Hepatocellular carcinoma (HCC) is the most frequent primary tumor of the liver. HCC in the noncirrhotic liver accounts for 15-20% of all HCC. Noncirrhotic HCC is a clinically different entity because of the non-neoplastic liver parenchyma involved. Our aim was to describe the presentation, treatment, and predictive survival results of noncirrhotic HCC in Veracruz.
Materials and methodsA retrospective study, spanning 13 years, was conducted on patients with noncirrhotic HCC. It analyzed their clinical characteristics, fibrosis/cirrhosis biologic index (NAFLD, MELD, ALBI, APRI, CDS, FIB-4, GUCI, LOK) results, disease treatment, and survival.
ResultsFrom a total of 168 cases of HCC, 33 (19.6%) noncirrhotic patients were included in the study. Of those patients, the mean patient age was 67.3 years (51.5% men), 9.1% had hepatitis C virus infection, and 27.3% were alcoholics. Less than 20% of the patients had biologic indexes suggestive of fibrosis/cirrhosis. Mean tumor size was 7.7 cm and 42.4% of the patients had alpha-fetoprotein (AFP) levels > 15 ng/mL. A total of 52.5% of the tumors were classified as Okuda II and 30.3% of the patients had advanced disease (the Milan criteria). Liver resection was performed on 51.5% of the patients, radiofrequency ablation on 18.2%, and transarterial chemoembolization on 9.1%. The overall 5-year survival rate was 55.4%. Liver resection resulted in the best 5-year survival rate (72.7%). Age > 67 years and elevated AFP levels were associated with poorer survival (p < 0.05, log-rank).
ConclusionsThe characteristics and survival rate of HCC in the noncirrhotic liver were similar to those reported in other studies. Liver resection provided the highest survival rates. The liver fibrosis biologic indexes were not risk factors for survival.
El carcinoma hepatocelular (CHC) es el tumor primario más frecuente del hígado. La incidencia de CHC en hígado no cirrótico es del 15-20% de todos los CHC. El parénquima hepático no tumoral hace que el CHC no cirrótico sea una entidad clínica diferente. El objetivo fue describir la presentación, tratamiento y resultados predictivos de sobrevida de CHC en hígado no cirrótico en nuestra población.
Material y métodosEstudio retrospectivo de 13 años en pacientes con CHC no cirrótico, analizando características clínicas e índices biológicos de fibrosis/cirrosis (NAFLD, MELD, ALBI, APRI, CDS, FIB-4, GUCI, LOK), tratamiento y sobrevida.
ResultadosSe incluyeron 33 pacientes con CHC en hígado no cirrótico de 168 CHC (19.6%); edad promedio 67.3 años (51.5% hombres), 9.1% con virus de hepatitis C y 27.3% con abuso de alcohol. El 20% tenía índices biológicos probables de fibrosis/cirrosis; el tamaño promedio tumoral fue 7.7 cm. y 42.4% tenían AFP > 15 ng/mL. Se clasificaron 52.5% Okuda II y 30.3% enfermedad avanzada (criterios de Milán). Se trataron 51.5% con resección hepática (RH), 18,2% ablación por radiofrecuencia y 9.1% quimioembolización transarterial. La sobrevida global a 5 años de 55,4%. La RH tuvo la mejor supervivencia a 5 años (72.7%). La edad > 67 años y el aumento de AFP se asoció con menor sobrevida (p < 0.05, log-rank).
ConclusionesEl CHC en hígado no cirrótico tiene características y sobrevida similar a otros sitios. La RH tiene la mayor sobrevida. Losíndices biológicos de fibrosis no fueron factores de riesgo para la sobrevida.
