Helicobacter pylori (H. pylori) is the main risk factor for the development of chronic gastritis, gastric ulcer, and gastric cancer. In H. pylori-infected individuals, the clinical result is dependent on various factors, among which are bacterial components, the immune response, and environmental influence.
AimsTo compare IFN-γ expression with the H. pylori vacA and cagA genotypes in patients with chronic gastritis and patients with gastric cancer.
MethodsNinety-five patients diagnosed with chronic gastritis and 20 with gastric cancer were included in the study. Three gastric biopsies were taken; one was used for the molecular detection and genotyping of H. pylori; another was fixed in absolute alcohol and histologic sections were made for determining IFN-γ expression through immunohistochemistry.
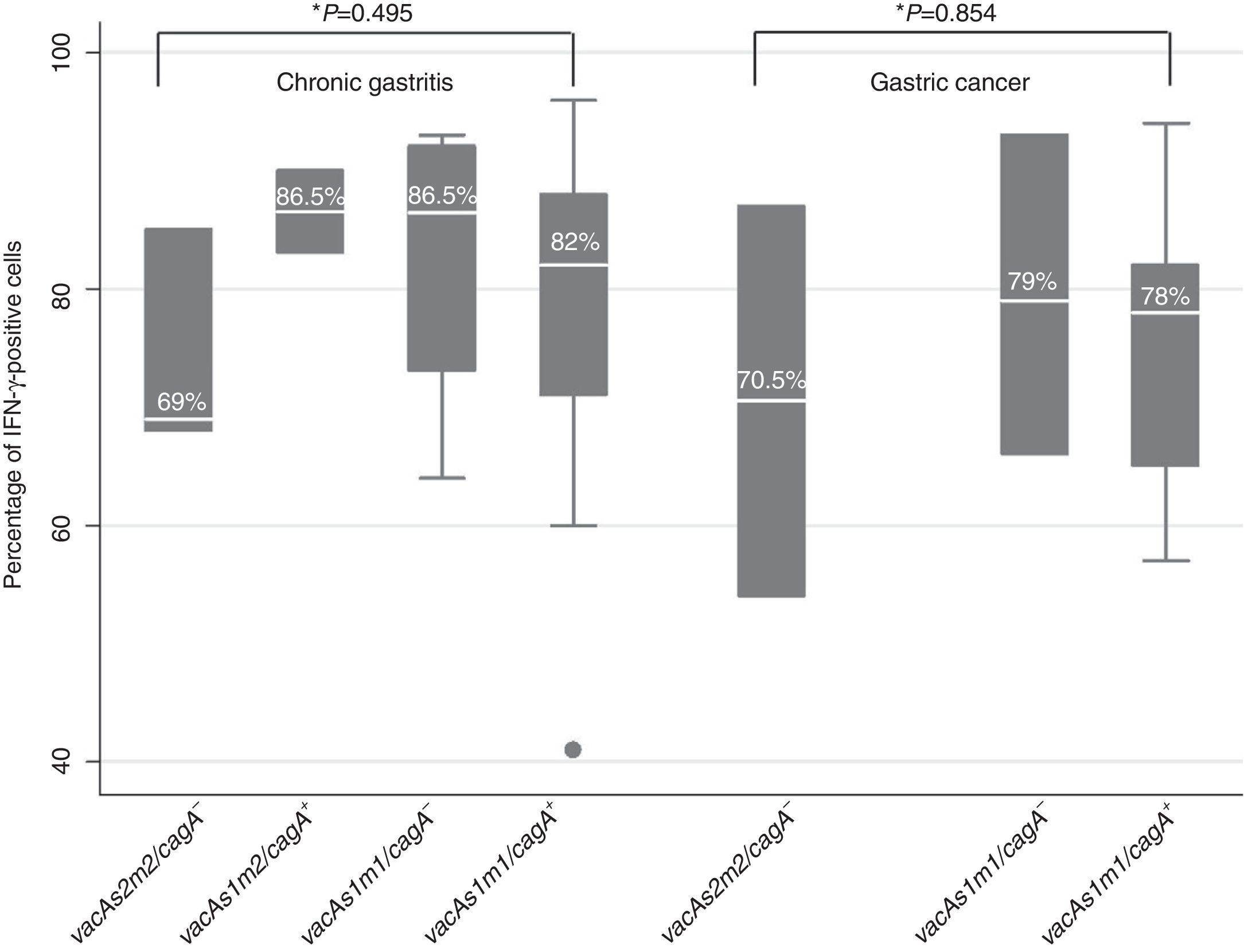
ResultsNo differences were found in the cells that expressed IFN-γ between the patients with chronic gastritis (median percentage of positive cells: 82.6% in patients without H. pylori and 82% in infected persons) and those with gastric cancer (70.5% in H. pylori-negative patients and 78.5% in infected persons). IFN-γ expression was 69% in chronic gastritis patients infected with H. pylori vacAs2m2/cagA− it was 86.5% in patients infected with H. pylori vacAs1m2/cagA-, 86.5% in vacAs1m1/cagA−, and 82% in vacAs1m1/cagA+. Similar data were found in the patients with gastric cancer.
