Antecedentes/Objetivo: La severidad de la gastritis crónica asociada con la infección por Heli-cobacter pylori (GCAHpI) juega un papel importante en la evaluación del riesgo potencial de desarrollar cáncer gástrico. Nuestro objetivo fue estimar el riesgo de cáncer gástrico de acuerdo con criterios histopatológicos y mediante la aplicación del índice de riesgo de cáncer gástrico (GCRI).
Métodos: Se realizó análisis histopatológico de biopsias gástricas (cuerpo y antro) de pacientes adultos consecutivos que fueron sometidos a panendoscopia y el GCRI se aplicó en pacientes que presentaban evidencia de GCAHpI.
Resultados: Se incluyeron 111 pacientes (77% mujeres) con una edad media de 38.6 ± 13.1. La infección activa por Helicobacter pylori (AHPI) se diagnosticó en 77 casos (69.40%). Del 45% de los casos con AHPI, 23% tuvieron pangastritis y 22% gastritis predominante en el cuerpo gástrico. Nueve casos fueron diagnosticados con metaplasia intestinal (8%), 7 de los cuales (77.70%) fueron del grupo AHPI. Veintiún por ciento de los pacientes con AHPI tenía un GCRI de 2 (18.10%) o 3 (2.50%) puntos (índice de riesgo alto), mientras que el 79.10% restante tuvieron un GCRI de 0 o 1 puntos (riesgo bajo índice). De los pacientes sin AHPI, ninguno de ellos tenía 3 puntos (p = 0.001). De los 18 pacientes que acumularon 2 o 3 puntos, 6 (33.30%) presentaron metaplasia intestinal (todos con pangastritis y gastritis en cuerpo), de los cuales 4 casos (66.60%) tenían AHPI.
Conclusiones: El riesgo estimado de cáncer gástrico en pacientes con GCAHpI en el entorno clínico estudiado fue relativamente bajo y 5% de los pacientes tenían un fenotipo histopatológico asociado con un riesgo elevado de desarrollar cáncer gástrico.
Background/Aim: Severity of chronic gastritis associated with Helicobacter pylori infection (CGAHpI) could play a role in evaluating the potential risk to develop gastric cancer. Our aim was to estimate the risk for gastric cancer in a clinical setting, according to histopathologic criteria, by applying the gastric cancer risk index (GCRI)
Methods: Histopathologic study of the gastric biopsies (corpus-antrum) from consecutive adult patients that underwent gastroesophageal duodenoscopy was carried out, and the GCRI was applied in patients presenting with CGAHpI.
Results: One hundred eleven patients (77% female) with a mean age of 38.6±13.1 years were included. Active Helicobacter pylori infection (aHpi) was diagnosed in 77 cases (69.40%). In 45% of the cases with aHpi, pangastritis (23%) or corpus-predominant gastritis (22%) was diagnosed. Nine cases were diagnosed with intestinal metaplasia (8%), 7 of which (77.70%) were in the aHpi group. Twenty one percent of the patients with aHpi had a GCRI of 2 (18.10%) or 3 (2.50%) points (high risk index), while 79.10% accumulated a GCRI of 0 or 1 points (low risk index). Of the patients with no aHpi, none of them had 3 points (p=0.001). Of the 18 patients that accumulated 2 or 3 points, 6 (33.30%) presented with intestinal metaplasia (all with pangastritis and corpus-predominant gastritis), of which 4 cases (66.60%) had aHpi.
Conclusions: The estimated gastric cancer risk in patients with CGAHpI in the clinical setting studied was relatively low and 5% of the patients had a histopathologic phenotype associated with an elevated risk for developing gastric cancer.
Introduction
Helicobacter pylori (H. pylori) is the principal etiologic agent associated with gastritis, peptic ulcer disease, gastric cancer, and primary gastric lymphoma. It is estimated that H. pylori infection affects approximately 50% of the world population,1 resulting in elevated morbidity and mortality figures. In 1994, the World Health Organization and the International Agency for Cancer Research classified this microorganism as a class I human carcinogen.2H. pylori infection is usually acquired during infancy and persists as chronic gastritis if the bacterium is not eradicated.3 According to the carcinogenesis model described by Correa for intestinal-type gastric adenocarcinoma,4 this disease begins with a chronic gastritis process, followed by glandular atrophy, intestinal metaplasia, dysplasia, and finally adenocarcinoma. However, this model cannot be routinely applied to all cases.5 Moreover, the proposed progression differs among countries and even among different regions in the same country.5,6
The pathologic study of endoscopic gastric biopsies is an excellent recourse for the diagnosis of gastritis and the visualization of H. pylori.7,8 Its findings are considered essential in patient clinical management. Recently, the combination of certain histopathologic characteristics has emerged as a promising tool for estimating the risk for developing gastric cancer12-19 in the clinical setting. It appears that the degree of severity of gastritis associated with H. pylori infection (GAHpI) in the antrum, as well as in the gastric corpus, may be a good marker for such risk in patients with chronic gastritis associated with H. pylori infection (CGAHpI).9-13
Aim
To estimate the risk for gastric cancer in a clinical setting according to histopathologic criteria in patients with CGAHpI, applying the gastric cancer risk index (GCRI) in patients with H. pylori infection, described by Meining et al.10
Population and sample
The population was made up of patients seen at the Gastroenterology Service of the Hospital Universitario de Maracaibo, in the State of Zulia, Venezuela, within the time frame of June 2007 to December 2009, that had undergone upper gastrointestinal endoscopy (esophagogastroduodenoscopy) for whatever reason. Consecutive patients were included regardless of sex, ethnic group, and age, and all were willing participants that signed statements of informed consent. Patients were excluded if they had severe systemic disease, active digestive hemorrhage or blood in the gastric cavity, or if they had been treated with anti-H. pylori antibiotics within the last 3 months or with H2 and/or proton pump inhibitors within the last 15 days.
