Neoadjuvant therapy in rectal cancer is associated with a decrease in tumor size and is the therapeutic indication for patients with T3 or T4 tumors or lymph node involvement. Our aim was to describe the frequency of pathologic response and the survival rate in patients that underwent neoadjuvant therapy for rectal cancer.
Materials and methodsA retrospective follow-up study with a survival analysis was conducted. Patients with locally advanced rectal cancer that received neoadjuvant treatment and were operated on at the Instituto de Cancerología Las Américas (Medellín-Colombia) were analyzed. Survival was calculated using the Kaplan-Meier method.
ResultsA total of 152 patients were included. Mean patient age was 59 years (12.8 SD), 53.9% were men, and 58.6% of the patients were diagnosed with stage IIIB disease. The pathologic complete response (pCR) was achieved in 17% of the patients. A total of 146 (96.1%) patients received the chemoradiotherapy protocol. Fifty-two (34.2%) patients developed metastasis and/or relapse, and one (3.8%) of those patients had presented with pCR. The median follow-up period was 33 months (Q1-Q3: 20-45), with an overall survival rate of 79.5% (95% CI: 70.9-85.8). The 5-year survival rate for the patients that had pCR was 80% (95% CI: 20.3-96.9).
ConclusionsThe frequency of pCR was similar to that in other published studies and disease recurrence was lower, compared with patients with no response. The 5-year survival rate in patients with pCR was high, albeit lower than that reported in other studies.
La terapia neoadyuvante en cáncer de recto se asocia con disminución del tamaño tumoral y es la indicación terapéutica para pacientes con tumores T3, T4 o con compromiso ganglionar. El objetivo fue describir la frecuencia de respuesta patológica y la supervivencia de los pacientes sometidos a neoadyuvancia para cáncer de recto.
Materiales y MétodosEstudio de seguimiento, retrospectivo, con análisis de supervivencia. Se incluyeron los pacientes con cáncer de recto localmente avanzado, que recibieron tratamiento neoadyuvante y se operaron en el Instituto de Cancerología Las Américas (Medellín-Colombia). La supervivencia se estimó por el método Kaplan-Meier.
ResultadosSe incluyeron 152 pacientes, promedio de edad 59 años (d.e. 12.8), 53.9% hombres, 58.6% diagnosticados en estadio IIIB. La respuesta patológica completa (pRC) se logró en 17% de los pacientes. El protocolo de quimiorradioterapia se dio a 146 (96.1%) pacientes; presentaron metástasis y/o recaída 52 (34.2%), de los cuales 1 (3.8%) había tenido pRC. La mediana de seguimiento fue 33 meses (Q1-Q3: 20-45) con una supervivencia global de 79.5% (IC95%: 70.9-85.8). Para quienes tuvieron pRC la supervivencia a 5 años fue 80% (IC95%: 20.3-96.9).
ConclusionesLa frecuencia de pRC fue similar a otros estudios publicados y su recidiva fue menor con respecto de quienes no tuvieron respuesta. La supervivencia a 5 años de los pacientes con pRC fue alta, aunque inferior a lo publicado en otros estudios.
Rectal cancer is a highly frequent disease, which in 2018, had an estimated 704,376 new cases, with incidence rates of 10 per 100,000 women and 5.6 per 100,000 men. In South America, the rates were 7.7 for women and 5.4 for men.1 According to the GLOBOCAN project, rectal cancer was the tenth cause of cancer in Colombia in 2018, with 2,922 new cases.2
Currently, neoadjuvant treatment is the therapeutic indication for patients with adenocarcinoma of the rectum that have T3 or T4 tumors or suspicion of regional lymph node involvement.3,4 The National Institute for Clinical Excellence (NICE) and National Comprehensive Cancer Network (NCCN) guidelines recommend offering preoperative chemoradiotherapy, with an interval before surgery, to enable a decrease in tumor size and better surgical results in high-risk patients with operable rectal cancer.4,5
Neoadjuvant therapy is associated with a significant reduction in tumor size, a decrease in the incidence of local recurrence, an increased probability of preserving the anal sphincter, and the possibility of achieving pathologic complete response (pCR).6
There are currently 5 different classifications for evaluating the pathologic response to chemoradiotherapy regarding neoadjuvant treatment results, and the American Joint Committee on Cancer classification is considered the standard.7–9 It consists of the following categorization: (0) complete response, when no tumor cells are observed, (1) moderate response, when only small tumor nests or single cells are observed, (2) minimal response, when there is residual cancer but predominant fibrosis, and (3) poor response when tumor regression is minimal or null.
The aim of the present study was to describe the pathologic response and survival rate of patients that underwent neoadjuvant therapy for rectal cancer.
Materials and methodsA retrospective follow-up study was conducted. The data were taken from the Information System for the Follow-up of Patients with Colorectal Cancer of the Instituto de Cancerología (IDC) Las Américas. Patients treated within the time frame of 2011-2017 for rectal cancer, with stage T3, T4, and/or lymph node involvement, and/or M1 with intention to cure, that received neoadjuvant chemotherapy and/or radiotherapy, and were operated on at the IDC were included in the study.
