Given the scarcity of organs worldwide, the medical community has developed different measures for increasing the number of donors,1–3 one of which is domino liver transplantation (DLT). The first DLT was performed in 1995 in Portugal by Furtado et al.,4 who transplanted a liver from a deceased donor into a patient with familial amyloidotic polyneuropathy (FAP), and the liver from the patient with FAP was assigned to another patient, 56 years of age, that presented with cirrhosis and hepatocellular carcinoma (HCC).
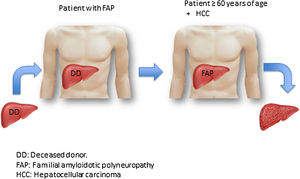
FAP is an autosomal-dominant inherited disorder caused by the mutation of the transthyretin (TTR) gene that codes for the TTR protein and is located on chromosome 18q. More than 100 mutations are known, any of which lead to instability of the corresponding protein and to the extracellular deposit of amyloid in several tissues (autonomous and peripheral nerves, wall of the gastrointestinal tract, heart, etc.). Symptom onset is between 25 and 35 years of age and the most common are peripheral neuropathy of the lower limbs, diarrhea, and diverse arrhythmias.5 TTR amyloid is predominantly produced in the liver and only 5% is produced in the retina and the choroid plexus. Thus, liver transplantation (LT) is the treatment of choice when systemic involvement begins, and before the appearance of incapacitating symptoms.6,7 Despite the presence of the genetic alteration, the morphology and function of the liver of a patient with FAP are completely normal and can therefore be transplanted into a patient with cirrhosis, with or without HCC, and with a certain urgency for LT (Fig. 1). We describe below the first two DLTs performed in Mexico.
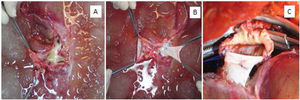
The first DLT was performed on a 41-year-old man, diagnosed in 2012 with FAP due to the TRR Ser50Arg mutation. He presented with segmental neuropathy in both median nerves, polyneuropathy disability (PND) scoring system stage 1, New York Heart Association (NYHA) class 1 amyloid infiltrative cardiomyopathy, ventricular extrasystoles, and a left ventricular ejection fraction (LEVF) of 61%. In 2013, the patient was the recipient of a liver from a 20-year-old deceased donor. The hepatectomy of the patient with FAP was performed with the total exclusion technique, in the usual manner, with the exception of the following differences: the biliary tract, portal vein, and suprahepatic and infrahepatic vena cava were divided at an equidistant point and the hepatic artery was divided at the bifurcation with the gastroduodenal artery. The explanted liver from the patient with FAP was perfused with 2L of Custodiol® (Germany) via the portal vein and 2L via the hepatic artery. To be able to utilize the liver from the FAP patient, complete reconstruction of the suprahepatic veins and the retrohepatic cava, with vessels from the deceased donor, was required (Fig. 2A, B).
A) Posterior view of the liver of the patient with FAP, after perfusion during the bench surgery. Significant loss of the retrohepatic and suprahepatic vena cava can be seen, as well as the ostium of the suprahepatic veins. B) Reconstruction of the retrohepatic cava with the inferior cava of the deceased donor. C) Anastomosis of the vascular repair with the suprahepatic cava in the first recipient.
The recipient of the FAP graft was a 60-year-old woman diagnosed with primary biliary cholangitis (PBC) and severe hemorrhagic portal hypertension, with TIPS placement for refractory ascites, and a model for end-stage liver disease (MELD) score of 15. The LT was performed in the usual manner, with the classic total exclusion technique.
The second DLT was performed in 2014, on a 31-year-old woman, diagnosed in 2012 with FAP, with the TTR Ser52Pro mutation. She presented with paresthesia in her upper limbs, with PND stage 1, mild infiltration in the left ventricle of the heart, revealed by magnetic resonance imaging, as well as perirectal fat, confirmed by biopsy. The brain-dead organ donor was a 41-year-old woman. The FAP patient underwent hepatectomy, in the same manner as the patient in the first DLT described above. There was no need for vascular reconstruction and only perfusion with Custodiol® was carried out. LT was then performed on a 59-year-old woman with cryptogenic cirrhosis and HCC, within the Milan criteria.
The 4 patients progressed adequately and were discharged with no complications.
The recipient of a liver with FAB should be around 60 years of age, because normally the estimated time interval after LT in which FAP can develop is between 10 and 15 years,8 albeit some authors report the appearance of symptoms taking place within a shorter period of time.9 Regarding our patients with FAP, they did not have the Val30Met mutation. More than 100 non-Val30Met mutations are known to exist and they present a higher risk for disease progression, with a mean survival of 7.1 years after transplantation. Heart complications are greater in those cases and are the main cause of death, if heart transplantation is not performed.10
The evolution of our patients with FAP, who had similar time intervals from diagnosis to transplantation and the same disease stage, was different. The male patient with the Ser50Arg mutation presented with severe heart disease progression at five years and died while waiting for a heart transplant. In the female patient with the Ser52Pro mutation, within the same period of time, the amyloid disease has remained stable. The first recipient of the liver with FAP has presented no manifestations of the disease, but the second recipient currently presents with mild amyloid infiltration of the heart.
Ethical considerationsIn this research informed consent was requested from the patients to review the case records. The work meets the current bioethics research regulations and does not require approval by the ethics committee, given that only the case records were reviewed, and patient privacy was not affected at any time. Neither of the patients can be identified in the images presented herein.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Vilatobá M, González-Duarte A, Cruz-Martínez R, García-Juárez I, Leal-Villalpando RP. Primeros dos casos de trasplante hepático dominó en México. Rev Gastroenterol Méx. 2022;87:386–388.