Oropharyngeal dysphagia (OD) occurs in children with cerebral palsy. It is important to investigate its relationship with some variables, and the objective of this study was to identify factors associated with OD.
Materials and methodsCase-control study in patients with cerebral palsy from 8months to 15years of age, from November 2018 to November 2019, approved by the Ethics Committee. The diagnosis of OD was made by videofluoroscopy when there was nasopharyngeal reflux, stagnation in the vallecular sinuses, in the piriformis sinuses, penetration, and aspiration. The independent variables were type of cerebral palsy, gross motor impairment classified into five levels, nutritional status and comorbidities. One case with OD was included and the next one without alterations in videofluoroscopy was control. The variables were compared with Chi square and Student's t. The association was measured with odds ratio. The confidence interval was 95%.
ResultsThirty patients with OD and 30without OD were studied. Sex, age, birth weight, and gestational age had a similar distribution in the two groups. From the data perceived by the mothers at the time of feeding, the greater frequency of the difficulty in the transfer of the food bolus in the group with OD showed a statistically significant difference (P<.001) and of the studied factors, the levelV of the gross motor involvement was associated with a higher frequency of OD.
ConclusionsOD was associated with level V of gross motor involvement.
La dysphagia (OD) occurs in children with cerebral palsy. It is important to investigate its relationship with some variables, and the objective of this study was to identify factors associated with OD.
Material y métodosEstudio de casos y controles en pacientes con parálisis cerebral de 8meses a 15años de edad, de noviembre de 2018 a noviembre de 2019, aprobado por el Comité de Ética. El diagnóstico de DOF se realizó por videofluoroscopia cuando hubo reflujo nasofaríngeo, estancamiento en valécula, en senos piriformes, penetración y aspiración. Las variables independientes fueron tipo de parálisis cerebral, afectación motora gruesa clasificada en cinco niveles, estado nutricional y comorbilidades. Se incluyó un caso con DOF, y el siguiente sin alteraciones en la videofluoroscopia fue control. Las variables se compararon con chi cuadrado (χ2) y t de Student. La asociación se midió con odds ratio. El intervalo de confianza fue del 95%.
ResultadosSe estudiaron 30 pacientes con DOF y 30 sin DOF. El sexo, la edad, el peso al nacer y la edad gestacional tuvieron distribución similar en los dos grupos. De los datos percibidos por las madres al momento de la alimentación, la mayor frecuencia de la dificultad en el traslado del bolo alimenticio en el grupo con DOF mostró diferencia estadística significativa (p<0.001); de los factores estudiados, el nivelV de la afectación motora gruesa se asoció con mayor frecuencia de DOF (OR: 5.7; IC95%: 1.8–17.5).
ConclusionesLa DOF se asoció con el nivelV de afectación motora gruesa.
Cerebral palsy (CP) is a group of nonprogressive neuromotor disorders that affect the development of the fetal brain. They are present at birth and persist throughout the patient’s lifetime. Prevalence is 2.1 per 1000 live births1.
CP involves limitations in the development of movement, posture, and motor function. It can also be accompanied by epilepsy; intellectual disability; impaired vision; cognitive, communication, and behavioral disorders; and swallowing alterations2.
Swallowing is a complex process that involves muscles of the mouth, larynx, and esophagus, with the participation of different levels of the central nervous system. It is made up of three phases that occur sequentially: the oral phase, the pharyngeal phase, and the esophageal phase3.
Dysphagia is difficulty swallowing and can be classified as oropharyngeal dysphagia (OPD) due to the dysfunction of the pharynx and the upper esophageal sphincter or as esophageal dysphagia due to dysfunction of the lower esophagus4.
The grouped prevalence of OPD in patients with CP reported in a meta-analysis was 50.4% and gradually increased with the severity of the CP5. OPD in patients with CP favors the appearance of infectious, respiratory, and nutritional complications that increase mortality6.
Videofluoroscopy (VFS) is the best radiologic study for diagnosing OPD. It enables visualization of the anatomy of the oral cavity, pharynx, larynx, and upper esophagus, as well as the function and integration of the phases of swallowing7,8.
At our institution, Quiñones-Pacheco et al.9 conducted a descriptive study on 25 patients with infantile CP that underwent VFS for OPD diagnosis. They found that 64% of the patients had vallecular residue and pyriform sinus stasis and 52% presented with pulmonary aspiration.