All the patients had a complete and recorded past medical history and underwent esophagogastroduodenoscopy with biopsies of the mucosa of the gastric corpus and antrum for histopathologic study, as well as a microbiologic culture for H. pylori, and a urease test.
Endoscopy of the upper gastrointestinal tract
This study was done on patients that, after a minimum of 10 hours of absolute fasting, received topical anesthesia of the pharyngeal and hypopharyngeal region and intravenous sedation with midazolam (3 to 5 mg intravenously).14 With continuous vital sign monitoring, a flexible endoscope was introduced through the mouth following a previously described technique.15 Upon reaching the gastric cavity, the fundus, corpus, and antrum were systematically examined. Endoscopic biopsy forceps were used for taking 3 samples of the antrum and one of the corpus (of approximately 3 mm in diameter, each one), giving preference to the zones of apparent lesion and taking care that the samples for the microbiologic culture and the rapid urease test were not impregnated with blood. The samples were placed into separate, previously labeled vials that contained the proper medium required for each test.
Urease test
The first biopsy from the gastric antrum was placed in an Eppendorf tube containing 1.5 ml of urea agar medium, prepared according to a previously described and standardized procedure for the population studied.16 The sample was incubated at 35º C for a maximum of 2 h, periodically verifying whether there were color changes in the agar. The test result was considered positive when, during the incubation, the phenol red indicator contained in the medium changed from light orange to pink or bright fuchsia. When there was no color change or changes occurred after the first 2 hours, the test result was considered negative.
Culture for Helicobacter pylori
The culture for this microorganism was carried out using the endoscopic biopsy of the gastric antrum mucosa, following a previously described procedure.17 In short, the biopsy was removed from the endoscopic grasper using a sterile needle and placed in an Eppendorf tube containing 0.5 ml of sterile saline solution, as the transport medium. It was processed no longer than 4 h after collection. First, the tissue was cut into thin pieces on a sterile watch glass, using a scalpel blade. This preparation was seeded in 2 enriched culture mediums, one selective and one non-selective, for primar y isolation, using the Brucella Agar base supplemented with 5% sheep blood. Three antimicrobial agents were added to the selective medium: vancomycin, trimethoprim, and amphotericin B. Both mediums were incubated in a humid microaerophilic environment (5% of O2, 10% of CO2, and 85% of N2), using anaerobic jars and Anaerocult® C gas-generating envelopes (Merck, Darmstadt, Germany) at a temperature of 35ºC. The cultures were inspected on days 3, 5, and 7. Isolate identification was based on the colony morphology and cellular characteristics of H. pylori, as well as through positive oxidase, catalase, and urease tests.
Histopathologic study
A biopsy of the gastric corpus and another of the antrum were fixed in a 10% formalin solution. The fragments of the gastric mucosa were then processed following the customary steps of dehydration and paraffin embedding, after which a series of slices were cut at a maximum of 5 mm, fixed on slides, and stained with hematoxylin-eosin.
Histopathologic evaluation
In all of the cases, the preparations were observed under a light microscope at ×10, ×40, and ×100 magnifications. When the presence of H. pylori was found, it was categorized as scant, moderate, or abundant. At the time of the histopathologic evaluation the pathologist had no knowledge of the clinical characteristics or the results of the urease test and microbiologic culture corresponding to each case.
All the biopsies were examined semi-quantitatively to establish the grade of mononuclear inflammation (plasmatic cells, lymphocytes, and histiocytes) and active inflammation (polymorphonuclear neutrophils) and the presence or absence of intestinal metaplasia according to the Sydney System.3
Criteria for diagnosing active Helicobacter pylori infection
Active H. pylori infection (aHpi) was diagnosed when one of the 3 tests carried out were positive. The absence of H. pylori in the 3 studies was considered to be a case with no active infection.
Gastritis classification
Gastritis was classified into 4 categories, according to the following histopathologic characteristics:
1. No Gastritis: Histologically normal antrum and corpus mucosa
2. Antrum-predominant gastritis: A greater degree of inflammation in the antrum, compared with the corpus
3. Pangastritis: Similar degree of inflammation in the antrum and corpus
4. Corpus-predominant gastritis: A greater degree of inflammation in the corpus, compared with the antrum
Gastric Cancer Risk Index
The GCRI proposed by Meining et al.10 was applied in patients with CGAHpI, which establishes the following criteria:
1. Mononuclear infiltration with plasmatic cells/lymphocytes is more pronounced in, or at least similar to, the mucosa of the corpus than the mucosa of the antrum: 1 point
2. Polymorphonuclear neutrophil invasion is more pronounced in, or at least similar to, the mucosa of the corpus than the mucosa of the antrum: 1 point
3. Intestinal metaplasia is present in the mucosa of the antrum or corpus: 1 point
The indexes of 0 and 1 are associated with a low positive predictive value for gastric cancer, whereas indices of 2 and 3 are associated with a high positive predictive value.10
Statistical analysis
The statistical significance of the qualitative differences was calculated using the chi-square test and the non-paired Student's t test. The Spearman correlation test was applied to establish the relation between gastritis phenotypes and the GCRI. Statistical significance was considered when p < 0.05.