ConclusionsIFN-γ expression varied depending on the H. pylori vacA and cagA genotype, but not in accordance with the presence of chronic gastritis or gastric cancer.
El H. pylori es el principal factor de riesgo para el desarrollo de gastritis crónica, úlcera gástrica y cáncer gástrico. El resultado clínico en infectados por esta bacteria depende de varios factores, entre ellos los componentes bacterianos, la respuesta inmune, y la influencia del medio ambiente.
ObjetivoComparar la expresión de IFN-γ con los genotipos vacA y cagA de H. pylori en pacientes con gastritis crónica y cáncer gástrico.
Pacientes y métodosSe incluyeron 95 pacientes con diagnóstico de gastritis crónica y 20 con cáncer gástrico. Se tomaron 3 biopsias gástricas, una se utilizó para la identificación molecular y genotipificación de H. pylori. Otra fue fijada en alcohol absoluto y realizaron cortes histológicos para determinar la expresión de IFN-γ por inmunohistoquímica.
ResultadosNo se encontraron diferencias en las células que expresaron IFN-γ entre pacientes con gastritis crónica (mediana del porcentaje de células positivas: 82.6% en pacientes sin H. pylori y 82% en personas infectadas) y cáncer gástrico (70.5% en pacientes H. pylori-negativos y 78.5% en infectados). En pacientes con gastritis crónica infectados por H. pylori vacAs2m2/cagA− la expresión de IFN-γ fue del 69%, en pacientes con H. pylori vacAs1m2/cagA− fue de 86.5%, en vacAs1m1/cagA− del 86.5%, y en vacAs1m1/cagA+ del 82%. En cáncer se encontraron datos similares.
ConclusiónLa expresión de INF-γ varía dependiendo del genotipo vacA y cagA de H. pylori, pero no de acuerdo a la presencia de gastritis crónica o cáncer gástrico.
Almost half the worldwide population is infected with Helicobacter pylori (H. pylori) and it is the main cause of chronic gastritis, gastric or duodenal ulcer, gastric cancer, and mucosa-associated lymphoid tissue (MALT) lymphoma, also known as extranodal marginal zone B cell lymphoma.1-3
The cytotoxin-associated gene A (CagA) and the vacuolating cytotoxin (VacA) are H. pylori virulence factors that have multiple effects on the human epithelial cell.4CagA-positive H. pylori strains are associated with more severe inflammation than the cagA-negative strains. H. pylori transfers the CagA protein and other soluble factors to the cytoplasm of the epithelial cell through its type IV secretion system.5 CagA activates intracellular signaling pathways that lead to the activation of transcription factors that modulate proinflammatory cytokine expression, to immune cell infiltration, to cell-to-cell adhesion damage, and to change in epithelial cell polarity and permeability.3 The VacA toxin induces vacuolation, apoptosis, and inhibition of cell proliferation.3,6 All the H. pylori strains contain the vacA gene, which is polymorphic in the signal (s1a, s1b, s1c, and s2 alleles) and mid (m1 and m2 alleles) regions. Each vacA gene contains an s allele and an m allele and the diversity in the sequence affects the vacuolating activity of the cytotoxin. The vacA s1m1 strains are associated with more severe disease.4,7
H. pylori induces a strong immunologic, humoral, and cellular response, characterized by the infiltration of neutrophils, macrophages, eosinophils, and lymphocytes into the infection site. Leukocyte migration is mediated by cytokines and chemokines released by the epithelial and immune cells. However, despite the high number of infiltrating leukocytes, the bacterium is not eliminated in the majority of the infected subjects, and the intensity of the inflammatory response contributes to the clinical result of the infection.8-9 In the gastric mucosa of adults with gastritis or peptic ulcer that are infected with H. pylori, there is a predominant Th1-type immune response, with a high level of expression of interferon gamma (IFN-γ) and interleukin-2 (IL-2) and a low level of expression of IL-4 and IL-10.9
IFN-γ is an important mediator of innate and adaptive immunity. It is produced mainly by T CD4+ and CD8+ cells and by natural killer (NK) cells. This cytokine is overexpressed in the stomach of humans and mice infected with Helicobacter spp.10 IFN-γ plays a dual role in response to H. pylori infection; on the one hand, it induces gastric inflammation and promotes the appearance of pre-neoplastic lesions caused by the infection, and on the other, IFN-γ reduces bacterial colonization and is essential for the elimination of the infection.11-12
Previous studies found that IFN-γ levels were higher in patients infected with H. pylori than in uninfected patients.9,13-15 In 2007, Wang et al. found that in patients infected with cagA+H. pylori, the cellular immune response mediated by Th1 cells was associated with earlier stages of gastric carcinogenesis, whereas humoral immunity mediated by Th2 cells predominated in the advanced stages.16 Despite the fact that increased IFN-γ expression has been observed in the gastric mucosa of H. pylori-positive patients, whether or not the expression of this cytokine varies with the vacA and cagA genotypes of the bacterium has not been studied. To the best of our knowledge, no data have been published documenting IFN-γ expression in Mexican patients with gastric diseases associated with H. pylori infection. The aim of this study was to relate IFN-γ expression with H. pylori infection and with the vacA genotypes and cagA status of the bacterium in patients with chronic gastritis and patients with cancer.