The relation of certain variables in children with CP and OPD has been studied in developed countries10–12, but the frequency and interaction of the variables can be different, according to region. Therefore, the aim of the present study was to determine the factors associated with OPD diagnosed through VFS in children with CP.
Materials and methodsStudy populationA case-control study13 was conducted on patients with CP between 8 months and 15 years of age, treated at the pediatric gastroenterology and neurology services of the Nuevo Hospital Civil de Guadalajara “Dr. Juan I. Menchaca”, within the time frame of November 2018 and November 2019.
The study included patients diagnosed with CP confirmed by the pediatric neurology service, whose swallowing coordination and synchrony with different food textures (liquid, nectar, puree) was evaluated by VFS, with barium as the contrast medium.
Patients with congenital facial, esophageal, or tracheal malformations or degenerative diseases of the central nervous system were excluded.
The two study groups were patients with OPD and patients with no OPD and they were consecutively included in the study. A patient with CP and alterations in the VFS suggestive of OPD was considered a case and the following patient with CP that did not have alterations in the VFS was considered a control.
Sample size was calculated with a 0.05% alpha error, a 20% beta error, a ratio of one control for every case, a hypothetic number of patients with OPD and gross motor function classification levels IV and V14,15 of 92%6, and a hypothetic number of patients with no OPD and gross motor function classification levels IV and V14,15 of 37%6. Twenty percent was added for possible exclusions, and so the suggested calculation was 15 cases and 15 controls. Those figures doubled due to the fact that the lowest odds ratio (OR) value detected was very high.
VariablesOPD was the dependent variable and was considered present when at least one of the following signs was detected through VFS, regardless of the clinical data: nasopharyngeal reflux, residue in the valleculae, residue in the pyriform sinuses, penetration, and aspiration.
The independent variables were the types of CP, according to their topographic classification, which could be spastic, dyskinetic, ataxic, hypotonic/flaccid, or mixed. The level of gross motor impairment was categorized into five levels, according to the gross motor function classification system (GMFCS)14,15, as well as additional variables, such as comorbidities and nutritional status.
Other study variables were sex, age at the time of the study, gestational age at birth, and clinical data perceived by the mothers of the patients with CP during at-home feeding.
ProceduresThe parents of the children diagnosed with CP seen at the pediatric gastroenterology and neurology services were invited to participate in the study, after the motivation behind the research, together with what was involved in a VFS, were explained. VFS is a procedure included within the diagnostic and therapeutic protocol of the pediatric gastroenterology service at the host institution, in patients with CP.
VFS was performed at the radiology service by the same certified radiologist (JJCA), through fluoroscopy and Multix Fusion Max, Siemens Icons 2008 imaging system equipment as follows: When possible, the patient was placed in the lateral position between the x-ray source and the image intensifier, with the equipment in the vertical position. If not possible, the patient was placed in a chair with his/her caretaker. The textures were prepared so they would be similar to those of the foods routinely given to the child orally. The preparation was administered with a plastic spoon, offering two to three swallows per texture, with the previous addition of 1–5ml of barium as the contrast agent. The first consistency was that of nectar, followed by that of liquid, and ending with the consistency of puree. The images were dynamically recorded. Enterex® was the commercial brand of the thickener employed to obtain the types of textures. The liquid texture was obtained by combining 90ml of water plus 13.5g of powdered barium, the texture of nectar was obtained by combining 90ml of water plus 9g of barium plus 2.25g of Enterex®, and the texture of puree was obtained by combining 90ml of water plus 9g of barium plus 9g of Enterex®. Parents were allowed to accompany the patient during the examination. They remained in the radiology room from the beginning to the end of the procedure and participated in carrying out the VFS to simulate the usual at-home feeding. The VFS findings were collected during the procedure on a form specifically designed for the present research.
One of the researchers (NGR) received first-hand information on the clinical data observed during at-home feeding from interviews with the parents. Simultaneously, the findings from the pediatric neurology evaluation were taken from the clinical case files so that the topographic forms of monoplegia, diplegia, quadriplegia, and hemiplegia could be classified. With respect to muscle tone, the CP was defined as spastic, dyskinetic, ataxic, hypotonic/flaccid, or mixed. The level of gross motor function was determined in five levels through the GMFCS14,15, ranging from level I, in which the child walks with no limitations, to level V, in which the child’s mobility is severely limited, requiring assisting devices.