Hepatocellular carcinoma (HCC) is the most common primary tumor of the liver worldwide and the fifth and ninth cancer in men and women, respectively.1 Globally, hepatitis B virus (HBV) is the main risk factor for HCC.2 In Europe and America, the main risk factors are hepatitis C virus (HCV) and alcohol consumption.3
HCC in the noncirrhotic liver accounts for 15-20% of all HCCs. If the estimated incidence of new cases of HCC across the globe is 600,000 cases per year, we can extrapolate an incidence of 90,000-120,000 new cases per year of HCC in the noncirrhotic liver. The "normal" quality of the non-neoplastic liver parenchyma is what makes noncirrhotic HCC a different entity, in relation to its epidemiology, clinical presentation, management, and outcome.4 Some reports on HCC in the noncirrhotic liver state that the preponderance of men (male:female ratio of 1.3-2:1) is lower than that reported for HCC in the cirrhotic liver. Mean patient age is generally lower in noncirrhotic patients than in cirrhotic ones.5
Nonalcoholic fatty liver disease is a metabolic disorder characterized by macrovesicular hepatic steatosis above 5% in individuals that do not consume a significant amount of alcohol, do not take medications that are toxic to the liver, or do not present with other causes of secondary steatosis. Its clinical spectrum includes simple steatosis, steatohepatitis, cirrhosis, and HCC. Nonalcoholic fatty liver disease is the manifestation of metabolic syndrome at the level of the liver.6 HCC is a late complication of nonalcoholic steatohepatitis, even in the noncirrhotic liver.7
HCC in the noncirrhotic liver is generally diagnosed when the tumor reaches a size that triggers symptoms.8 It is also an incidental diagnosis in imaging studies or from abnormal laboratory findings.9 The level of serum alpha-fetoprotein (AFP) considered diagnostic for HCC (400 ng/dl) is similar in cirrhotic and noncirrhotic patients, albeit AFP > 20 ng/dl is less frequent in noncirrhotic HCC.10,11
Liver resection (LR) is the treatment of choice in noncirrhotic HCC due to the low risk for postoperative liver failure, a lower recurrence rate, and lower morbidity and mortality rates than in the cirrhotic liver. Despite having a larger tumor burden, the conserved function of the noncirrhotic liver generally makes large resections possible and safe.12,13
There are biologic indexes for establishing liver fibrosis in nonalcoholic fatty liver disease that are determined in serum and are diagnostically acceptable. Among the most widely studied are the nonalcoholic fatty liver disease (NAFLD) fibrosis score, the fibrosis 4 calculator (FIB-4), and the AST-to-platelet ratio index (APRI).6 Some of those indexes have been used as outcome predictors in HCC, especially in patients undergoing surgical resection.14–17 Other indexes, such as the albumin-bilirubin (ALBI) score, have been proposed for evaluating liver reserve in patients undergoing surgery for HCC.15 In addition, the cirrhosis determinant score (CDS), the LOK index, and the Göteborg University cirrhosis index (GUCI) have been studied as survival outcome factors in patients with HCC.14,16–18
Given the different presentation pattern of noncirrhotic HCC, the aim of the present study was to describe the presentation characteristics, treatment, and survival rate of HCC in the noncirrhotic liver in patients seen at our healthcare institution, applying the fibrosis biologic indexes and the liver reserve indexes as survival outcome factors in noncirrhotic HCC.
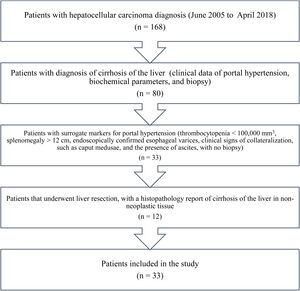
Materials and methodsAll the patients in our database diagnosed with HCC within the time frame of June 2005 and April 2018 were identified. A total of 168 patients with HCC were registered within that time period. The patients diagnosed with cirrhosis of the liver (clinical data of portal hypertension, biochemical parameters, and biopsy) and the patients that had surrogate markers for portal hypertension (thrombocytopenia < 100,000 mm3, splenomegaly > 12 cm, endoscopically confirmed esophageal varices, and ascites, with no biopsy)19 were excluded from the study. The patients that underwent LR, with a histopathologic report of liver cirrhosis in non-neoplastic tissue, were also excluded. The remaining patients were identified and included in the study (Fig. 1).
The variables analyzed included age, sex, body mass index, comorbidities (diabetes and/or high blood pressure), viral hepatitis, and abusive alcohol ingestion (excessive alcohol consumption: 4 drinks for women and 5 or more drinks for men within approximately 3 hours or drinking for 5 or more days within the past month).20 The presence of metabolic syndrome was documented, according to the criteria of the American Heart Association and the National Heart, Lung, and Blood Institute.21
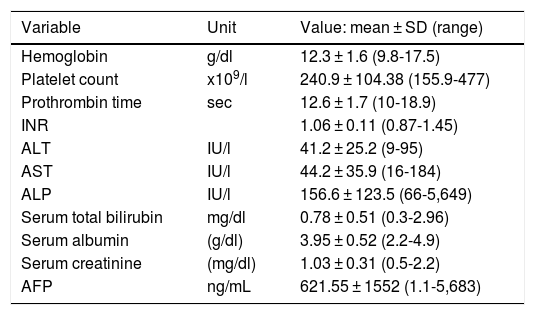
The laboratory data analyzed were hemoglobin, platelet count, prothrombin time, international normalized ratio, alanine transaminase, aspartate transaminase, alkaline phosphatase, total bilirubin, serum albumin, serum creatinine, and AFP. The following liver fibrosis/cirrhosis biologic indexes were calculated at the time of diagnosis: the NAFLD fibrosis score, APRI, FIB-4, Lok Index, CDS, and GUCI. Those indexes were classified into grades: grade 1: fibrosis was unlikely; grade 2: undetermined; grade 3: fibrosis/cirrhosis was very likely.14 In addition, the ALBI scores were registered as indicators of liver disease severity and categorized into: grade 1: good liver reserve; grade 2: intermediate liver reserve; and grade 3: poor liver reserve. When obtained, fibrosis of non-neoplastic liver tissue was histopathologically evaluated through the METAVIR score.22
Hepatocellular carcinoma diagnosis and stagingAll the patients were diagnosed through computed tomography, utilizing the HCC diagnostic criteria,23 with or without an AFP value > 200 ng/mL. HCC diagnosis was histologically confirmed through tumor biopsy or surgical sample. Patients with fibrolamellar HCC were excluded from the study. Tumor size, number of tumors, and portal vein thrombosis were documented. Metastasis was evaluated through computed tomography images. The patients were classified according to the Okuda staging system (tumor size > 50%, ascites, albumin < 3 mg/dl, bilirubin > 3 mg/dl; stage I: no adverse factors, stage II: 1-2 adverse factors, stage III: 3-4 adverse factors), the Cancer of the Liver Italian Program (CLIP) (clinical factors: Child-Pugh score, number of tumor nodules and whether the tumor extends beyond 50% of the liver, AFP, and portal vein thrombosis; assigning 0-2 points, obtaining a 0-6 score),24 and the Milan criteria staging systems for HCC (not advanced: single HCC tumor < 5 cm, 2 or 3 nodules with a diameter < 3 cm, and with no lymph node involvement, vascular extension, or distant metastasis; advanced: tumor larger than those just mentioned).25
Active treatment was defined as: LR, radiofrequency ablation (RFA), and transarterial chemoembolization (TACE). Sorafenib use was also registered. Patient survival (in months) was determined from the time of diagnosis to loss to follow-up or death.