MethodsPatientsPatients that underwent an upper gastrointestinal endoscopy at the Hospital General «Raymundo Abarca Alarcón» and the Unidad Especializada en Gastroenterología Endoscopía in the city of Chilpancingo and the Endoscopy Service of the Instituto Estatal de Cancerología «Arturo Beltrán Ortega» in Acapulco, Guerrero, Mexico, within the time frame of August 2011 and March 2013 were included in the study. The selected patients had undergone no H. pylori eradication treatment during the month prior to the endoscopic procedure. Patients with immunosuppressant or nonsteroidal anti-inflammatory treatment were excluded from the study. The patients or their parents signed statements of informed consent. A questionnaire was applied to the patients that agreed to participate in the study in order to record general data and information related to the disease. The project was approved by the Bioethics Committee of the Universidad Autónoma de Guerrero and the participating hospitals.
Endoscopy and biopsy collectionThe endoscopy was performed with a video processor and video gastroscope (Fujinon, Wayne, NJ, USA), after a night of fasting. In each patient, three gastric biopsies were taken from the antrum, body, or the tumor; one was placed in a buffering solution (Tris 10mM pH 8.0, EDTA 20mM pH 8.0, SDS 0.5%) for the molecular diagnosis of H. pylori. Another was fixed in absolute alcohol to verify IFN-γ expression through immunohistochemistry. The last biopsy was fixed in buffered formalin for the histopathologic study. The histologic slices were stained with hematoxylin and eosin and evaluated by a pathologist using the updated Sydney system criteria17 or the Lauren classification.18
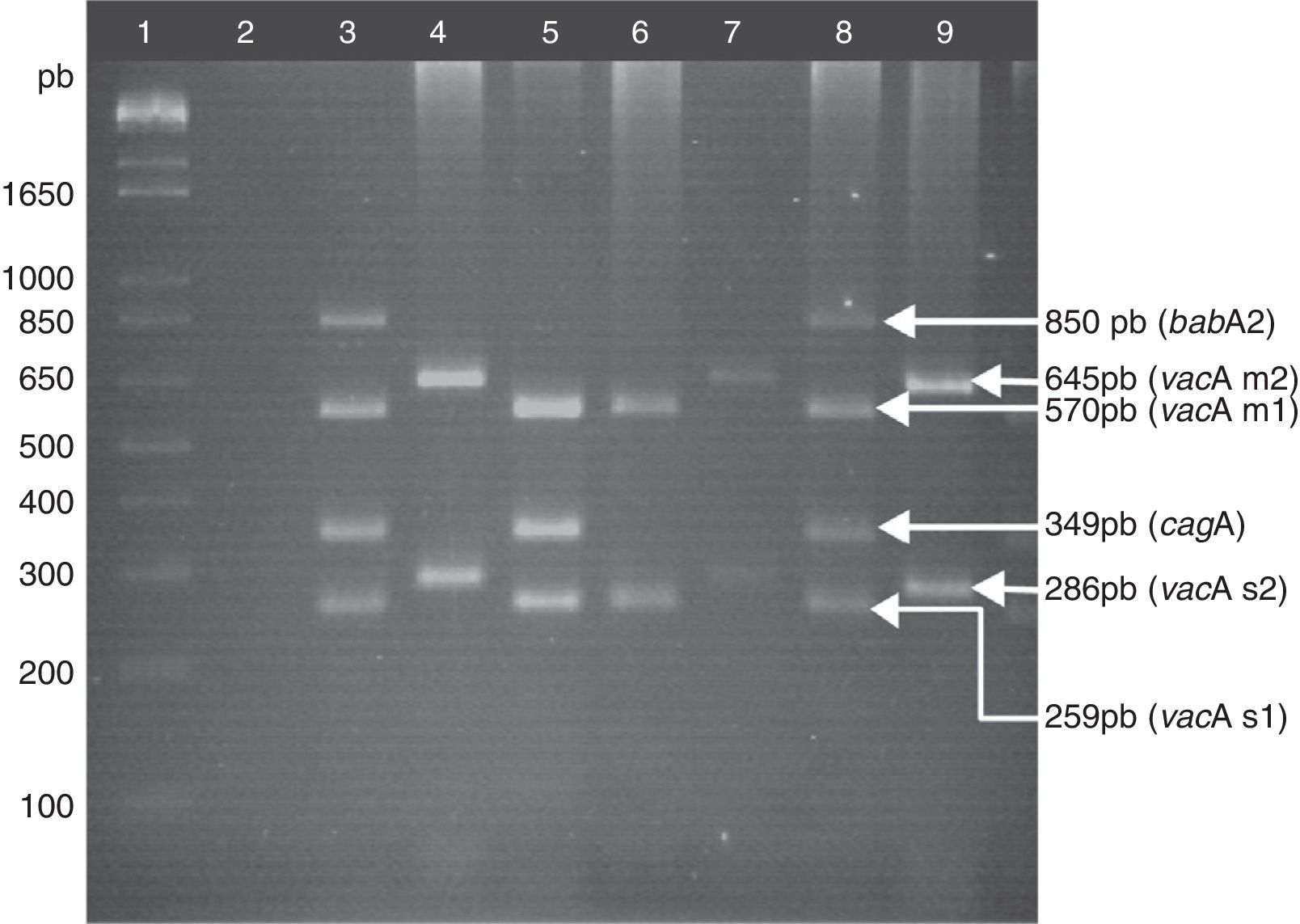
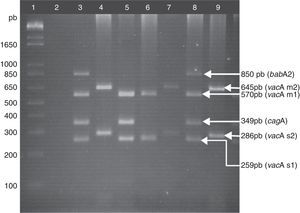
Helicobacter pylori detection and genotypingTotal DNA was extracted from the gastric biopsies using the phenol-chloroform-isoamyl alcohol technique after proteinase K digestion.19H. pylori detection was carried out through PCR, amplifying a fragment of the 16S rRNA gene, following the methodology previously described by Martínez-Carrillo et al.20 The H. pylori-positive samples underwent a second PCR with starters for amplifying the s and m region of the vacA gene,21-22 the cagA gene,23 and the babA2 gene.24 The following were mixed in a final volume of 25μL: 500 ng of DNA, 1.5mM of MgCl2, 0.15mM of dNTPs (Invitrogen, Carlsbad, CA, USA), 1.3 U of Platinum® Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA), and the required oligonucleotide concentrations of 2.5 pM for vacA, 5 pM for cagA, and 12.5 pM for