The CP topographic classification was diagnosed by the pediatric neurologist that participated in the present research.
A certified nutritionist diagnosed the nutritional status and included patient weight, height, and body mass index (BMI) calculated with the weight (kg)/height (m2) formula. The measurements were carried out utilizing calibrated instruments, with the patients in lightweight underwear.
Patients that could not stand or sit independently were weighed in the supine decubitus position on a Seca infantometer (50g accuracy) and those that could stand independently were weighed on a Seca scale (0.1kg accuracy). The child was placed at the center of the scale (standing or in the supine decubitus position) with arms hanging at each side of the body, looking straight ahead. The weight of the children that could not stand unassisted was obtained after subtracting the mother’s weight16.
To measure height, the patients stood upright on a horizontal plane with head, back, buttocks, and calves against the vertical bar of the instrument. The head (with the Frankfort horizontal as the reference plane) touched the mobile bar of the measuring scale, applying slight pressure to the mastoid processes. The patients were measured using a Seca Harpenden stadiometer (0.1cm accuracy). The children whose condition did not allow them to be measured were evaluated using formulas for estimating height with the length of the tibia (cm) and height of the knee (cm)16.
The anthropometric indexes of weight-for-age (W/A), height-for-age (H/A), weight-for-height (W/H), and BMI, utilizing growth charts for children with infantile CP16, were calculated to classify nutritional status according to the World Health Organization (WHO)17,18.
The research data were collected on a form specifically designed for the present research. A pilot test of data capture and entry was first carried out to correct errors.
Statistical analysisKurtosis and asymmetry parameters were calculated for the quantitative variables. If distribution was symmetric, mean and standard deviation were determined and they were compared using the Student’s t test for two independent samples. The qualitative variables were expressed in percentages and contrasted using the chi-square test or the Fisher’s exact test. The association between OPD and the study factors was measured using ORs.
A 95% confidence interval (CI) was employed. The statistical analysis was performed using the statistics for social sciences program (SPSS Statistics for Macintosh, Version 22.0. Armonk, NY, IBM Corp.)
Ethical considerationsWritten statements of informed consent to participate in the research described was requested from the persons responsible for the patients.
The research meets the current bioethics research regulations and was authorized by the Ethics Committee of the host institution (Folio 000138).
ResultsSixty-four patients with CP between 8 months and 15 years of age were invited, through their parents, to participate in the study. Four patients were excluded; three due to lack of cooperation by the children and one because the parent did not accept barium administration for the child. The final sample was made up of 60 subjects: 30 patients with OPD and 30 patients with no OPD.
In the 30 cases with OPD, the signs of the disorder identified by VFS were vallecular residue, stagnation in the pyriform sinuses, nasopharyngeal reflux, penetration, aspiration, and silent aspiration. Twenty-six patients (87%) had more than one sign. Three signs were simultaneously identified per patient on average. Six patients (20%) had the maximum number of signs in a single patient and 4 patients (13%) had the minimum of one sign in a single patient.
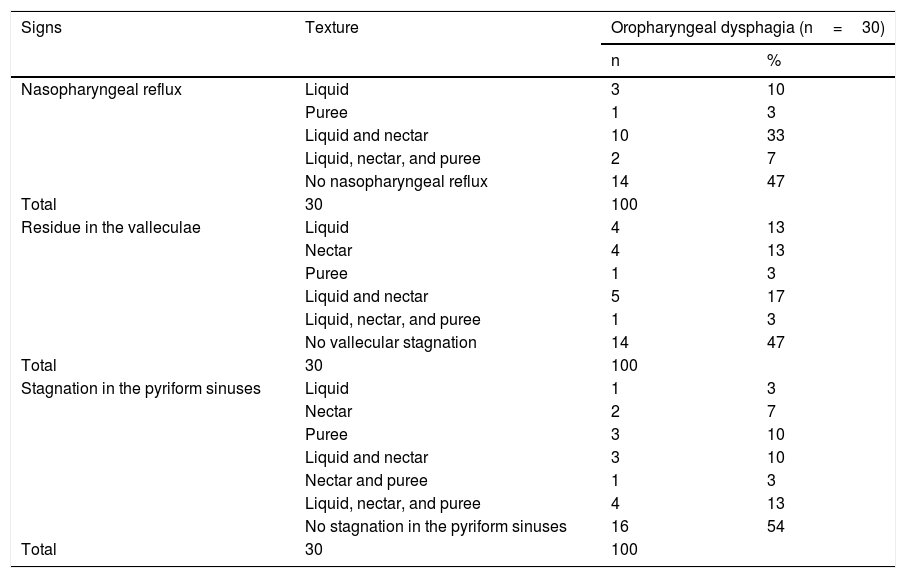
In the VFS, the most frequent texture utilized was nectar and was accompanied by nasopharyngeal reflux, vallecular residue, and stagnation in the pyriform sinuses. However, the liquid texture was the most common in all the signs evaluated, when it was included in the combination of textures (Table 1).