Statistical analysisData were expressed as mean ± standard deviation and range for the continuous variables, and as frequencies and percentages for the categorical variables. Patient survival was calculated using the Kaplan-Meier method and the log-rank test was utilized to compare the differences in survival. The continuous variables were categorized for patient survival using mean and median, based on data asymmetry. The multivariate analysis was performed utilizing the Cox proportional risk regression model to adjust the effect of the confounding risk factors on patient survival. Variables with a p ≤ 0.1 were included in the multivariate analysis. Statistical significance was set at a p < 0.05. The data were analyzed using the SPSS version 25 (Chicago, IL, USA) software.
Ethical considerationsThe study was previously approved by the institutional local research and ethics committee.
The authors declare that this article contains no personal information that could identify the patients. Therefore, requesting patient informed consent for the publication of this article was not required.
ResultsThirty-three patients with noncirrhotic HCC were included in the study (Fig. 1), resulting in an incidence of 19.6% (17 men [51.5%] and 16 women [48.5%]). Mean patient age was 67.3 ± 14.8 years (range: 29-88). Mean body mass index was 26.2 ± 4 kg/m2 (range: 19.7-36). Ten patients (30.3%) had diabetes mellitus and 11 (33.3%) had high blood pressure. Two patients (6.1%) met the criteria for metabolic syndrome and they had no history of HCV or alcohol consumption. Three patients (9.1%) were HCV carriers and 9 (27.3%) had abusive alcohol consumption. Twenty-one patients (63.3%) had possible nonalcoholic fatty liver disease. Table 1 shows the main laboratory work-up characteristics of the patients. There were no significant differences in the sociodemographic data between the patients that were HCV carriers, alcohol consumers, or those that presented with nonalcoholic fatty liver disease.
Laboratory results of the patients.
| Variable | Unit | Value: mean ± SD (range) |
|---|---|---|
| Hemoglobin | g/dl | 12.3 ± 1.6 (9.8-17.5) |
| Platelet count | x109/l | 240.9 ± 104.38 (155.9-477) |
| Prothrombin time | sec | 12.6 ± 1.7 (10-18.9) |
| INR | 1.06 ± 0.11 (0.87-1.45) | |
| ALT | IU/l | 41.2 ± 25.2 (9-95) |
| AST | IU/l | 44.2 ± 35.9 (16-184) |
| ALP | IU/l | 156.6 ± 123.5 (66-5,649) |
| Serum total bilirubin | mg/dl | 0.78 ± 0.51 (0.3-2.96) |
| Serum albumin | (g/dl) | 3.95 ± 0.52 (2.2-4.9) |
| Serum creatinine | (mg/dl) | 1.03 ± 0.31 (0.5-2.2) |
| AFP | ng/mL | 621.55 ± 1552 (1.1-5,683) |
AFP: alpha-fetoprotein; ALP: alkaline phosphatase; ALT: alanine transaminase, AST: aspartate transaminase; INR: international normalized ratio; SD: standard deviation.
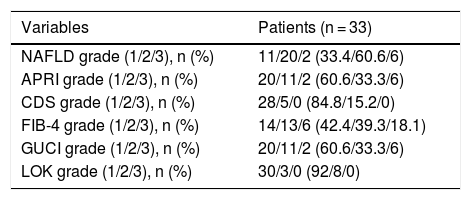
According to the fibrosis/cirrhosis biologic indexes, less than 20% of the patients had grade 3 (very likely fibrosis/likely cirrhosis) (Table 2). Twenty-one patients (66.6%) had grade 1 (adequate liver reserve), 11 (33.3%) had grade 2, and one patient (3.3%) had grade 3 (poor liver reserve, according to the ALBI score).
Fibrosis/cirrhosis biologic indexes.
| Variables | Patients (n = 33) |
|---|---|
| NAFLD grade (1/2/3), n (%) | 11/20/2 (33.4/60.6/6) |
| APRI grade (1/2/3), n (%) | 20/11/2 (60.6/33.3/6) |
| CDS grade (1/2/3), n (%) | 28/5/0 (84.8/15.2/0) |
| FIB-4 grade (1/2/3), n (%) | 14/13/6 (42.4/39.3/18.1) |
| GUCI grade (1/2/3), n (%) | 20/11/2 (60.6/33.3/6) |
| LOK grade (1/2/3), n (%) | 30/3/0 (92/8/0) |
APRI: Aspartate Transaminase to Platelet Ratio Index; CDS: Cirrhosis Discriminant Score; FIB-4: Fibrosis-4 Score; GUCI: Göteborg University Cirrhosis Index; NAFLD: nonalcoholic fatty liver disease.