babA2. The amplification program included an initial denaturalization step at 94°C for 10min, 35 cycles at 94°C for 1min, 57°C for 1min, 72°C for 1min, and a final extension step at 72°C for 10min (figure 1). In each PCR, DNA from the J99 strain was used, with the vacAs1m1/cagA+/babA2+ genotype as the positive control. Sterile deionized water was used instead of DNA for the negative control. All the reactions were made in a thermal cycler Mastercycler Ep gradient (Eppendorf, Hamburg, Germany).
Genotyping of H. pylori. Lane 1: 1kb plus molecular weight marker; lane 2: negative control; lane 3: positive control, DNA from the H. pylori strain J99, vacAs1m1/cagA+/babA2+ genotype; lanes 4, 7, and 9: clinical samples of vacAs2m2/cagA−/babA2− genotype; lane 5: clinical sample of vacAs1m1/cagA+/babA2− genotype; lane 6: clinical sample of vacAs1m1/cagA−/babA2− genotype; lane 8: clinical sample of vacAs1m1/cagA+/babA2+genotype. Agarose gel at 2.5%.
The samples fixed in absolute alcohol were embedded in paraffin and sliced at a thickness of 3μm. Each tissue section was deparaffinized in xylene and rehydrated with descending grades of alcohol. The slides were boiled in citrate buffer (Declere 1X, Cell Marque, Rocklin, CA, USA) for 20min in an autoclave for antigen retrieval. After permeabilization and the blocking of endogenous peroxidase, the slices were incubated all night with mouse anti-human IFN-γ monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, USA) at a 1:50 dilution. Antibody binding was detected with the Mouse/Rabbit ImmunoDetector HRP/DAB Detection System Kit (Bio SB, Santa Barbara, CA, USA). The slices were counter-stained with hematoxylin (Biocare Medical, Concord, CA, USA). A total of 100 mononuclear cells were counted in 5 randomly selected fields and those with a brown cytoplasmic or nuclear stain were considered positive. The data were expressed as positive cell percentages. To validate the results of the manual counting of the IFN-γ+ cells, a random number of samples equivalent to 10% were selected and the IFN-γ+ cell percentage was verified using Leica Microsystems CMS GmbH version 4.3.0 software.
Statistical analysisThe frequency of the qualitative variables, the mean ± standard deviation of the parametric quantitative variables, and median and interquartile range for the nonparametric variables were determined. The p value was obtained with the chi-square test or the Fisher exact test for qualitative variables and the Student's t test, ANOVA, Mann-Whitney, or Kruskal-Wallis tests for quantitative variables. Statistical significance was set at a p<0.05.