The sociodemographic variables of age, sex, and type of health insurance were considered, as well as clinical conditions, such as medical comorbidities (high blood pressure, diabetes, chronic obstructive pulmonary disease, chronic kidney disease, and heart diseases), histologic diagnosis, clinical stage, neoadjuvant treatment, chemotherapy regimen, radiotherapy dose, type of surgery, months between diagnosis and neoadjuvant therapy, time interval between neoadjuvant therapy and surgery, and complications (during neoadjuvant therapy, complications were considered severe if treatment was suspended, and postoperative complications were those that required reintervention). In addition, the recurrence variables of metastasis and/or relapse, months between surgery and metastasis, and vital status were included.
The American Joint Committee on Cancer staging system was employed. Clinical staging was carried out through colonoscopy, contrast-enhanced chest computed tomography, and contrast-enhanced abdominal and pelvic magnetic resonance imaging.
The chemoradiotherapy protocol was three-dimensional conformal radiation therapy. Gross tumor volume was defined as macroscopic disease identified through physical examination and contrast-enhanced pelvic tomography or pelvic magnetic resonance imaging. Clinical tumor volume covered the totality of the mesorectum and the right and left internal iliac lymph nodes for T3 tumors, and the right and left external iliac lymph nodes for T4 tumors. The total dose was 45 Gy to 1.8 Gy for the lymph node chains and 50.4 Gy to 1.8 Gy for the tumor.
The chemotherapy regimen was intravenous 5 FU 350 mg/m2 daily plus intravenous leucovorin 20 mg/m2 daily for 5 days on the first and fifth week of radiotherapy, and in case of shortage, oral capecitabin 1,650 mg/m2/day every 12 h every day during radiotherapy.
Surgery included total mesorectal excision in the majority of patients. Preserving the anal sphincter or not depended on the resection level of the mass. Low anterior resection, either with the open or laparoscopic approach, and abdominoperineal resection, either with the open or laparoscopic approach, were contemplated. In patients that refused mesorectal excision, local resection with the transanal approach was performed.
All the resection specimens were evaluated and staged by pathologists at the Las Américas pathology laboratory, following the guidelines of the College of American Pathologists and the American Joint Committee on Cancer.8,9 Representative tumor sections of the specimens with macroscopically visible disease were processed for microscopic evaluation. In the cases in which the tumor was not macroscopically observed, the tumor bed, ulcerated areas, fibrotic areas, or areas suspected of tumor, were processed in their totality in 4 mm-thick serial slices in paraffin blocks and then evaluated microscopically. The lymph nodes were processed and evaluated in their totality.
pCR was defined as the absence of viable tumor cells in the surgical specimen (ypT0ypN0) and incomplete response was considered when almost complete, partial, poor, or no response was classified.
Overall survival (OS) was determined as length of time from diagnosis to death by any cause and disease-free survival (DFS) was defined as the length of time from diagnosis to the appearance of signs and/or symptoms of disease or death. The date of death was obtained from the clinical history, or from the database of the Registraduría Nacional del Estado Civil or the Administradora de Recursos del Sistema. The closing date for obtaining information needed for OS and DFS was August 30, 2018. Patients whose vital status could not be collected from the abovementioned sources were censored, utilizing the last contact date at the IDC.
Statistical analysisThe quantitative variables were expressed as mean and standard deviation (SD) or median and interquartile range (IQR), according to the symmetry of the variable. The Kolmogorov-Smirnov test was utilized to test for normality. The categorical variables were expressed through absolute frequencies and percentages. The Pearson’s chi-square test, Student’s t test, and Mann-Whitney U test were employed to determine the association between 2 variables, as required. Survival was calculated through the Kaplan-Meier method and the survival curves were compared using the log-rank test. The data were analyzed using the STATA v12 program.
Ethical considerationsThe protocol was carried out according to international ethics regulations and the Colombian legislature, and the study and its execution were approved and overseen by the Independent Ethics Committee of the IDC Las Américas, which meets the Good Clinical Practice norms in all its activities.
The Ethics Committee exempted the researcher from obtaining informed consent, given that the research was classified as No Risk, according to Colombian regulations. The authors followed the protocols of patient data publication, maintaining patient anonymity at all times.