Grade 1: unlikely fibrosis; Grade 2: undetermined; Grade 3: very likely fibrosis/possible cirrhosis.
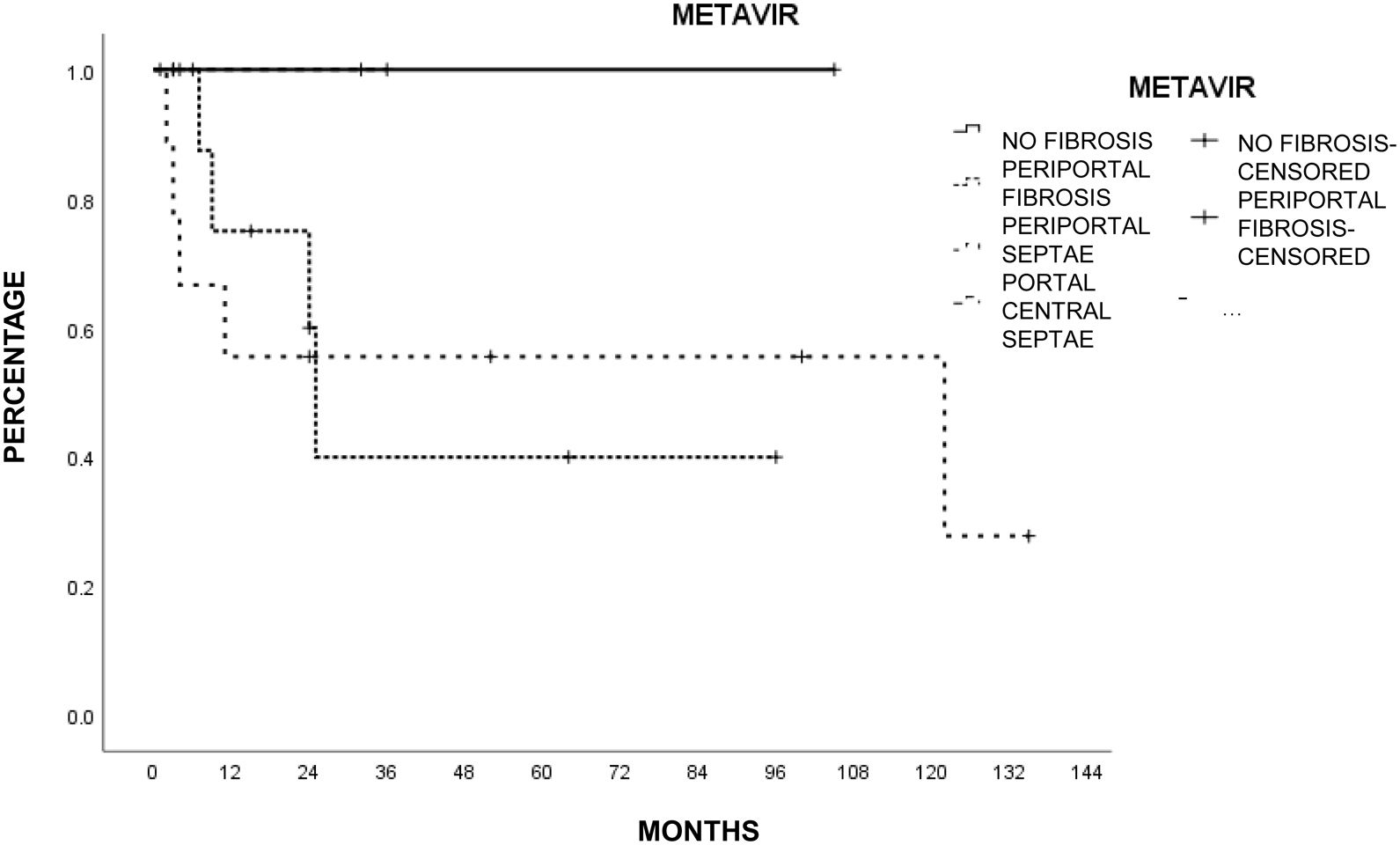
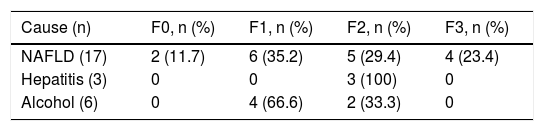
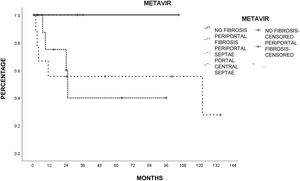
Liver histology was determined according to the METAVIR fibrosis score in 26 patients (78.7%). The most common score was mild portal fibrosis with no septa (F1) and moderate portal fibrosis with few septa (F2) in 10 patients (46.2%), followed by severe fibrosis and numerous septa without cirrhosis (F3) in 4 patients (15.4%) and no fibrosis (F0) in 2 patients (7.7%). Table 3 shows the METAVIR score distribution in relation to the cause of liver damage.