Results
A total of 111 patients were studied; 85 of them were women (76.6%) and 26 were men (23.4%), with ages from 16 to 76 years (mean ± standard deviation: 38.6 ± 13.1 years), with no significant variations in the mean age between the sexes. Active H. pylori infection (aHpi) was diagnosed in 77 cases (69.4%); in the 34 remaining cases (30.6%), the 3 diagnostic tests were negative (H. pylori negative). Diagnosis of aHpi was made in 68.2% of the female patients and 63.3% of the male patients, with no significant differences between them.
Gastritis classification
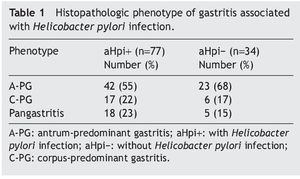
Table 1 shows the histopathologic phenotype of the gastritis diagnosed in the patients with and without aHpi. In 45% of the cases with aHpi, pangastritis (23%) or corpus-predominant gastritis (22%) was diagnosed, whereas 32% of the cases without aHpi developed pangastritis (15%) or corpus-predominant gastritis (17%). Figure 1 shows the histopathologic aspects of a case of antrum-predominant gastritis, of pangastritis, and of corpus-predominant gastritis. Figure 2 shows the presence of H. pylori, intestinal metaplasia, and polymorphonuclear neutrophil infiltration.
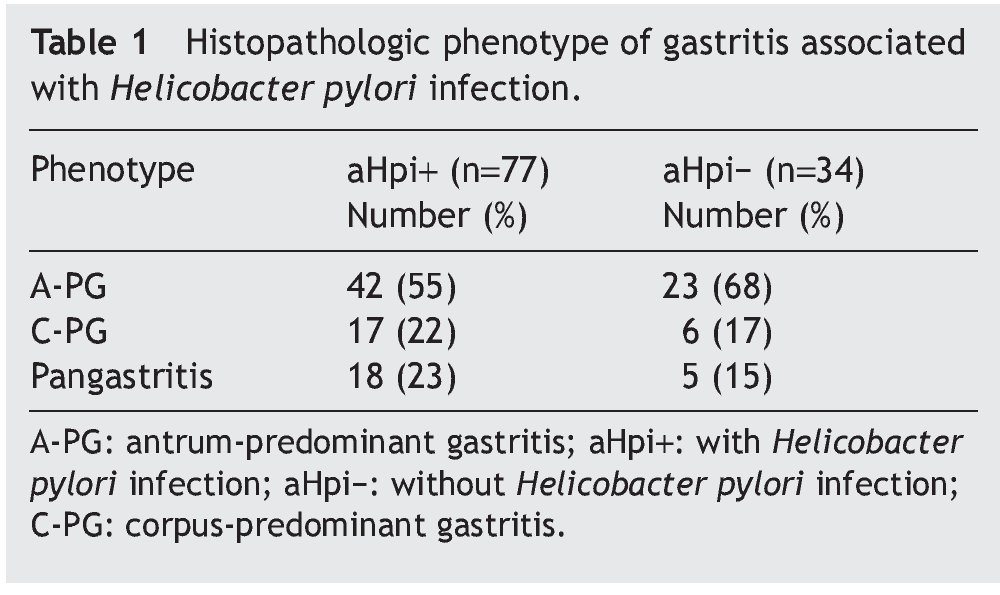
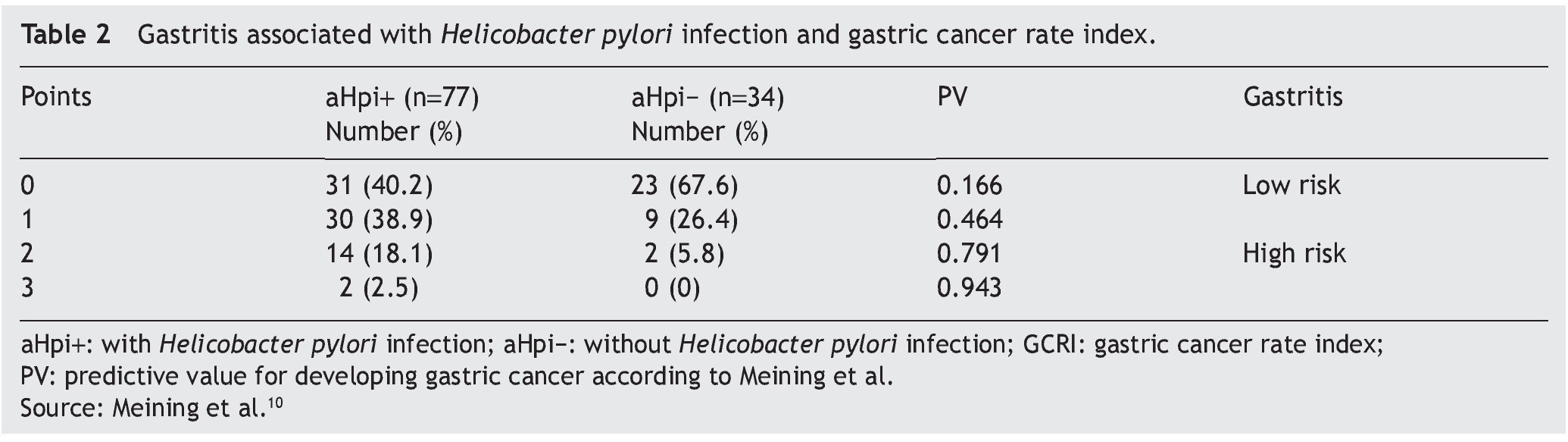
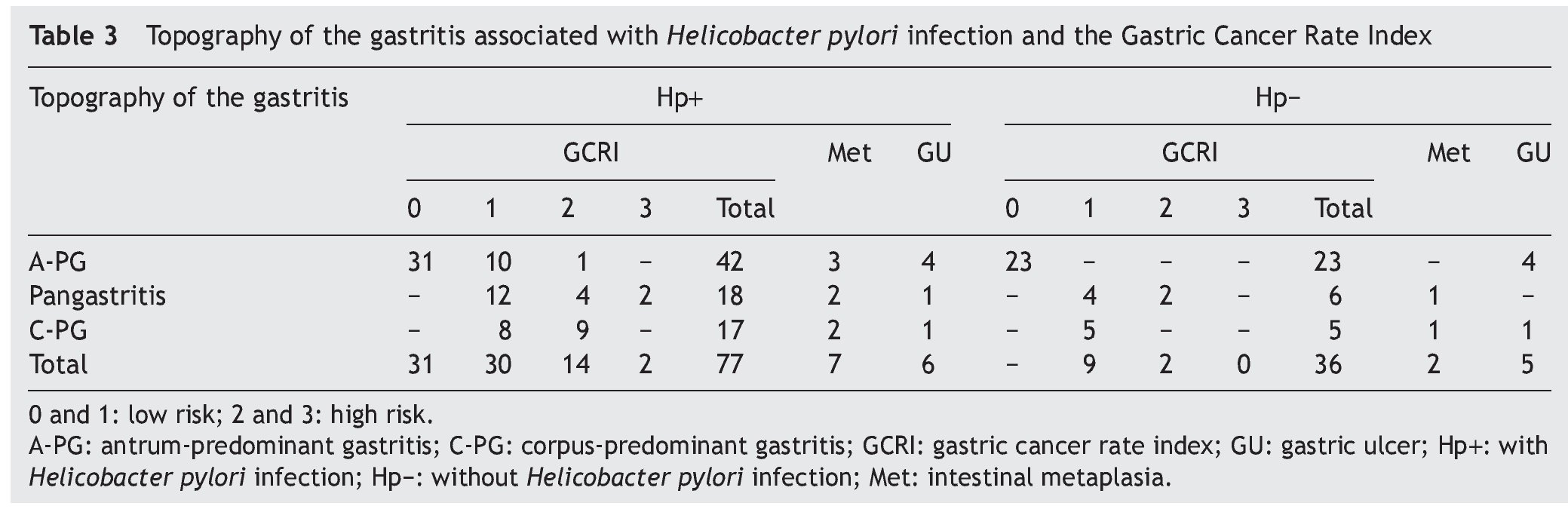
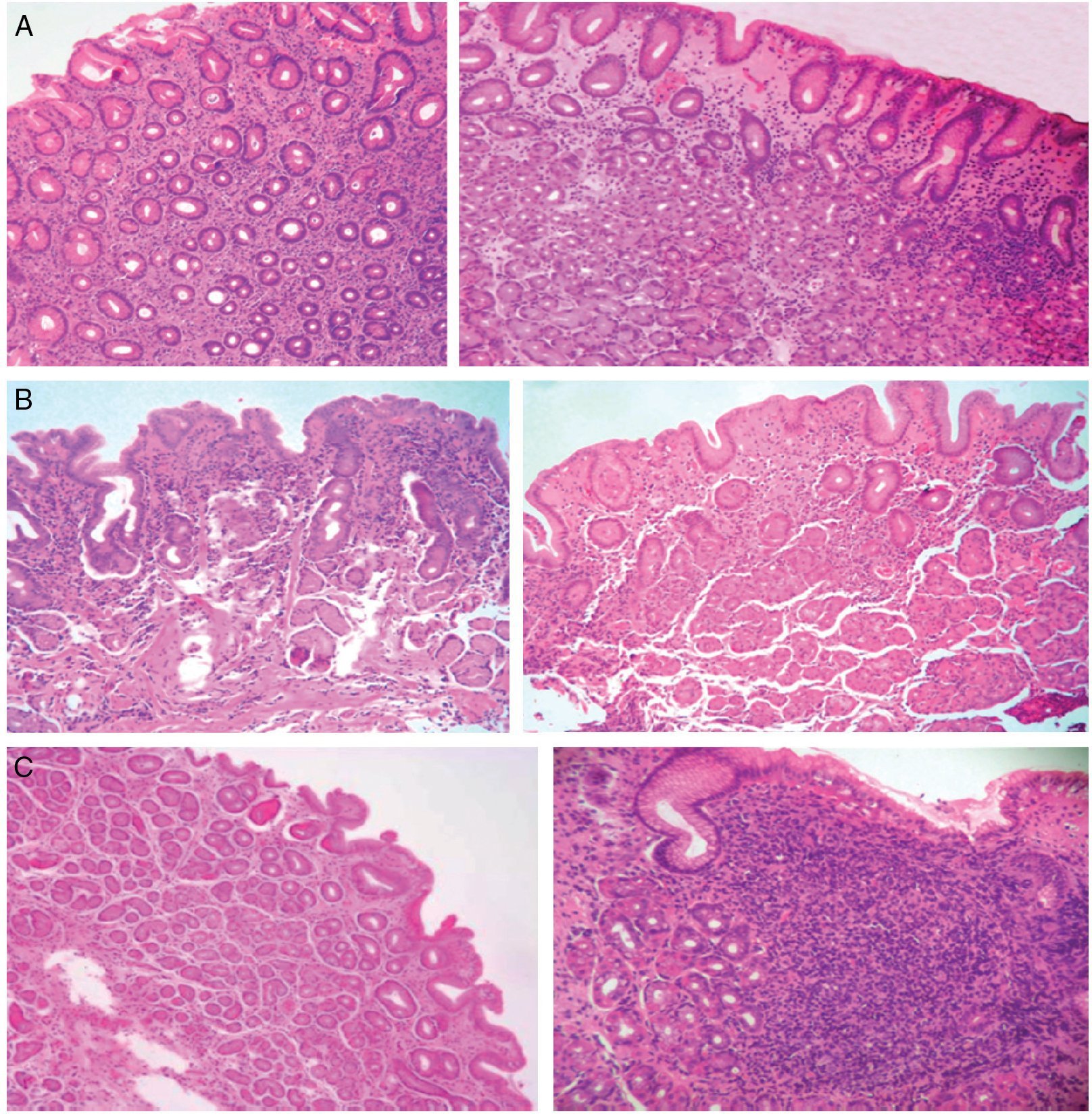
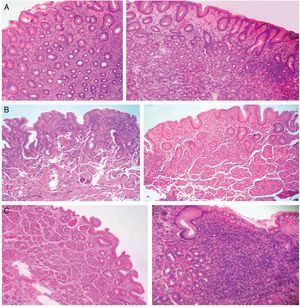
Figure 1. A) Antrum-predominant gastritis (×40/×40). B) Pangastritis (×40/×40). C) Corpus-predominant gastritis (×40/×100). Antrum mucosa: the microphotographs on the left. Corpus mucosa: the microphotographs on the right. Stain: Hematoxylin-eosin.