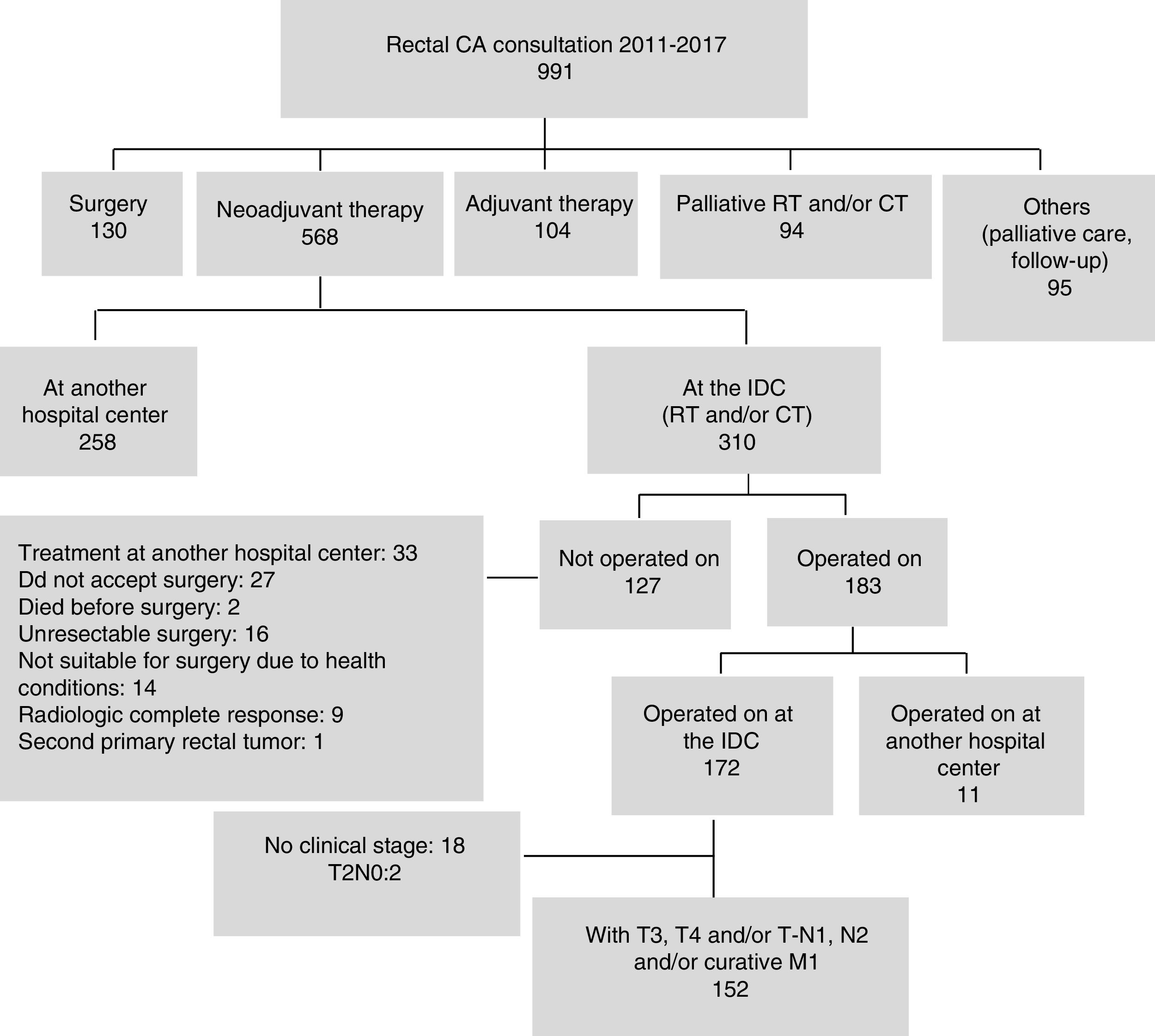
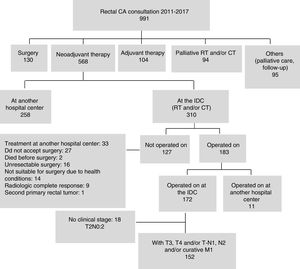
ResultsOf the 991 patients with rectal cancer treated within the study period, 310 (31.3%) received neoadjuvant treatment at the IDC, resulting in the inclusion of 152 patients in the study. Fig. 1 shows the reasons 127 (40.9%) patients were not operated on. Nine patients with response identified by imaging studies requested a multidisciplinary evaluation: 2 of them presented with metastasis, one died, and 7 continue with clinical complete response.