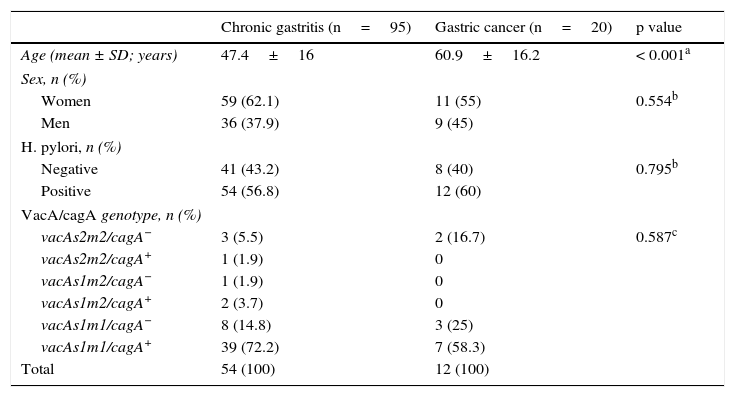
ResultsPrevalence of Helicobacter pylori and vacA/cagA genotype infectionNinety-five patients with confirmed histologic diagnosis of chronic gastritis and 20 with histopathologic gastric adenocarcinoma results were studied. The mean age for the chronic gastritis group was 47.4 years and 60.9 years for the gastric cancer group (p<0.001, table 1). Of the 115 patients included in the study, 66 (57.4%) were H. pylori-positive and vacAs1m1/cagA+ was the most frequent genotype at 69.7% (46/66). The prevalence of H. pylori and vacA/cagA genotype infection varied between the groups (table 1).
Prevalence of H. pylori and vacA/cagA genotype infection.
| Chronic gastritis (n=95) | Gastric cancer (n=20) | p value | |
|---|---|---|---|
| Age (mean ± SD; years) | 47.4±16 | 60.9±16.2 | < 0.001a |
| Sex, n (%) | |||
| Women | 59 (62.1) | 11 (55) | 0.554b |
| Men | 36 (37.9) | 9 (45) | |
| H. pylori, n (%) | |||
| Negative | 41 (43.2) | 8 (40) | 0.795b |
| Positive | 54 (56.8) | 12 (60) | |
| VacA/cagA genotype, n (%) | |||
| vacAs2m2/cagA− | 3 (5.5) | 2 (16.7) | 0.587c |
| vacAs2m2/cagA+ | 1 (1.9) | 0 | |
| vacAs1m2/cagA− | 1 (1.9) | 0 | |
| vacAs1m2/cagA+ | 2 (3.7) | 0 | |
| vacAs1m1/cagA− | 8 (14.8) | 3 (25) | |
| vacAs1m1/cagA+ | 39 (72.2) | 7 (58.3) | |
| Total | 54 (100) | 12 (100) | |
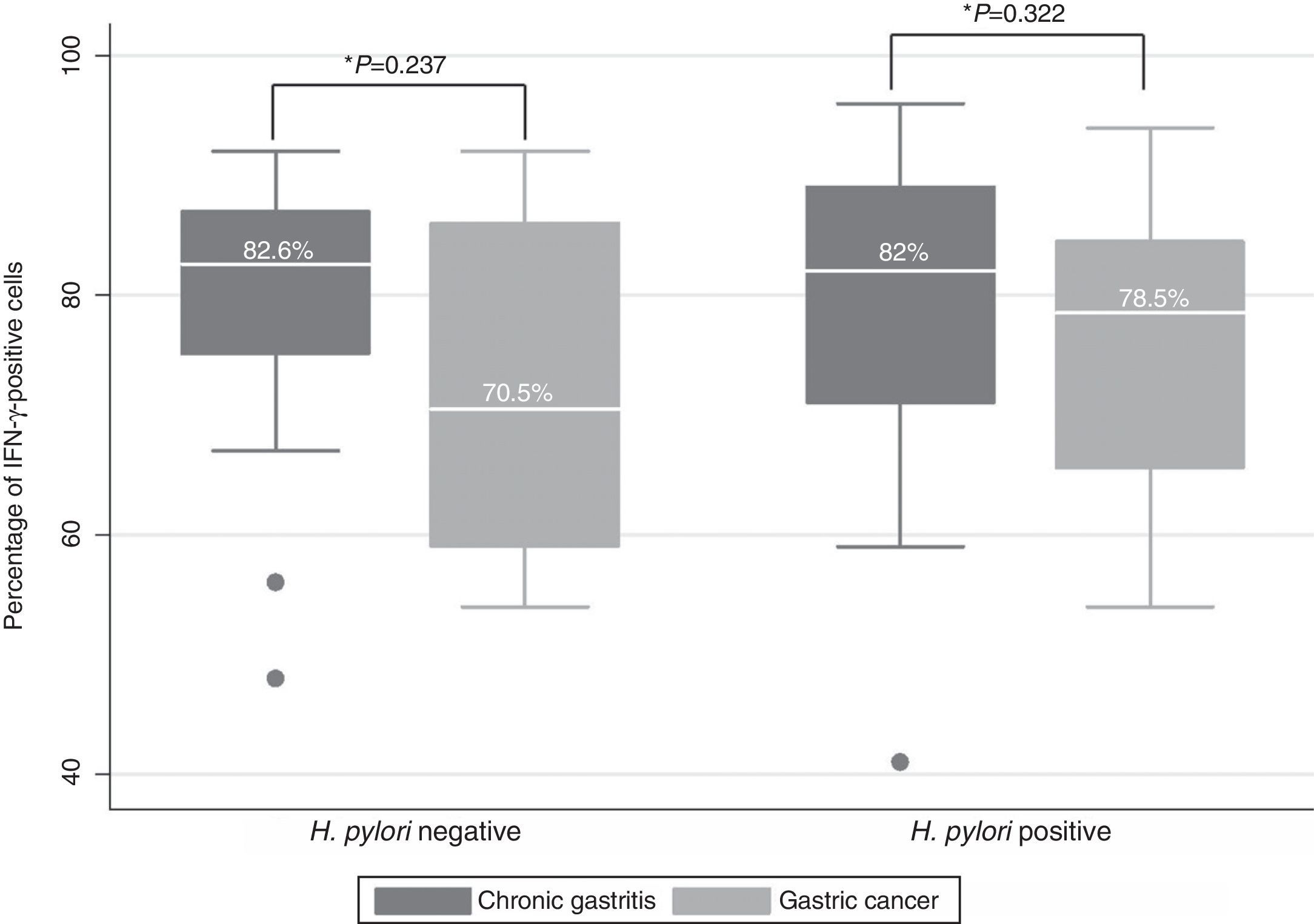
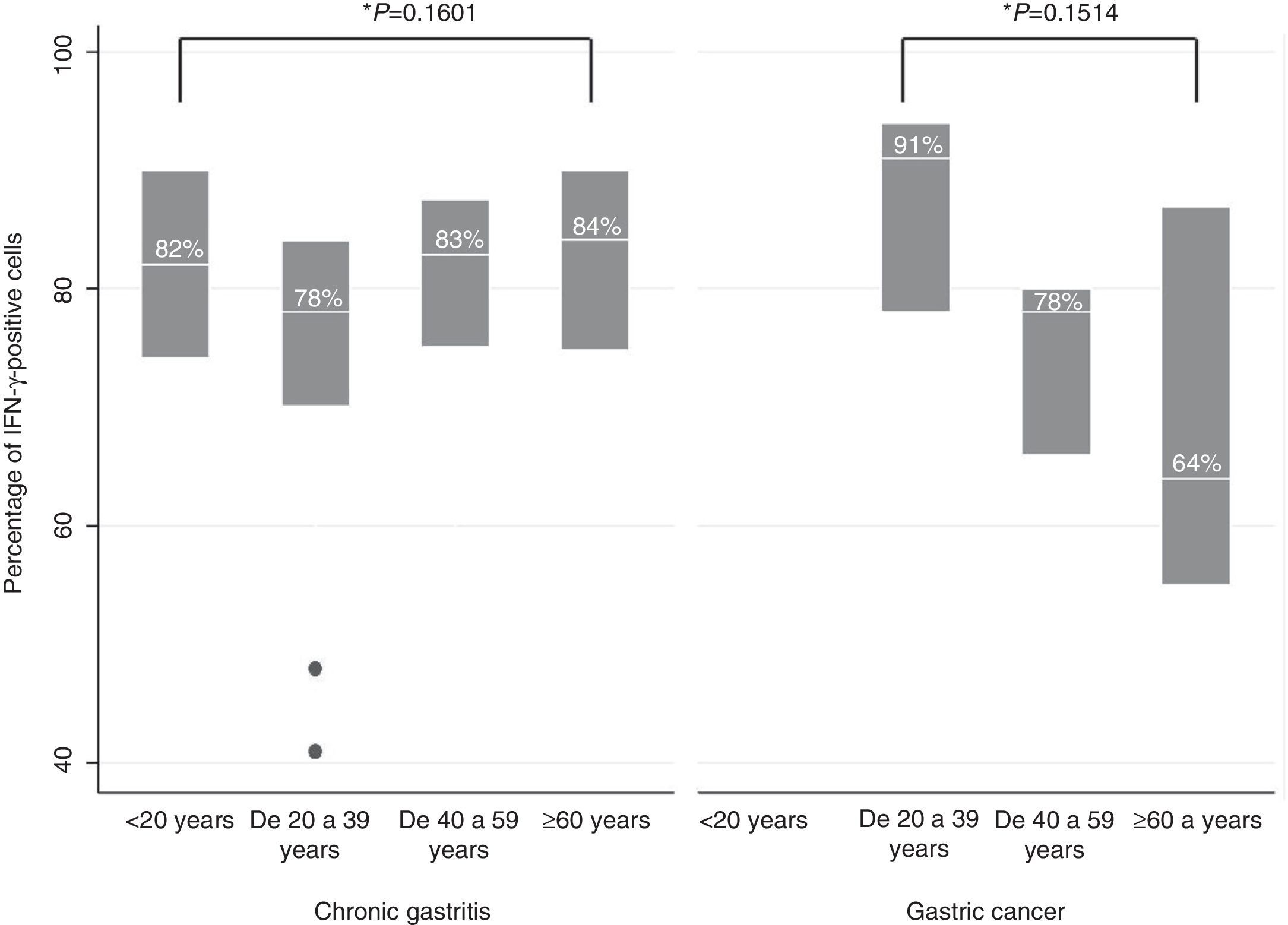
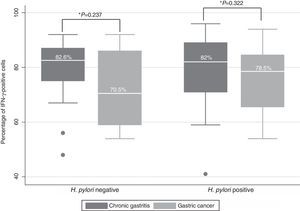
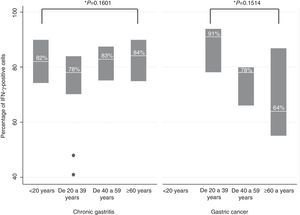