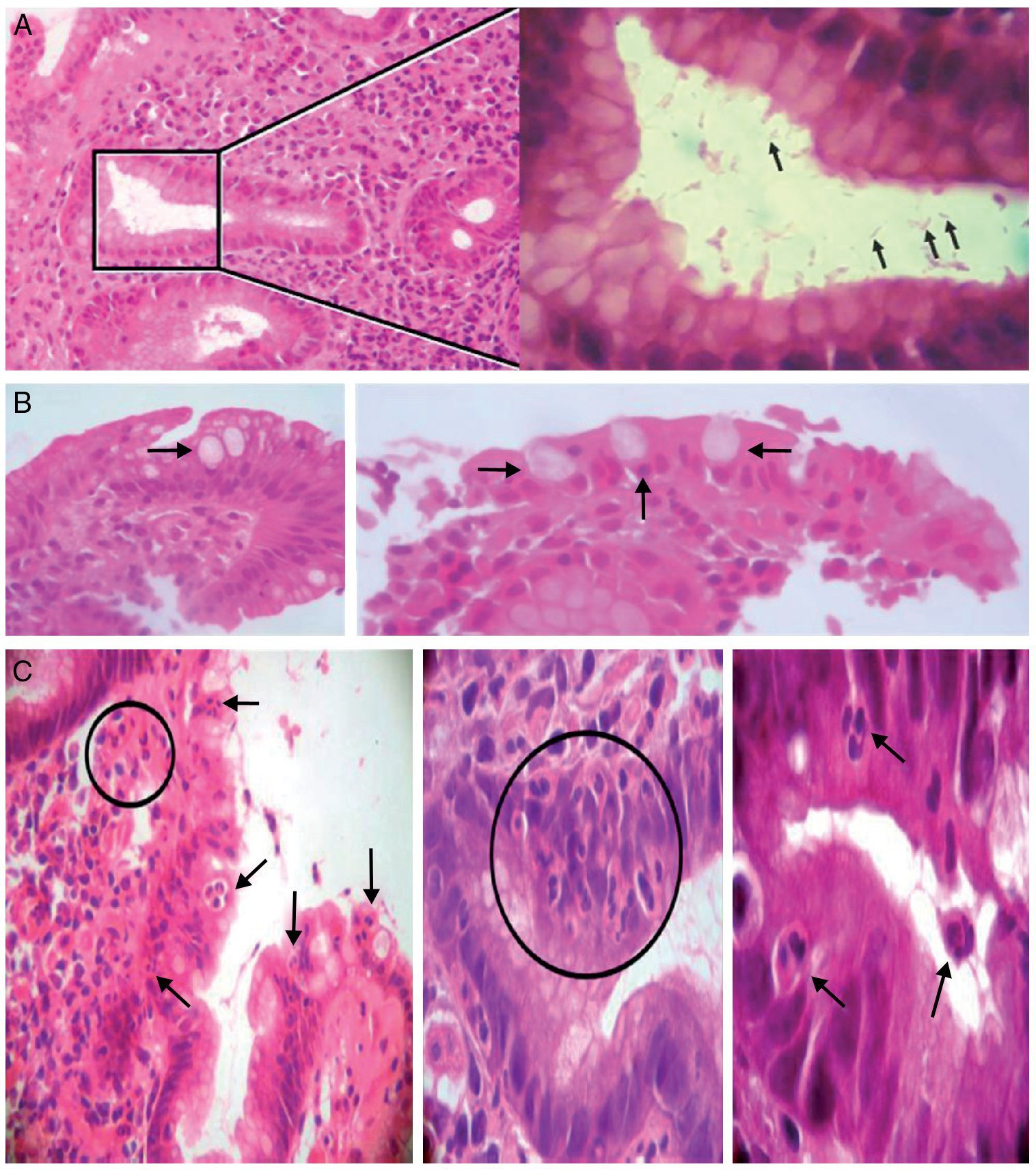
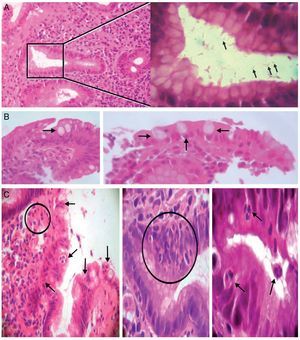
Figure 2. A) Left: chronic antrum gastritis with the presence of Helicobacter pylori in a glandular lumen indicated by a square (left). Stain: hematoxylin-eosin. (×100). Right: greater magnification of the area of the square showing some Helicobacter pylori bacilli (arrows) (immersion). B) Intestinal metaplasia. Presence of goblet cells (arrows) (×40/×100; left and right, respectively). C) Presence of polymorphonuclear inflammatory neutrophils infiltrating the lamina propria, the epithelial covering, and the glandular lumen (arrows and circles) (×40/×400/immersion; from left to right, respectively).