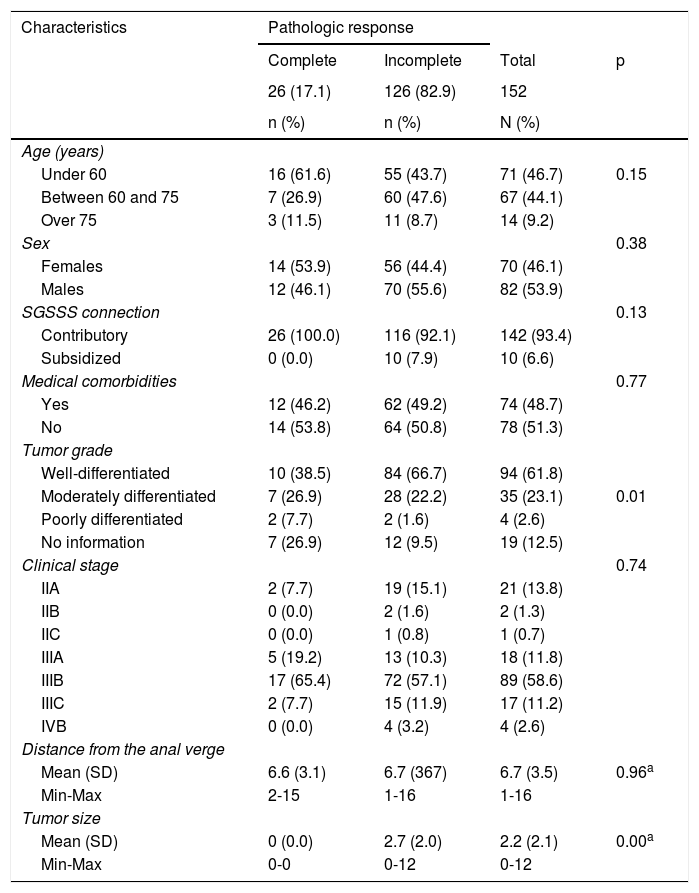
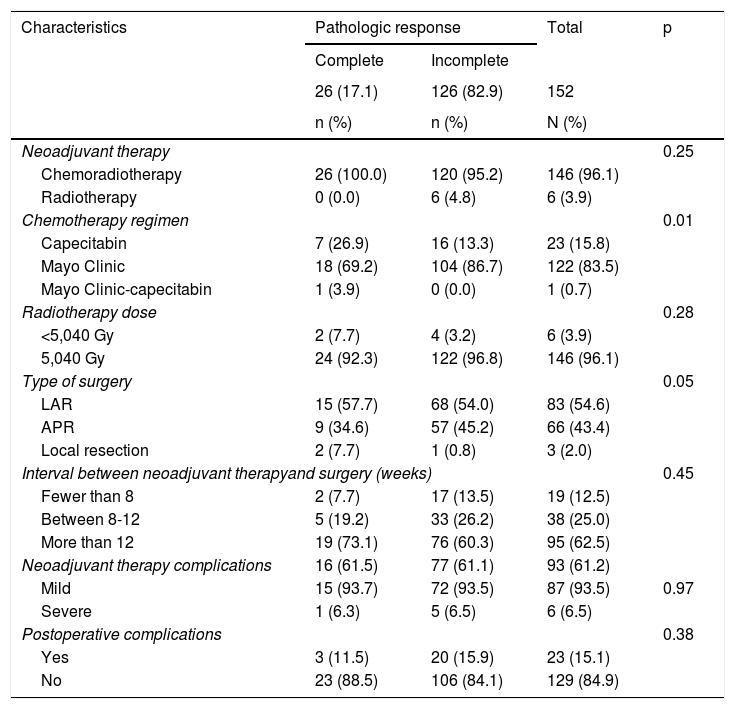
Mean patient age was 59 years (12.8 SD), with a range of 18 to 84 years, and male sex predominated (n = 82; 53.9%). Seventy-four patients (48.7%) presented with medical comorbidities. Eighty-nine patients (58.6%) had clinical stage IIIB disease. The histologic type of all the tumors was adenocarcinoma. The metastasis sites in the 4 patients with stage IV disease were the liver (n = 2) and the lung (n = 2), and their treatment was surgery and chemotherapy. Twenty-six patients (17%) achieved pCR after neoadjuvant treatment, with a mean age of 56 years (13.8 SD) and a predominance of female sex (n = 14; 53.9%) (Table 1).
Sociodemographic and clinical characteristics.
| Characteristics | Pathologic response | |||
|---|---|---|---|---|
| Complete | Incomplete | Total | p | |
| 26 (17.1) | 126 (82.9) | 152 | ||
| n (%) | n (%) | N (%) | ||
| Age (years) | ||||
| Under 60 | 16 (61.6) | 55 (43.7) | 71 (46.7) | 0.15 |
| Between 60 and 75 | 7 (26.9) | 60 (47.6) | 67 (44.1) | |
| Over 75 | 3 (11.5) | 11 (8.7) | 14 (9.2) | |
| Sex | 0.38 | |||
| Females | 14 (53.9) | 56 (44.4) | 70 (46.1) | |
| Males | 12 (46.1) | 70 (55.6) | 82 (53.9) | |
| SGSSS connection | 0.13 | |||
| Contributory | 26 (100.0) | 116 (92.1) | 142 (93.4) | |
| Subsidized | 0 (0.0) | 10 (7.9) | 10 (6.6) | |
| Medical comorbidities | 0.77 | |||
| Yes | 12 (46.2) | 62 (49.2) | 74 (48.7) | |
| No | 14 (53.8) | 64 (50.8) | 78 (51.3) | |
| Tumor grade | ||||
| Well-differentiated | 10 (38.5) | 84 (66.7) | 94 (61.8) | |
| Moderately differentiated | 7 (26.9) | 28 (22.2) | 35 (23.1) | 0.01 |
| Poorly differentiated | 2 (7.7) | 2 (1.6) | 4 (2.6) | |
| No information | 7 (26.9) | 12 (9.5) | 19 (12.5) | |
| Clinical stage | 0.74 | |||
| IIA | 2 (7.7) | 19 (15.1) | 21 (13.8) | |
| IIB | 0 (0.0) | 2 (1.6) | 2 (1.3) | |
| IIC | 0 (0.0) | 1 (0.8) | 1 (0.7) | |
| IIIA | 5 (19.2) | 13 (10.3) | 18 (11.8) | |
| IIIB | 17 (65.4) | 72 (57.1) | 89 (58.6) | |
| IIIC | 2 (7.7) | 15 (11.9) | 17 (11.2) | |
| IVB | 0 (0.0) | 4 (3.2) | 4 (2.6) | |
| Distance from the anal verge | ||||
| Mean (SD) | 6.6 (3.1) | 6.7 (367) | 6.7 (3.5) | 0.96a |
| Min-Max | 2-15 | 1-16 | 1-16 | |
| Tumor size | ||||
| Mean (SD) | 0 (0.0) | 2.7 (2.0) | 2.2 (2.1) | 0.00a |
| Min-Max | 0-0 | 0-12 | 0-12 | |
Max: maximum; Min: minimum; SD: standard deviation; SGSSS: Sistema General de Seguridad Social en Salud (General Social Security Health System).