Frequency of nasopharyngeal reflux and stagnation in the valleculae and pyriform sinuses, in patients with oropharyngeal dysphagia.
| Signs | Texture | Oropharyngeal dysphagia (n=30) | |
|---|---|---|---|
| n | % | ||
| Nasopharyngeal reflux | Liquid | 3 | 10 |
| Puree | 1 | 3 | |
| Liquid and nectar | 10 | 33 | |
| Liquid, nectar, and puree | 2 | 7 | |
| No nasopharyngeal reflux | 14 | 47 | |
| Total | 30 | 100 | |
| Residue in the valleculae | Liquid | 4 | 13 |
| Nectar | 4 | 13 | |
| Puree | 1 | 3 | |
| Liquid and nectar | 5 | 17 | |
| Liquid, nectar, and puree | 1 | 3 | |
| No vallecular stagnation | 14 | 47 | |
| Total | 30 | 100 | |
| Stagnation in the pyriform sinuses | Liquid | 1 | 3 |
| Nectar | 2 | 7 | |
| Puree | 3 | 10 | |
| Liquid and nectar | 3 | 10 | |
| Nectar and puree | 1 | 3 | |
| Liquid, nectar, and puree | 4 | 13 | |
| No stagnation in the pyriform sinuses | 16 | 54 | |
| Total | 30 | 100 | |
n: number of patients; %: percentage of patients.
Seventeen of the patients with OPD (57%) and 16 of the patients with no OPD (53%) were females (p=0.795).
The distribution of age at the time of the study was symmetric between the two groups and ranged from 8 months to 15 years.
The mean age was 6.7±1.7 years in the patients with OPD and 7.8±1.1 years in the patients with no OPD (p=0.972). The age of 26 patients with OPD (86%) and 20 with no OPD (66%) was between 2 and 12 years. Two cases (7%) and 2 controls (7%) were under 2 years of age and 2 cases with OPD (7%) and 8 with no OPD (27%) were above 12 years of age.
Fifteen patients with OPD (50%) and 22 with no OPD (73%) had a low middle socioeconomic status (p=0.063) and all the other patients belonged to the middle socioeconomic group.
The gestational age at birth had a normal distribution, with a mean age of 34.7±3.7 months in the neonates with OPD and 35.2±2.9 months in the neonates with no OPD (p=0.517).
Birthweight was symmetrically distributed, with a mean weight of 2299±829g in the cases and 2330±572 g in the controls (p=0.863).
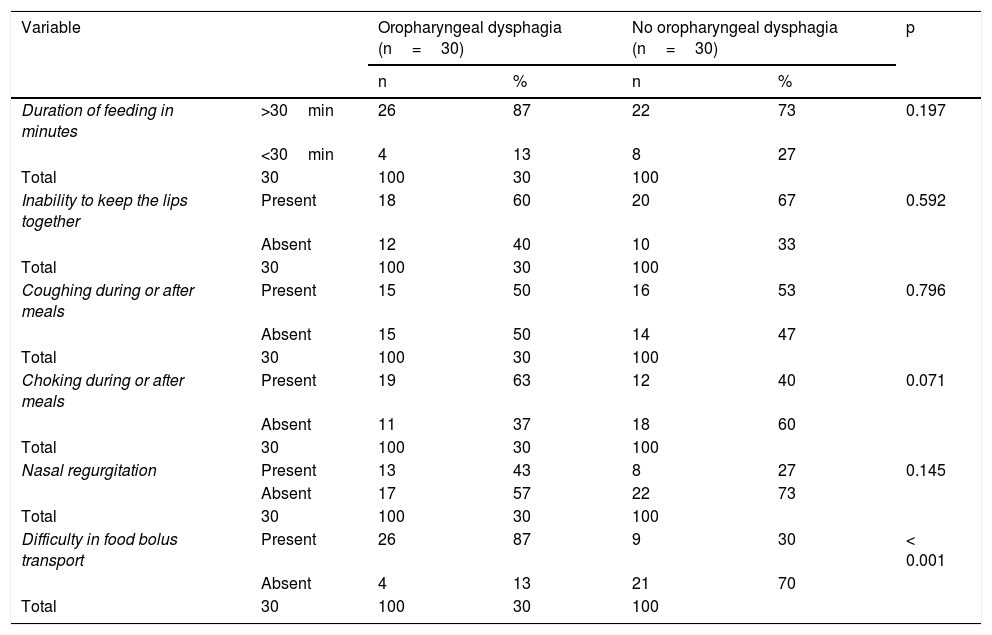
There was a similar distribution between the two study groups regarding the frequency of the data perceived by the mothers during at-home feeding, such as feeding duration above 30 min, inability to keep the lips together, coughing during or after meals, and choking during or after meals. Only the higher frequency of difficulty in food bolus transport in the OPD group showed a statistically significant difference (Table 2).