Cases with intestinal metaplasia
Nine cases were diagnosed with intestinal metaplasia (8%), 7 of which (77.7%) were in the aHpi group. Of the latter, 3 corresponded to intestinal metaplasia in the antrum mucosa and 4 in the corpus mucosa. There was significant statistical difference between the patients with and without aHpi (p = 0.04; χ2: 8.196). None of the patients with intestinal metaplasia presented with duodenal or gastric ulcer and there was no relation between the presence of intestinal metaplasia and the demographic or clinical aspects of the patients.
Gastric Cancer Rate Index
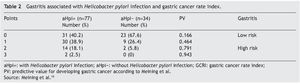
Table 2 presents the data related to the GCRI in the patients with and without aHpi. A total of 20.6% of the patients with aHpi had a GCRI of 2 (18.1%) or 3 (2.5%) points (high risk index according to Meining et al.10), whereas 79.1% accumulated a GCRI of 0 or 1 points (low risk index according Meining et al.10). Of the patients with no aHpi, 5.8% had 2 points, none of them presented with 3 points, and 94% had 0 or 1 points. There were statistically significant differences between the two groups (p = 0.001; χ2: 22.951). The patients with aHpi had a mean GCRI of 1.83 ± 0.8 points, whereas the patients without aHpi had a mean GCRI of 1.38 ± 0.6 points and the difference was statistically significant (p < 0.05).
Topography of gastritis associated with Helicobacter pylori infection and the Gastric Cancer Rate Index
Table 3 shows the relation between the topography of GAHpI and the GCRI, the presence of intestinal metaplasia, and the presence of gastric ulcer. Of the 18 patients that accumulated 2 or 3 points, 6 (33.3%) presented with intestinal metaplasia (all with pangastritis and corpus-predominant gastritis), of which 4 cases (66.6%) belonged to the aHpi group.
In the group of patients with aHpi (77 patients), all that developed pangastritis and corpus-predominant gastritis (n=35) accumulated 1 or more points, 15 of them reached 2 or 3 points (43%), and 4 presented with intestinal metaplasia (11.4%). Of the patients that presented with antrum-predominant gastritis, 1 had 2 points (2.3%). Four cases of antrum-predominant gastritis had gastric ulcer (9.5%), whereas 2 patients (6%) with pangastritis or with corpus-predominant gastritis had gastric ulcer.
In the group of patients without aHpi, none had a GCRI of 3 points and 2 patients (6%) diagnosed with pangastritis had a GCRI of 2 points, one of whom presented with intestinal metaplasia. All the patients that developed antrum-predominant gastritis had a GCRI of 0 points. Four patients diagnosed with antrum-predominant gastritis presented with gastric ulcer (17%), as did one patient (9%) diagnosed with pangastritis.
The Spearman correlation between the gastritis phenotype and the GCRI produced a value of 0.715 (a high correlation, with a statistical significance of p = 0.01).
Discussion
There was a high frequency of CGAHpI, affecting a little over two thirds of the study population (69.4%), of which slightly less than half (45%) presented with pangastritis (23%) and/or chronic corpus-predominant gastritis (22%). In Japan, Uemura et al.12 reported similar results in 1,246 patients with CGAHpI; 27% of them with pangastritis and 17% with chronic corpus-predominant gastritis. They reported a relative risk for developing gastric cancer of 15.6 for the patients with pangastritis and 34.5 for those with chronic corpus-predominant gastritis.
Various authors9,11,12 have observed that patients with CGAHpI presenting with pangastritis, chronic corpus-predominant gastritis, severe atrophy, and/or intestinal metaplasia are at high risk for developing intestinal-type gastric carcinoma. In our study, of the 35 patients with CGAHpI that presented with the phenotype of pangastritis and/or corpus-predominant gastritis, 15 (43%) had 2 or 3 points on the GCRI, of whom 4 (11.4%) presented with intestinal metaplasia; in other words, metaplastic-atrophic pangastritis and/or metaplastic-atrophic corpus-predominant gastritis.