A total of 146 patients (96.1%) underwent the chemoradiotherapy protocol. One hundred twenty-two patients (83.5%) received the Mayo Clinic chemotherapy regimen, and 146 patients (96.1%) received a radiotherapy dose of 50.4 Gy. The majority of patients underwent low anterior resection of the rectum (n = 83; 54.6%). A total of 62.5% of the patients were operated on after 12 weeks due to administrative delays, such as authorizations, documentation receipt, procedure programming, concomitant treatment of another disease, or unwillingness to undergo surgery, among others. All the patients with pCR received chemoradiotherapy, the mean time between diagnosis and the start of neoadjuvant therapy was 1.8 months (IQR: 1.5-3.6), which was lower than the 2.5 months (IQR: 1.7-3.2) found in the patients that did not have complete response (Table 2).
Treatment and complications.
| Characteristics | Pathologic response | Total | p | |
|---|---|---|---|---|
| Complete | Incomplete | |||
| 26 (17.1) | 126 (82.9) | 152 | ||
| n (%) | n (%) | N (%) | ||
| Neoadjuvant therapy | 0.25 | |||
| Chemoradiotherapy | 26 (100.0) | 120 (95.2) | 146 (96.1) | |
| Radiotherapy | 0 (0.0) | 6 (4.8) | 6 (3.9) | |
| Chemotherapy regimen | 0.01 | |||
| Capecitabin | 7 (26.9) | 16 (13.3) | 23 (15.8) | |
| Mayo Clinic | 18 (69.2) | 104 (86.7) | 122 (83.5) | |
| Mayo Clinic-capecitabin | 1 (3.9) | 0 (0.0) | 1 (0.7) | |
| Radiotherapy dose | 0.28 | |||
| <5,040 Gy | 2 (7.7) | 4 (3.2) | 6 (3.9) | |
| 5,040 Gy | 24 (92.3) | 122 (96.8) | 146 (96.1) | |
| Type of surgery | 0.05 | |||
| LAR | 15 (57.7) | 68 (54.0) | 83 (54.6) | |
| APR | 9 (34.6) | 57 (45.2) | 66 (43.4) | |
| Local resection | 2 (7.7) | 1 (0.8) | 3 (2.0) | |
| Interval between neoadjuvant therapyand surgery (weeks) | 0.45 | |||
| Fewer than 8 | 2 (7.7) | 17 (13.5) | 19 (12.5) | |
| Between 8-12 | 5 (19.2) | 33 (26.2) | 38 (25.0) | |
| More than 12 | 19 (73.1) | 76 (60.3) | 95 (62.5) | |
| Neoadjuvant therapy complications | 16 (61.5) | 77 (61.1) | 93 (61.2) | |
| Mild | 15 (93.7) | 72 (93.5) | 87 (93.5) | 0.97 |
| Severe | 1 (6.3) | 5 (6.5) | 6 (6.5) | |
| Postoperative complications | 0.38 | |||
| Yes | 3 (11.5) | 20 (15.9) | 23 (15.1) | |
| No | 23 (88.5) | 106 (84.1) | 129 (84.9) | |
APR: abdominoperineal resection; LAR: low anterior resection.
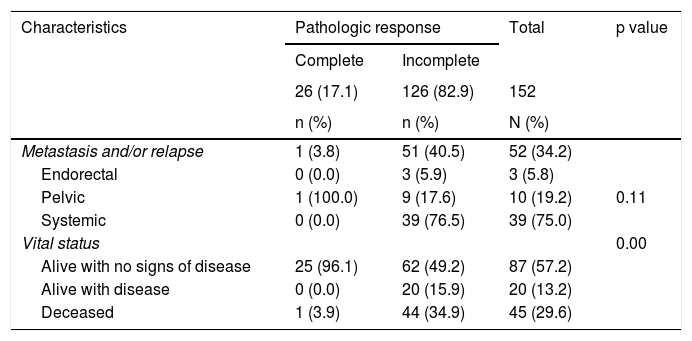
A total of 34.2% of the patients had disease progression and it presented before surgery in 9 of those patients. Of the patients with pCR, only one patient had relapse and it was located at the pelvic site. On the other hand, the group with no response to neoadjuvant therapy had more frequent systemic recurrence (n = 39; 76.5%). The median of time between surgery and metastasis and/or relapse was 13.5 weeks (IQR: 6-19) (Table 3).