IFN-γ expression was located predominantly in the cytoplasm of the infiltrating mononuclear cells. IFN-γ expression was detected in the majority of the H. pylori-positive and H. pylori-negative patients (99/115, data not shown). No differences were found in relation to IFN-γ-positive cells between the patients with chronic gastritis (median percentage of positive cells: 82.6% in patients without H. pylori and 82% in infected persons) and those with gastric cancer (70.5% in H. pylori-negative patients and 78.5% in infected persons) (figure 2). In chronic gastritis, the median of the percentage of IFN-γ-positive cells by age group varied between 78 and 84% (interquartile range of 70 to 90%); in gastric cancer the median was from 64 to 90% (interquartile range of 55 to 94%) and no statistically significant differences were observed in the percentage of IFN-γ-positive cells in the age groups of patients with chronic gastritis (p=0.1601) or in the age groups of patients with gastric cancer (p=0.1514) (figure 3); nor were any differences found in the percentage of IFN-γ-positive cells between patients with chronic gastritis and those with gastric cancer (p=0.8781) (data not shown).
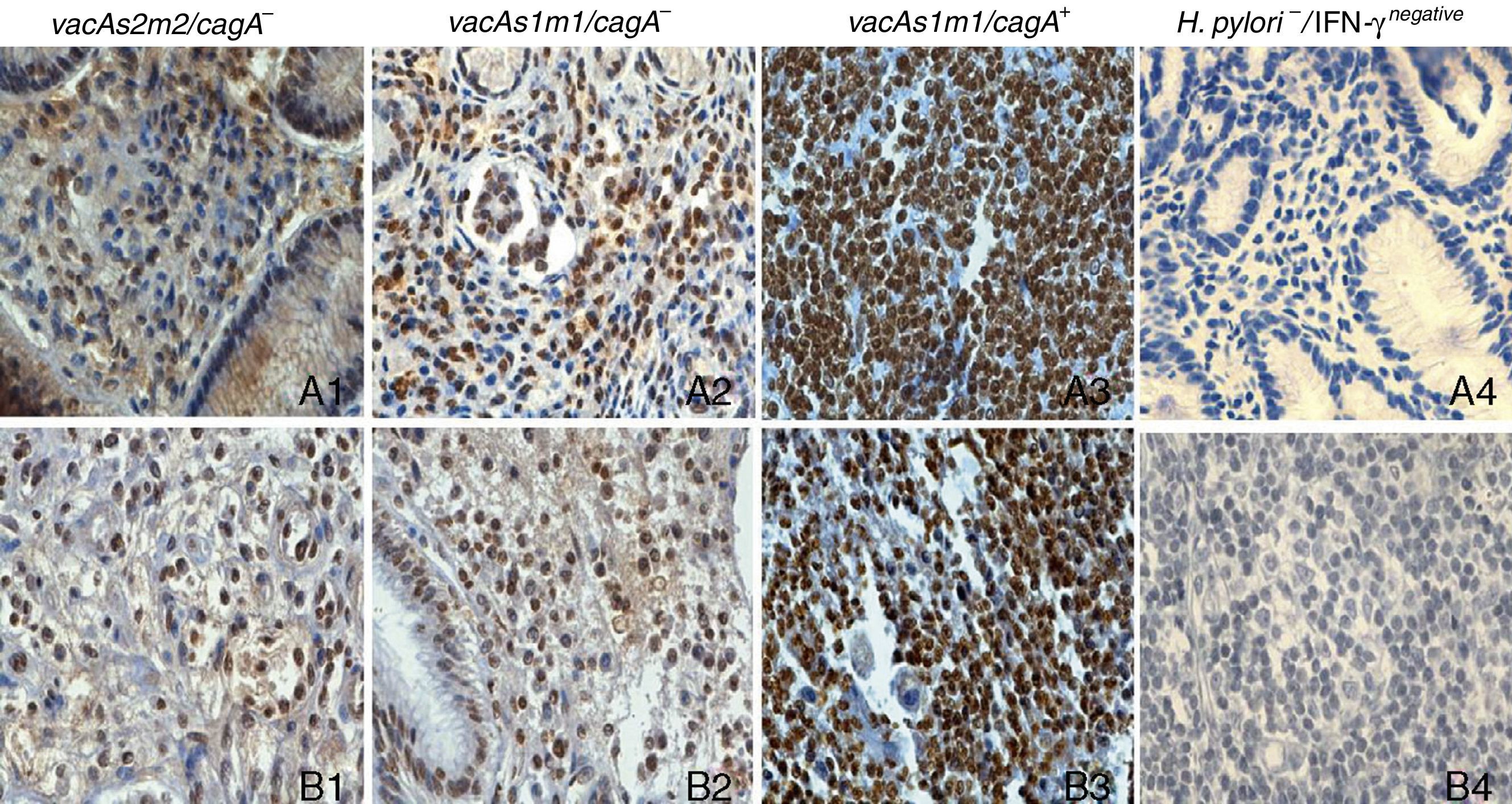
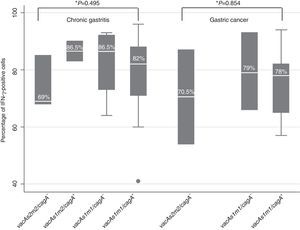
IFN-γ expression varied depending on the H. pylori genotype in patients with chronic gastritis and in those with gastric cancer (figure 4). In the chronic gastritis group, those infected with H. pylori vacAs2m2/cagA- presented with a lower percentage of IFN-γ-positive cells, (69%), compared with the patients with H. pylori vacAs1m2/cagA- (86.5%), vacAs1m1/cagA- (86.5%), and vacAs1m1/cagA+ (82%) (figure 5). Interestingly, of 5 patients with the H. pylori vacAs1m1/cagA+ genotype, 93% of the cells were IFN-γ-positive in 4 of them, and 96% in one patient. In the patients with gastric cancer that were infected with the vacAs2m2/cagA− genotype, 70.5% of the cells were