Data perceived by the mothers of the patients, with or without oropharyngeal dysphagia, during at-home feeding.
| Variable | Oropharyngeal dysphagia (n=30) | No oropharyngeal dysphagia (n=30) | p | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Duration of feeding in minutes | >30min | 26 | 87 | 22 | 73 | 0.197 |
| <30min | 4 | 13 | 8 | 27 | ||
| Total | 30 | 100 | 30 | 100 | ||
| Inability to keep the lips together | Present | 18 | 60 | 20 | 67 | 0.592 |
| Absent | 12 | 40 | 10 | 33 | ||
| Total | 30 | 100 | 30 | 100 | ||
| Coughing during or after meals | Present | 15 | 50 | 16 | 53 | 0.796 |
| Absent | 15 | 50 | 14 | 47 | ||
| Total | 30 | 100 | 30 | 100 | ||
| Choking during or after meals | Present | 19 | 63 | 12 | 40 | 0.071 |
| Absent | 11 | 37 | 18 | 60 | ||
| Total | 30 | 100 | 30 | 100 | ||
| Nasal regurgitation | Present | 13 | 43 | 8 | 27 | 0.145 |
| Absent | 17 | 57 | 22 | 73 | ||
| Total | 30 | 100 | 30 | 100 | ||
| Difficulty in food bolus transport | Present | 26 | 87 | 9 | 30 | < 0.001 |
| Absent | 4 | 13 | 21 | 70 | ||
| Total | 30 | 100 | 30 | 100 | ||
p value upon comparing the frequencies with the chi-square test or the Fisher’s exact test.
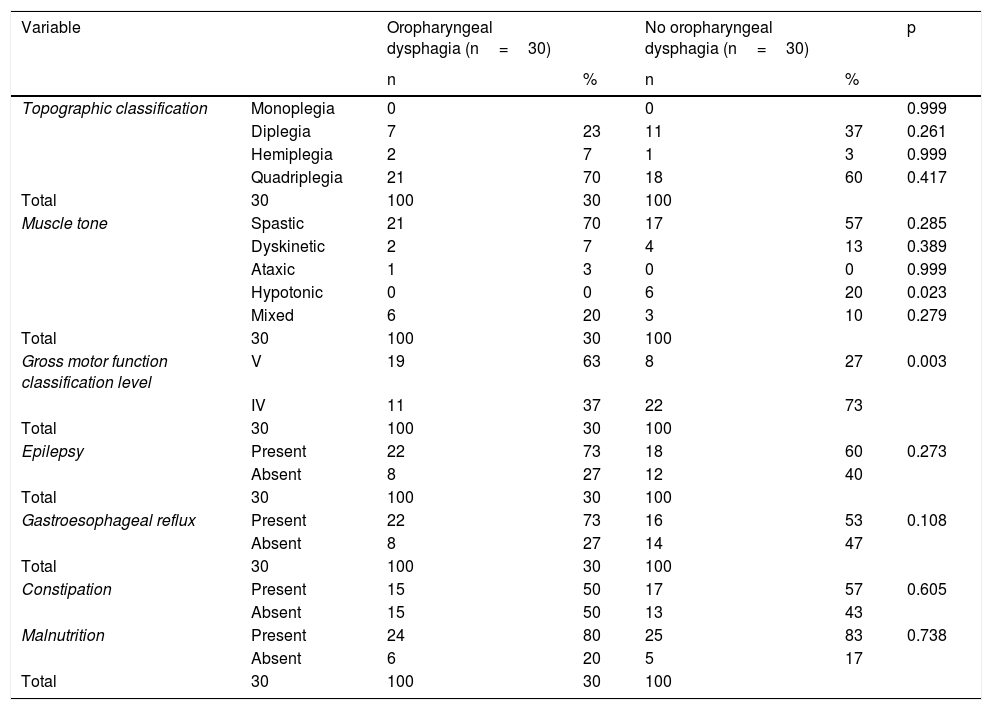
Distribution was similar between the two study groups, regarding the factors that could be associated with OPD, such as the frequency of the manifestations related to the topographic CP classification (monoplegia, diplegia, quadriplegia, and hemiplegia), muscle tone types (spastic, dyskinetic, ataxic, and mixed), and comorbidities (epilepsy, gastroesophageal reflux, constipation, and malnutrition). In addition, there was a statistically significant higher number of patients with OPD with level V of the GMFCS13,14, compared with level IV (p=0.003) (Table 3) and level V was associated with a greater frequency of OPD (OR 5.7; 95% CI 1.8–17.5).