Metastasis and/or relapse and vital status.
| Characteristics | Pathologic response | Total | p value | |
|---|---|---|---|---|
| Complete | Incomplete | |||
| 26 (17.1) | 126 (82.9) | 152 | ||
| n (%) | n (%) | N (%) | ||
| Metastasis and/or relapse | 1 (3.8) | 51 (40.5) | 52 (34.2) | |
| Endorectal | 0 (0.0) | 3 (5.9) | 3 (5.8) | |
| Pelvic | 1 (100.0) | 9 (17.6) | 10 (19.2) | 0.11 |
| Systemic | 0 (0.0) | 39 (76.5) | 39 (75.0) | |
| Vital status | 0.00 | |||
| Alive with no signs of disease | 25 (96.1) | 62 (49.2) | 87 (57.2) | |
| Alive with disease | 0 (0.0) | 20 (15.9) | 20 (13.2) | |
| Deceased | 1 (3.9) | 44 (34.9) | 45 (29.6) | |
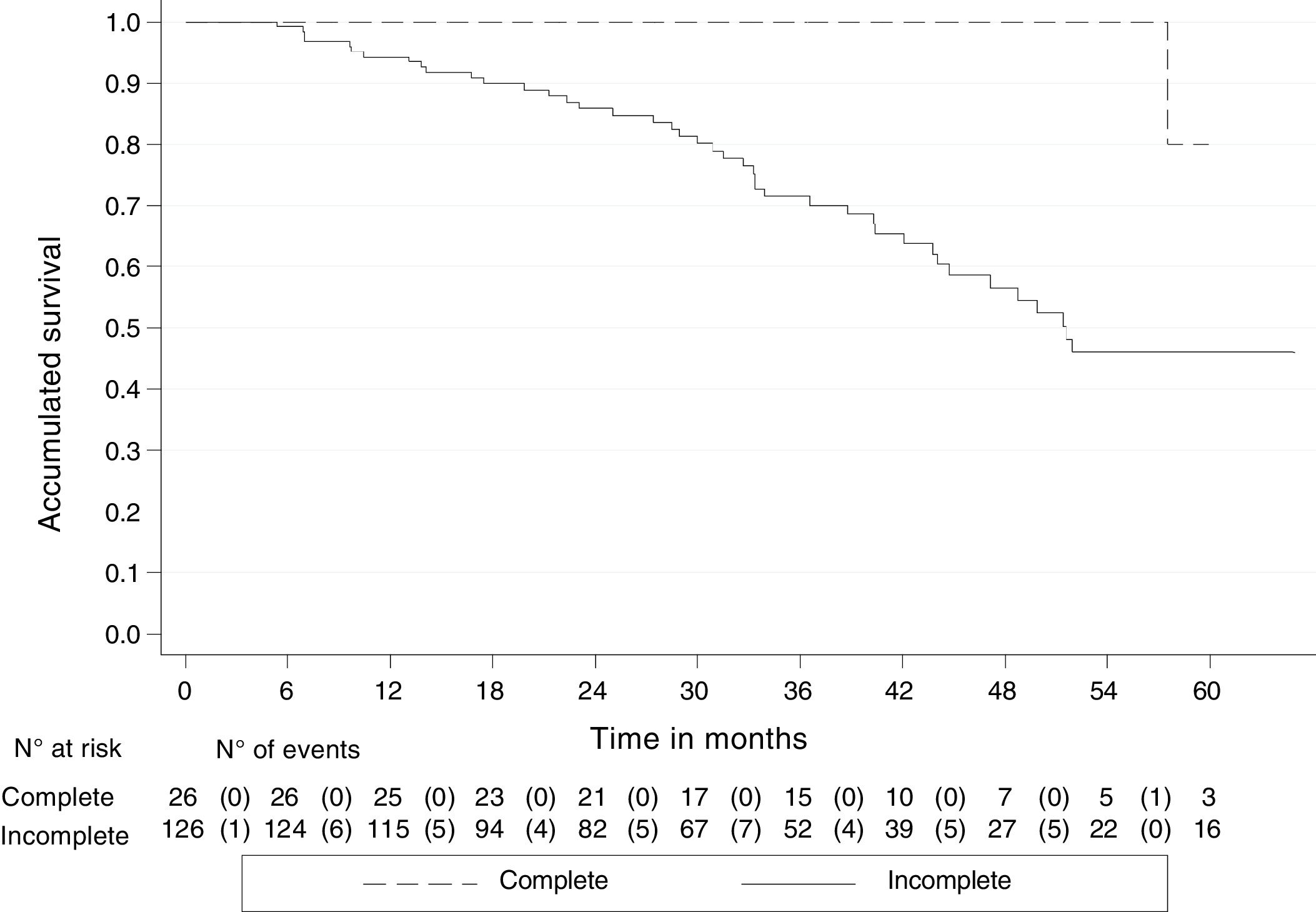
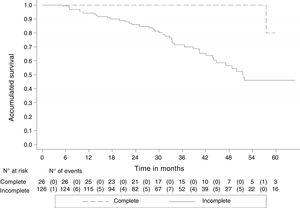
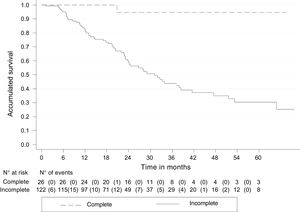
By August 30, 2018, 45 (29.6%) of the 152 patients had died, one (3.9%) with pCR and 44 (34.9%) with incomplete response. The median follow-up of all the patients was 33.3 months (IQR: 20-45), with an OS rate of 79.5% (95% CI 70.9-85.8). The 5-year OS rate was 51.2% (95% CI 38.6-62.4) and 80% of those cases had pCR (95% CI: 20.3-96.9), whereas 46.1% had incomplete response (95% CI: 33.3-57.8). The Kaplan-Meier curves showed better OS in the patients with pCR (p < 0.05) (Fig. 2).