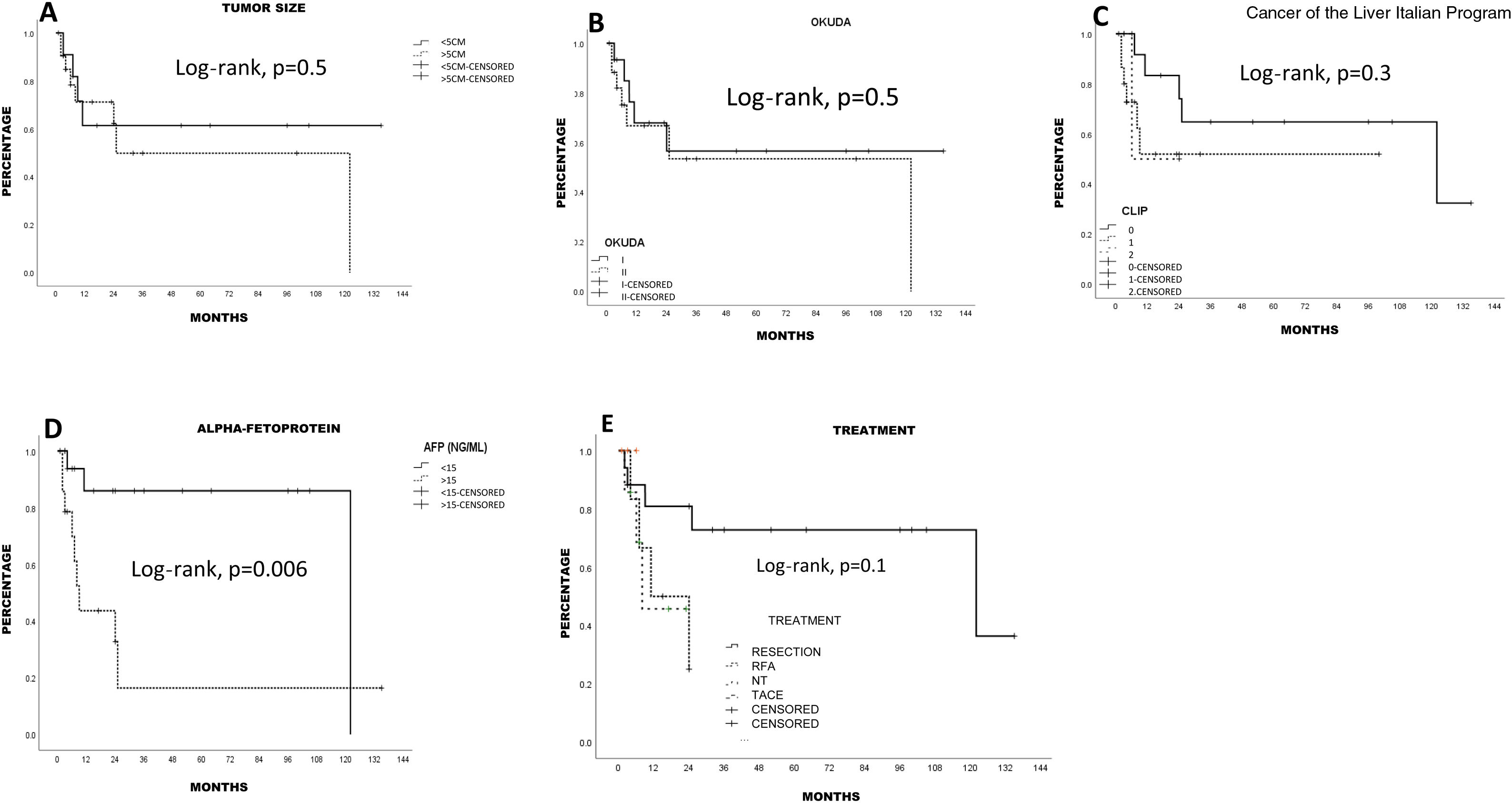
Tumor characteristics and treatmentThe majority of the patients (n = 31, 93.9%) had a single lesion, one patient (3.3%) had 2 lesions, and one patient (3.3%) had 3 lesions. Mean tumor size of the larger lesions was 7.7 ± 2.9 cm (range: 3-15) and only 11 patients (33.3%) had a tumor smaller than 5 cm in diameter. Two patients (6.6%) had portal vein thrombosis and 42.4% (n = 14) had an increase in AFP (> 15 ng/mL from a local laboratory). Eighteen patients (48.5%) were classified as stage I Okuda and 15 patients (52.5%) as stage II Okuda. According to the CLIP classification, 45.5% patients (n = 15) were CLIP 0, 16 (48.5%) were CLIP 1, and one patient (6.1%) was CLIP 2. Only 10 patients (30.3%) presented with non-advanced disease, according to the Milan criteria.