IFN-γ-positive; in those infected with the vacAs1m1/cagA- genotype, the percentage of IFN-γ-positive cells reached 79%; and in the patients infected with the H. pylori vacAs1m1/cagA+ genotype, the percentage of IFN-γ-positive cells was 78%, reaching 94% in one of the 7 patients with this genotype (figure 5).
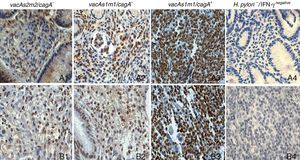
Immunohistochemistry of IFN-γ expression in gastric biopsies from patients with chronic gastritis and patients with gastric cancer, counterstained with hematoxylin (x10). A1-A4) Gastric biopsies of patients with chronic gastritis. B1-B3) Gastric biopsies from patients with gastric cancer. B4) Amygdala biopsy, negative reaction control (the primary antibody was omitted).
There was a variation between the percentage of IFN-γ+ cells counted by microscopic observation and those verified with the Leica Microsystems software of ± 4.4 cells.
DiscussionThe incidence and/or severity of the pathologies related to H. pylori may vary among geographic areas31 and the prevalence of infection and genotype distribution varies among countries, regions, and ethnic groups. In Mexico, a variation in the prevalence of H. pylori has been reported that ranges from 86.1% in the southeast, to 47.1% in children in the northeast of the country, and to 66% in a population of the Tepehuano ethnic group in northern Mexico.32-34 In 2005, Torres et al. reported that in Mexico, the prevalence of H. pylori cagA+ varied from 47.6% to 63.4%.35