In the stage comparison, the 5-year OS rate was 58.6% (95% CI 33.3-.77.2), 48.3% (95% CI 33.1-61.9), and 50% (95% CI 0.6-.91.0) in the patients with stages II, III, and IV of the disease, respectively.
There were no differences in 5-year OS in the interval of time between the completion of neoadjuvant therapy and the performance of surgery. The patients operated on before 8 weeks had an OS rate of 51.7% (95% CI 25.9-72.4), 58.2% (95% CI 32.1-77.3) for those operated on between 8 and 12 weeks, and 46.5% (95% CI 27.4-63.5) for those operated on after 12 weeks. There were also no differences in 5-year OS rates, regarding the time from neoadjuvant treatment to surgery, between the patients with and without pCR.
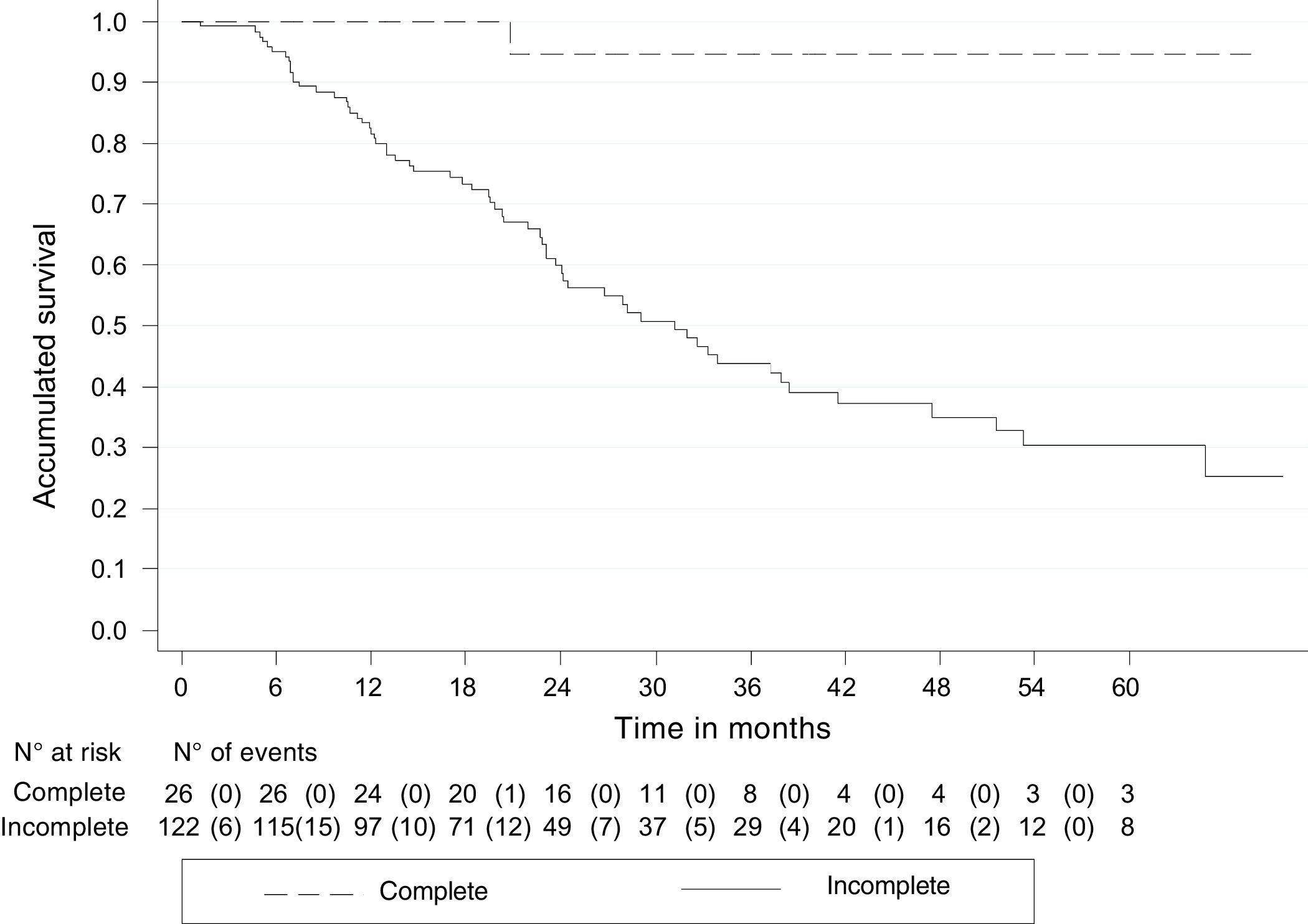
DFS was 71.7% (95% CI 63.2-78.6) and 5-year DFS was 38.4% (95% CI 27.1-49.6). The patients with pCR had a higher DFS rate, compared with those with partial response or no response: 94.7% (95% CI 68.1-99.2) and 30.1% (95% CI 19.4-41.6), respectively (Fig. 3).