Active treatment was carried out on 26 patients (78.7%): 17 (51.5%) underwent LR, 6 (18.2%) had RFA, and 3 (9.1%) underwent TACE. The majority of indications for RFA were age above 70 years (4 patients) and associated comorbidities (diabetes in 3 patients). All the patients that underwent TACE were over 70 years of age and presented with diabetes mellitus and high blood pressure as comorbidities. All the LRs were R0 (microscopically negative surgical margin). Six patients (18.18%) did not receive active treatment (LR, RFA, or TACE) due to: portal vein thrombosis (n = 2), cachexia (n = 2), number of lesions (3) (n = 1), and extrahepatic metastasis (costal margin) (n = 1). Recurrence in the patients with LR was 11.7% (n = 2) and 33% (n = 2) in the patients with RFA (24 ± 17.4 months after treatment). Mean recurrence time was 22.8 ± 15.3 months (range: 14-50). Sorafenib was administered to 7 patients (21.2%): for recurrence after LR in 2 patients, recurrence after RFA in 2 patients, after TACE in one patient, and in 2 patients that did not have active treatment.
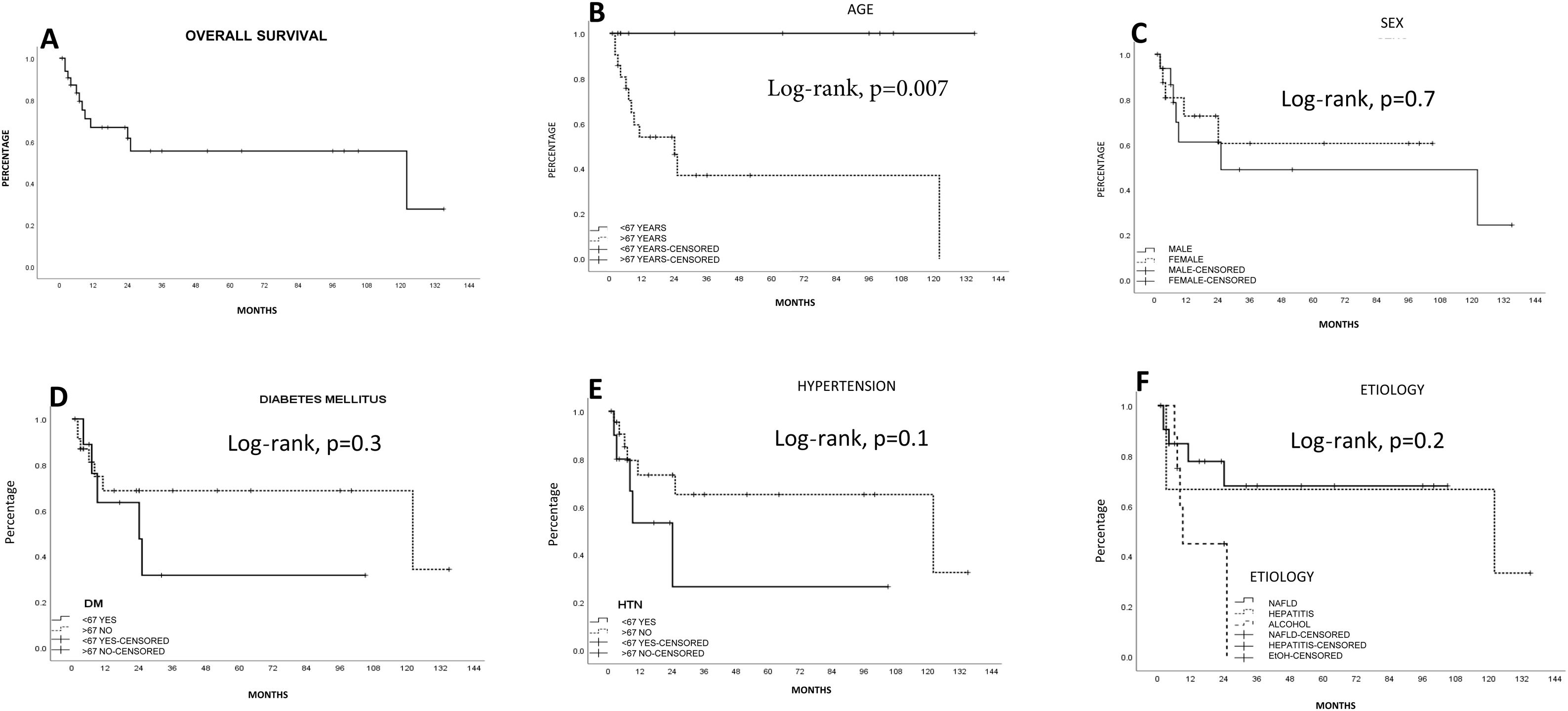
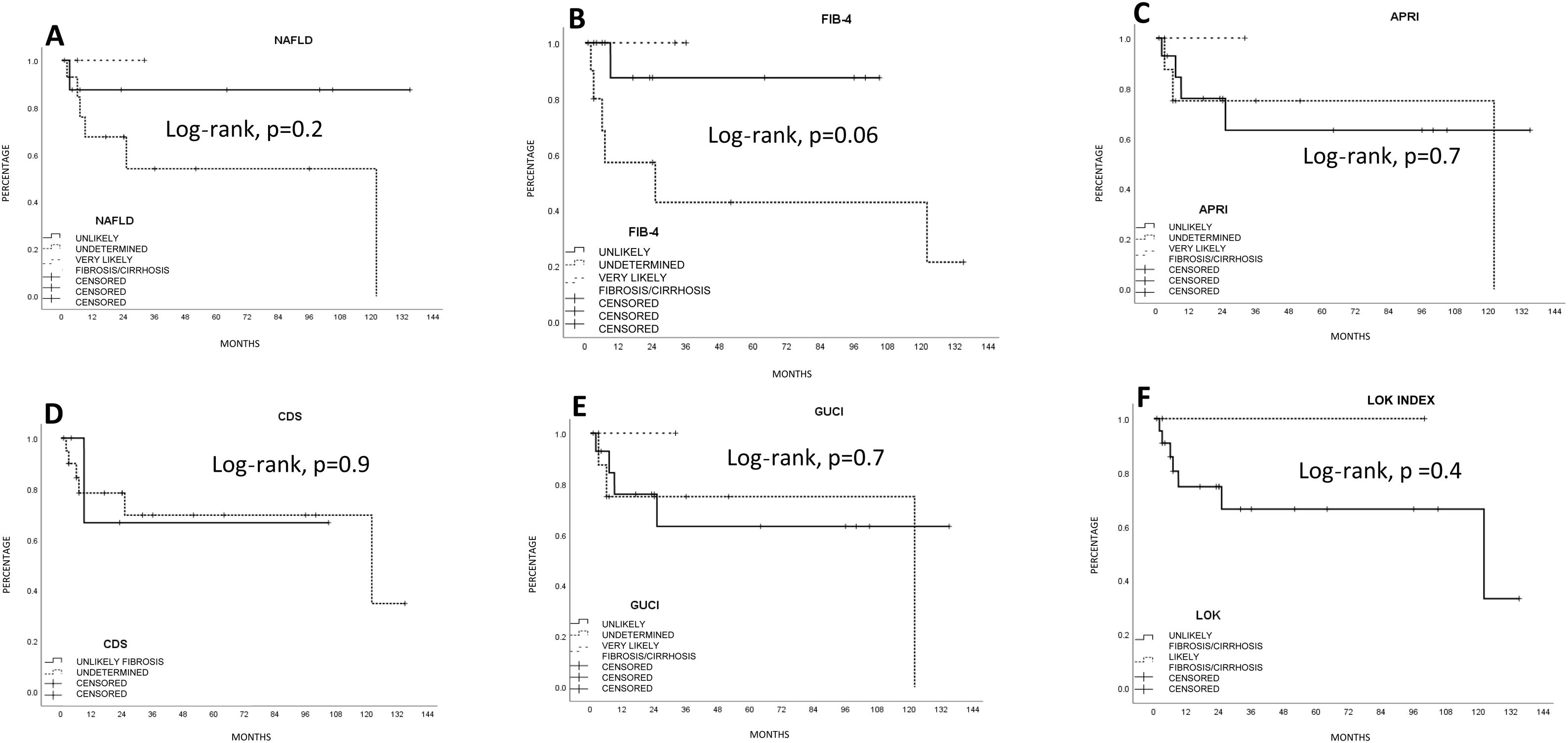
SurvivalMean survival in all the patients calculated using the Kaplan-Meier method was 76.1 ± 12.5 months (95% CI: 51.5-100.7) (66.8% at one year and 55.4% at 5 years). LR produced the highest survival calculation (96.5 ± 14.2 months; 95% CI: 68.6-124.4), compared with RFA (15.6 ± 4 months; 95% CI: 7.7-23.5) and TACE (13.6 ± 3.8 months; 95% CI: 6.2-21.1), but with no statistical significance (p = 0.1, log-rank). Survival was lower, and statistically significant, in the patients above 67 years of age, compared with those under 67 years of age (p < 0.05, log-rank). The patients with a better FIB-4 biologic index had a statistical trend toward better survival (p = 0.06, log-rank). No other fibrosis/cirrhosis biologic index had an influence on patient survival.
The patients with less severe liver disease (ALBI grade 1) had better one-year survival (90% vs 68%) and 5-year survival (59% vs 57%) than the patients with more severe liver disease (ALBI grade 2; one year: 26.9%, 5 years: not registered), but with no statistical significance. Neither fibrosis grade determined through biopsy nor tumor characteristics had an influence on patient survival. Survival was lower in patients with AFP levels > 15 ng/mL, with statistical significance (p < 0.05, log-rank). Survival calculated with the Kaplan-Meier method, in relation to the variables analyzed, are shown in the corresponding figures (Figs. 2–5).
Estimated survival by the Kaplan-Meier method, according to the fibrosis biologic index. A) Nonalcoholic fatty liver disease (NAFLD). (B) Fibrosis-4 score (FIB-4). C) AST/Platelet index (APRI). D) Cirrhosis discriminant score (CDS). E) Göteborg University Cirrhosis Index (GUCI). F) Lok index. The FIB-4 index had a statistically significant trend (p = 0.06).
The Cox regression analysis showed no statistical significance regarding age (< 67 years: p = 0.61), AFP > 15 ng/mL (p = 0.65), FIB-4 model (p = 0.65), or treatment (p = 0.83).