Thus, it can be derived that there is a group of patients with CGAHpI that develops pangastritis or corpus-predominant gastritis with a high GCRI and no intestinal metaplasia and another group with a high GCRI that has intestinal metaplasia. In our study, 19% of the patients (15/77) corresponded to this first group, and 5% (4/77) to the second. On the other hand, in the group of patients with no H. pylori infection, only 5.8% (2/34) presented with pangastritis or corpus-predominant gastritis with a high GCRI without intestinal metaplasia and 3% (1/34) with metaplastic-atrophic pangastritis.
Therefore, the data analysis indicated that in the group of patients with the histopathologic phenotype associated with the greatest risk for developing gastric cancer, this risk decreased progressively when the respective combinations of the GCRI score and the presence of intestinal metaplasia were considered.
In this study only 2 patients with CGAHpI (2.5%) obtained a score of 3 on the GCRI and 7 patients (9%) had intestinal metaplasia. It is striking that the GCRI score of 3 and the presence of intestinal metaplasia were observed in patients with pangastritis or chronic corpus-dominant gastritis. Several authors have shown that these 3 characteristics are individually considered to be factors that are connected with a greater risk for developing gastric cancer. In a study of 2,000 patients, Meining and Stolte18 observed a strong correlation between the degree of activity of corpus gastritis associated with H. pylori and the presence of intestinal metaplasia. They also noticed that severe corpus gastritis and intestinal metaplasia were predominant in patients with gastric cancer and gastric ulcers, and so they concluded that severe corpus gastritis could be a gastric cancer marker. Afterwards, Meining et al.20 retrospectively applied the GCRI to the gastric biopsies of 415 patients that developed gastric cancer and established that a score of 3 had a 93% sensitivity and an 85% specificity for gastric cancer. In our study, only 2.5% of the patients with CGAHpI had 3 points and none of the patients without H. pylori infection did. Finally, Hsu et al.20 studied 1,225 patients with dyspepsia and aHpi through a multivariate analysis and determined that intestinal metaplasia was the only factor that independently predicted the development of gastric cancer. In the present study, intestinal metaplasia was diagnosed in 9 patients (8%), 7 of whom (77.7%) were in the aHpi group.
In general, in accordance with the abovementioned and in the cases analyzed in this study, the estimated gastric cancer risk in patients with CGAHpI is relatively low, which is similar to that reported by other authors.9,11,12,18,20,21 In addition, the risk could be connected to pangastritis or corpus-predominant gastritis with an elevated GSRI and/or intestinal metaplasia.
It can therefore be opportune to combine the histopathologic phenotype of the CGAHpI and the GCRI in order to obtain a more precise individual estimate of the risk for gastric cancer in CGAHpI patients.
Regarding the present study, other relevant aspects to consider in the integral risk estimate are patient age, the presence of aHpi, duodenal ulcer, and the geographic location of the patients. The mean age of the patients in this study was 38.6 ± 13.1 years. Leodolter et al.9 referred to a directly proportional relation between age, GCRI score, and the presence of pangastritis and corpus-predominant gastritis. Some studies have presented results of case series of patients with a mean age above 50 years.9-11,21-24
In our study, none of the patients with intestinal metaplasia presented with duodenal ulcer. This phenomenon could be linked to that which was reported by Tsukui et al.,24 who suggested that the presence of duodenal ulcer in patients with dyspepsia could reduce the risk for developing atrophic gastritis and intestinal metaplasia in the gastric corpus. The presence of duodenal ulcer is associated with a low level of atrophic changes, antrum-predominant gastritis, and a low risk for developing gastric cancer.25