Neurologic factors and comorbidities possibly related to oropharyngeal dysphagia diagnosed by videofluoroscopy in the two study groups.
| Variable | Oropharyngeal dysphagia (n=30) | No oropharyngeal dysphagia (n=30) | p | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Topographic classification | Monoplegia | 0 | 0 | 0.999 | ||
| Diplegia | 7 | 23 | 11 | 37 | 0.261 | |
| Hemiplegia | 2 | 7 | 1 | 3 | 0.999 | |
| Quadriplegia | 21 | 70 | 18 | 60 | 0.417 | |
| Total | 30 | 100 | 30 | 100 | ||
| Muscle tone | Spastic | 21 | 70 | 17 | 57 | 0.285 |
| Dyskinetic | 2 | 7 | 4 | 13 | 0.389 | |
| Ataxic | 1 | 3 | 0 | 0 | 0.999 | |
| Hypotonic | 0 | 0 | 6 | 20 | 0.023 | |
| Mixed | 6 | 20 | 3 | 10 | 0.279 | |
| Total | 30 | 100 | 30 | 100 | ||
| Gross motor function classification level | V | 19 | 63 | 8 | 27 | 0.003 |
| IV | 11 | 37 | 22 | 73 | ||
| Total | 30 | 100 | 30 | 100 | ||
| Epilepsy | Present | 22 | 73 | 18 | 60 | 0.273 |
| Absent | 8 | 27 | 12 | 40 | ||
| Total | 30 | 100 | 30 | 100 | ||
| Gastroesophageal reflux | Present | 22 | 73 | 16 | 53 | 0.108 |
| Absent | 8 | 27 | 14 | 47 | ||
| Total | 30 | 100 | 30 | 100 | ||
| Constipation | Present | 15 | 50 | 17 | 57 | 0.605 |
| Absent | 15 | 50 | 13 | 43 | ||
| Malnutrition | Present | 24 | 80 | 25 | 83 | 0.738 |
| Absent | 6 | 20 | 5 | 17 | ||
| Total | 30 | 100 | 30 | 100 |
*p value upon comparing the frequencies with the chi-square test or the Fisher’s exact test.
The frequency of hypotonic muscle tone was higher in the patients with no OPD and was statistically significant (Table 3).
Other comorbidities, such as constipation and orthopedic, auditory, and endocrine problems, were similarly distributed in the two study groups.
Twenty-four patients with OPD (80%) and 25 with no OPD (83%) had some degree of malnutrition but the differences in the frequencies of mild, moderate, and severe malnutrition between the two study groups were not statistically significant. Six patients (20%) in the OPD group and 5 patients in the group with no OPD (155) had severe malnutrition and the majority of the patients in both study groups were diagnosed with moderate malnutrition.
Discussion and conclusionsThe present study showed that OPD was associated with level V of the five-level GMFCS14,15. Difficulty in food bolus transport perceived by the mothers of the patients with OPD during at-home feeding was also statistically significant.
In the present study, OPD was associated with greater severity measured by the GMFCS, as proposed by Palisano et al.14,15 Said finding has also been reported by other authors that documented OPD through questionnaires19−21 and by researchers that diagnosed OPD through VFS6,22,23.
