disclosureNone.
AuthorshipGuarantor of the article: Ron Schey. Author contributions: Alexandra H. Weiss: article selection, article retrieval, manuscript writing. Natalya Iorio: article retrieval, article selection, manuscript writing. Ron Schey: critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Weiss AH, Iorio N, Schey R. La motilidad esofágica en la esofagitis eosinofílica. Revista de Gastroenterología de México. 2015;80:205–213.