DiscussionOur study results show that the incidence of noncirrhotic HCC, its clinical characteristics, and its survival rate are similar to those reported worldwide. There were differences, especially in HCV infection, the rate of diabetes mellitus, and recurrence. Surgical treatment resulted in the greatest survival, compared with the other treatments included in our study. Survival was lower in patients above 67 years of age and in those with elevated AFP. The fibrosis/liver cirrhosis biologic indexes were not identified as risk factors for survival.
The incidence of noncirrhotic HCC in our study (20%) was similar to that previously described in the literature, although it can vary geographically from 5 to 54%.4,5,12
No HBV infection and 27% of cases of HCV infection were the inverse of Asian (HBV 50-70% and HCV: 3-4%)9,14 and different European (HBV:1 1-2 0% and HCV: 9-46%) study results.5,12 Contrastingly, diabetes mellitus (associated with nonalcoholic steatohepatitis and the later development of HCC)5 was higher than the noncirrhotic HCC rates reported in Chinese (20%)14 and Spanish (3%) studies. Our results regarding alcohol consumption abuse were similar to those reported at other European centers (2 0-2 7%).8,12 The natural development of HCC is complicated by presenting in a diseased liver. Up to a 10-fold increase of HCC in patients with nonalcoholic fatty liver disease has been reported.6 Fatty liver disease is one of the main causes for referral for liver transplantation due to HCC.26 When facing the possible presence of nonalcoholic fatty liver, we must contemplate strategies for carrying out greater scanning for the disease and its possible development into HCC.
Curiously, the presence of histologic liver fibrosis in our results (approximately 95% combining the METAVIR fibrosis score [F1-F-3]) was high, compared with 60-70% of patients with fibrosis in Italian and German populations,8,12 even though non-tumor histology was not analyzed in 20% of our population (one of our study’s main limitations). However, the fibrosis/cirrhosis biologic indexes had no specific correlation with the histologic results, with the exception of FIB-4, because the indexes reflected fibrosis that was unlikely or undetermined in up to 80% of the cases. Other studies that have evaluated those indexes in patients with HCC have reported similar results.14 A bidirectional explanation for the discrepancy between the biologic indexes and histopathologic studies could be poor reading of the non-neoplastic tissue or biochemical parameters that are altered by the tumor itself.
The tumor characteristics of size, number of lesions, portal vein thrombosis, elevated AFP, and evaluation of the Milan criteria in our study were similar to those in other case series.4,12,13 As previously described, LR is the best therapeutic option and the only curative option for those patients.4,5,8,9,14 Nevertheless, not all patients undergo LR. Forty to 65% of patients are offered other treatments, such as RFA or TACE.10,12 LR was performed in 51% of our patients, with the highest survival results, compared with the other therapies.
In general, approximately 50% of patients have 5-year survival, which is a better result than in cirrhotic patients with HCC.4,5,8–12,14 Some groups have stated that noncirrhotic HCC outcome is affected by small lesions with no vascular invasion,4,14 satellite nodules,4 R0 resections4,8 patient age,8 diabetes,14 tumor stage (TNM),8,9 tumor size > 10 cm,8 tumor stage outside of the Milan criteria,10 elevated serum AFP,14 ALBI score,14 and tumor recurrence.4–14 We reached a 55% 5-year survival rate and found that advanced patient age (> 67 years), serum AFP (> 15 ng/mL), and perhaps FIB-4 (p = 0.06) were associated with a poor survival rate in the univariate analysis (log-rank), but those findings were not confirmed in the multivariate analysis. Unfortunately, due to the limitations of the study’s retrospective design, the incomplete histopathologic examination of the tumor samples, and the fact that different pathologists performed them, we could not include other histopathologic characteristics, such as vascular invasion or other forms of control of non-neoplastic tissue.
We had a lower recurrence rate (12%), compared with previously described percentages (27-73%)4,8,9,12 and said recurrence did not have a statistically significant impact on survival, unlike that found in other studies.12,14 Our aggressiveness, in terms of performing R0 LR, produced a recurrence rate of 11% but RFA had a 3-fold increase in recurrence (33%), causing concern as to its indications and use in our case series.
Finally, there is little information on noncirrhotic HCC in the Mexican population. Recent Mexican case series at other hospital centers only refer to a 27-33% rate of noncirrhotic HCC, when included in the studies,27–30 without a complete characterization of that subgroup of patients. Others only give a detailed description of fibrolamellar HCC,30 which is a clinically different entity, in terms of outcome and survival.
ConclusionsIn our study, the incidence, clinical characteristics, and survival of HCC in the noncirrhotic liver were similar to those reported in other studies. LR provided the highest survival rates. None of the invasive liver fibrosis biologic indexes were associated as risk factors in relation to patient survival.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Martínez-Mier G, Esquivel-Torres S, Casanova-Sánchez IE, Escobar-Ríos AY, Troche-Gutiérrez JM, Yoldi-Aguirre CA. Carcinoma hepatocelular en hígado no cirrótico: características clínicas y resultados en Veracruz, México. Revista de Gastroenterología de México. 2021;86:4–12.