Regarding the diagnosis of OPD through questionnaires, in a Dutch study conducted by Calis et al.19 on 166 children from 2 to 19 years of age with CP levels IV and V measured by the GMFCS, they found that level V was more frequent in patients that had greater OPD severity (p<0.001). In Northern Ireland, Parkes et al.20 reported that patients with level IV measured by the GMFCS had nearly 5-times more probability of presenting with OPD (OR=4.8, p<0.001) and those with level V had 15-times more probability of presenting with OPD (OR=15.7, p<0.001). In an Australian study, Benfer et al.21 described an incremental relation of OPD severity to the level of gross motor function impairment and the children with GMFCS level V had 17-times more probability of presenting with OPD than the patients with level I (OR=17.9, p=0.036).
In addition, researchers that diagnosed OPD by VFS have reported a relation of OPD to the severity of motor function impairment. In a South Korean study, Kim et al.6 found alterations in the oral and pharyngeal phases of swallowing in 20% of the subjects with levels I and II, in 28.6% of the subjects with level III, and in 91.7% of the subjects with levels IV and V, measured by the GMFCS (p<0.001). In their Iranian study, Asgarshirazi et al.22 reported that 73% of the patients with GMFCS levels IV and V had important symptoms of OPD, compared with 21% of the patients with levels I and II (p<0.001). In Australia and Bangladesh, Benfer et al.23 found that the probability of presenting with OPD was nearly 1.9–3.5-times greater with each increase in GMFCS level in children with CP between 18 and 36 months of age, across all gross motor severity levels.
The age of the patients in the present study was similar to that reported in previous studies6,19,20,22, with only Benfer et al.21,23 including younger patients (18–36 months of age). Benfer et al.24 were also the only authors that quantified the intensity of the association of OPD diagnosed by VFS with greater severity of motor function impairment (OR 3.8; 95% CI 3.2–4.3), a lower figure than that found in the present study (OR 5.7; 95% CI 1.8–17.5).
The largely voluntary alterations in the oral phase of swallowing are a result of damage to the cortical neuronal networks and problems in the pharyngeal phase of swallowing result from disorders in the automatic swallowing components. Both situations can occur due to subcortical cerebral and basal ganglia injury24. Therefore, the association of OPD severity with higher levels of gross motor function impairment is likely due to the fact that when there is greater neurologic injury from CP, there is also less control of voluntary and involuntary movements of the buccofacial muscles and the tongue.
The frequency of difficulty in food bolus transport perceived by the mothers of the patients during at-home feeding (87% in the cases and 30% in the controls) documented in the present study was higher than the results reported by other researchers6,12, who found a frequency ranging from 33% to 52% in patients with OPD. Those differences could be due in part to the different manners of collecting said datum and the heterogeneity of the different grades of CP and OPD severity.
Notably, 87% of the patients with OPD in our study presented with two or more signs of alteration at the pharyngeal level, signifying a high probability of presenting with the serious problem of airway aspiration.
The results of the present analysis are important, given that, to the best of our knowledge, there are no studies in Latin America that investigate the factors associated with OPD diagnosed by VFS in children with CP. In addition, the case-control design enabled us to have more evenly matched study groups, better measure the differences of the frequencies of the variables between the study groups, and to explore the possible association of a dependent variable with the independent variables. Studies conducted in other countries6,8,10–12,19–23 have descriptive and cross-sectional designs.
A limitation of the present research was the fact that the clinical data perceived by the mothers of the patients was obtained through questionnaires, as occurred in some of the studies reviewed19–21, but having video recordings of the at-home feedings of the patients would have been more reliable. A larger sample size would also have enabled the effect on OPD of the less frequent factors to be analyzed, rather than just the level of gross motor impairment.
Despite the abovementioned limitations, the present study concluded that there is an association between OPD documented by VFS and the severity of gross motor function impairment, which could be due to the relation of greater neurologic injury to increased alteration of the control of voluntary and involuntary movements of the buccofacial muscles and the tongue.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Author contributionsAll authors declare that each of them meets the authorship requisites and has reviewed and approved the final version of the manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: González-Rozo N, Pérez-Molina JJ, Quiñones-Pacheco YB, Flores-Fong LE, Rea-Rosas A, Cabrales-deAnda JL. Factores asociados a disfagia orofaríngea diagnosticada por videofluoroscopia en niños con parálisis cerebral. Revista de Gastroenterología de México. 2022;87:44–51.