Discussion and conclusionsSeveral studies have described the results of treatment with neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer in relation to pCR, local control, locoregional control, and survival.
In the present study, the sociodemographic characteristics of the cohort were very similar to those reported in other studies, with a mean age that varied from 58 to 65 years and a predominance of male sex.10–13
The frequency of pCR in our study was 17%, which concurs with that published in other studies. In the study by Banurra et al.,14 with a 13-year follow-up and very similar cohort, pCR was 12.5%. In 500 patients with follow-up, currently the largest cohort reported, Cienfuegos et al.10 described a pCR of 12%. Codina-Cazador et al.,11 Espinola et al.,12 and Omejec and Potisek,13 with cohorts of 162, 119, and 202 patients, reported pCR in 11.7%, 15.1%, and 14.8% of the patients, respectively.
In general, treatment of rectal cancer in stage II (T3-T4, disease with negative lymph nodes and penetration of the tumor through the muscle wall) or stage III (disease with positive lymph nodes with no distant metastasis) includes locoregional treatment due to the relatively high risk for recurrence. That risk is associated with the proximity of the rectum to the pelvic structures and organs, the absence of serosa surrounding the rectum, and the technical difficulties associated with obtaining wide surgical margins in the resection.4 Sauer et al.15 reported an incidence of local relapse at 10 years of 7.1%. In contrast, in another study by Cienfuegos et al.,10 they described tumor recurrence in 28% of the patients, of which 25.6% corresponded to distant recurrences. Likewise, in our case series, the 34% recurrence rate was high. Nevertheless, in the group with complete response, there was only one case with pelvic relapse. Finally, the results of a 12-study meta-analysis16 published in 2012 showed a 5-year local recurrence rate of 0% in the patients with pCR. The overall weighted average of the local recurrence rate in all those studies was 7%. The patients with pCR had 4 times less probability of developing recurrence, compared with the patients that had a partial response or no response. Distant relapses or metastases were observed in 8.7% of the patients that had complete response.
The majority of authors have described a correlation between the degree of response and survival. In their institutional, comparative, retrospective study, Lee et al.17 reported a 5-year OS rate of 76.2% and a 5-year DFS rate of 72.1%. Cienfugos et al.10 reported a 5-year OS rate of 76.9% and a DFS rate of 66%. In the meta-analysis,16 there was a 5-year OS rate of 90.2% for the patients with pCR. Those patients had a 3.3 times higher OS advantage, compared with the patients that did not have complete response. The 5-year DFS rate was 87% in the patients with pCR. That group had 4.3 times more probability of being disease-free at 5 years than the patients without complete response. Other studies11,12,14 described a 100% 5-year OS rate. In our study, the 5-year OS rate was 80% and the DFS rate was 94.7%.
Researchers have attempted to identify the optimum time interval between neoadjuvant treatment and surgery, for increasing the complete response rates, but the results have been contradictory and inconsistent. In addition, whether patients with pCR should undergo adjuvant chemotherapy is still a subject of debate.
The strength of the present study is that it is the first analysis of its kind in Colombia. Even though it was limited by the number of patients, the short follow-up period, and the fact that it was conducted at a single cancer center, our patients were shown to have results similar to those of other studies. Therefore, we conclude that long-term oncologic control is excellent in patients that receive neoadjuvant treatment with chemoradiotherapy and achieve pCR, resulting in reduced distant and locoregional recurrence rates, as well as improved OS and DFS rates.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Palacios-Fuenmayor LJ, Naranjo-Isaza AM, Serna-Ortiz CA, Mosquera-Castro DA, Arbeláez-Leon LM, Gómez-Wolff LR, et al. Evaluación de la respuesta patológica al tratamiento neoadyuvante en cáncer de recto. Experiencia del Instituto de Cancerología de Medellín (Colombia, 2011-2017). Revista de Gastroenterología de México. 2021;86:13–20.