Patients with aHpi had a significantly higher mean GCRI score (1.83 ± 0.8 points vs. 1.38 ± 0.6 points) and a higher incidence of intestinal metaplasia than the patients with no aHpi (p < 0.05 in both aspects). The patients with metaplasia and high GCRI scores were considered at high risk for developing gastric cancer.10,26 In this context, the eradication of H. pylori could be advisable in these patients, considering that it could reduce the risk for developing gastric cancer26-28 and generally stop or delay the progression of the histopathologic changes associated with the carcinogenesis process, which finally, could reduce the risk for developing cancer.26-30 Another potentially relevant aspect connected to H. pylori eradication in the patients studied is the fact that in 2 Venezuelan cities (San Cristóbal and Caracas), the CagA+/s1m1 genotype is the most prevalent.31 In a study conducted in the city of Maracaibo on 32 patients — 16 mestizos and 16 ethnic Wayuus — serum IgG anti-CagA positivity was 94 and 75%, respectively.32 Infection with these types of strains is linked to the development of precancerous lesions and cancer.33,34 In a study with Colombian patients, it was reported that strains of H. pylori expressing high levels of CagA were associated with more advanced precancerous lesions.35
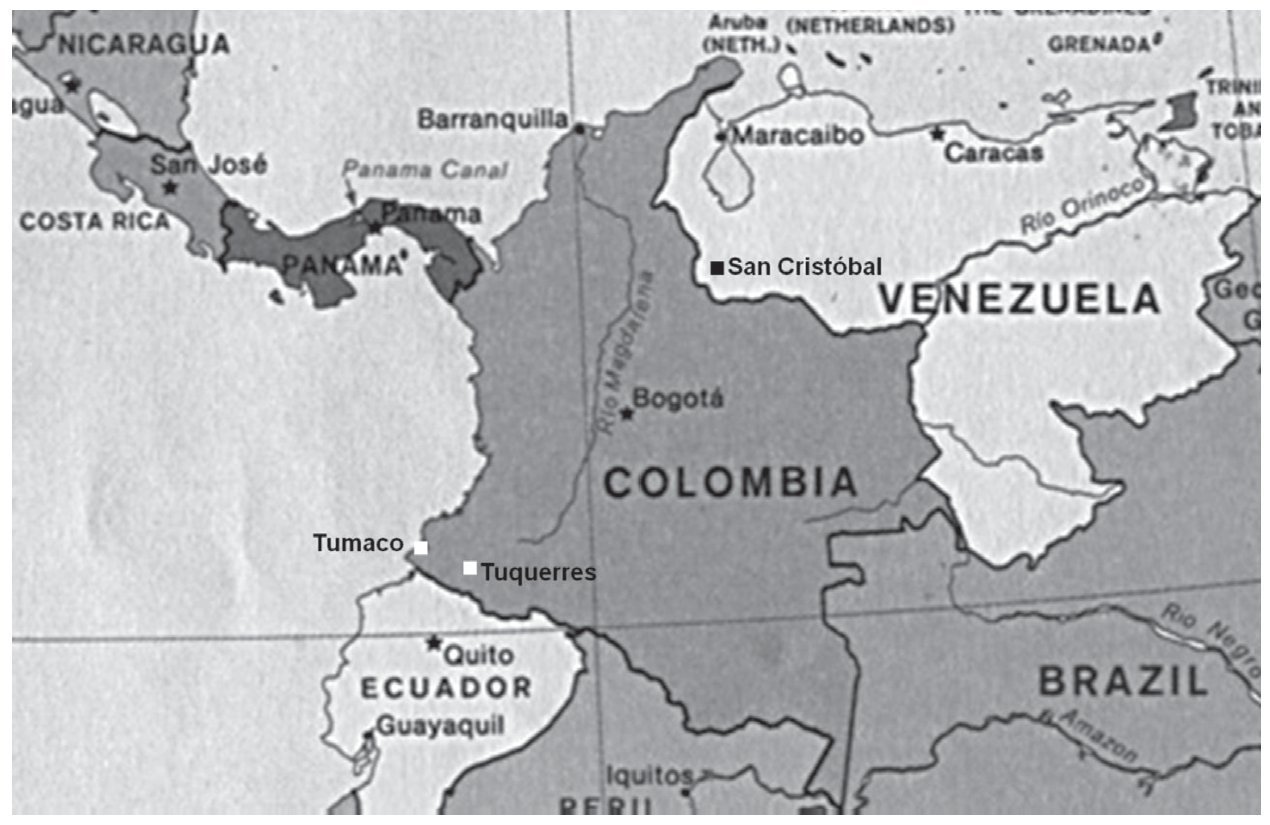
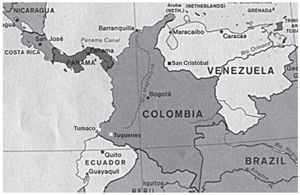
From the geographic perspective, the study patients are inhabitants of a South American city situated at sea level (Maracaibo, Zulia State, Venezuela, see map, Fig. 3). In Colombia and Costa Rica, much lower gastric cancer rates have been reported in populations that live at sea level, when compared with populations living in the mountains.6 These differences could perhaps be due to the differences in dietary habits and to the exposure to or infestation of helminths. Reports state that populations located at sea level consume greater quantities of fish, vegetables, and fresh fruits and that they have higher rates of intestinal helminthic parasitosis.6,36 This latter circumstance produces a predominantly Th2-type immune response that is connected to preventing the development of atrophic gastritis and a lower anti-secretory effect of certain proinflammatory cytokines.6,25,36
Figure 3. Map showing the location of the cities of Maracaibo, Caracas, San Cristóbal (Venezuela) and Tumaco and Tuquerres (Colombia).
Finally, follow-up should be carried out on the progression of the endoscopic findings, H. pylori infection, and the anatomy and pathology of gastritis, at least in the patients with the histopathologic phenotype associated with a higher risk for developing gastric cancer (pangastritis and corpus-predominant gastritis with or without intestinal metaplasia) and 2 or more points in the GCRI.
Conclusions
It appears to be advantageous to combine the histopathologic phenotype of CGAHpI, the GCRI, and certain non-histopathologic aspects such as age, aHpi, the presence of duodenal ulcer, and geographic location for obtaining a more precise individual estimate of the risk for gastric cancer in patients with CGAHpI. The combination of these different aspects could be used to design a particular clinicopathologic gastric cancer risk index that would be suitable for each country or geographic location.
Financial disclosure
This research was partially financed by the Consejo de Desarrollo Científico y Humanístico de la Universidad del Zulia (CONDES-LUZ, Venezuela); Proyecto#CC-0340-10.
Conflict of interests
The authors declare that there is no conflict of interest.
Received 6 November 2012;
accepted 21 January 2013
* Corresponding author at:
Laboratorio de Investigaciones Gastrointestinales, Instituto de Investigaciones Biológicas. Facultad de Medicina. Universidad del Zulia. Maracaibo, Venezuela.
Tel.: +58 0261 7597249; fax: +58 0261 7597250.
E-mail address:gabrielarismendi@gmail.com (G. Arismendi-Morillo).