Nevertheless, there are only a few reports on the prevalence of infection from H. pylori and its genotypes in the Mexican population. In this study, 60% of the cases of gastric cancer were H. pylori-positive, and the vacAs1m1/cagA+ genotype was the most frequent, at 58.3% (7/12). The frequency of H. pylori found in our study was the same as that reported by Morales-Espinosa et al., but we reported a higher frequency than that found in 2008 by López-Vidal et al. in a Mexican population; they detected the presence of H. pylori in 38% of the samples of patients with gastric cancer. However, López-Vidal et al. reported a higher prevalence of H. pylori cagA+ (72%) than what was found in our study on patients from the State of Guerrero with gastric cancer (58%). These differences may be due to the different geographical regions the patients come from. H. pylori genotypes have been reported to circulate differentially between populations and geographic zones. In a Northeastern Brazilian population in 2012, Figueiredo Cavalcante et al. found that 83.3% of the H. pylori strains were vacAs1, 53.3% were vacAm1, and 96.7% were cagA+ in patients with gastric cancer.25-28
Furthermore, we found that in the cases of chronic gastritis, 56.8% were H. pylori-positive and the most frequent genotypes were vacAs1m1, with 87%, and cagA+, with 77.8%. The frequency of the vacAs1m1 genotype in our study was higher than the 43.4% reported by Román-Román et al. in 2013 on a population from the State of Guerrero with chronic gastritis, and it was also higher than that found by Paniagua et al. in 2009 in patients from the State of Mexico; they reported that of the patients with chronic gastritis that were H. pylori-positive (60.1%), 40% of them had the vacAs1m1 genotype and 52% were cagA+. In 2012, Figueiredo Cavalcante et al. found that the s1 and m1 allelotypes were the most frequent in patients with chronic gastritis at 72.4% and 51.3%, respectively, and that 73.7% were H. pylori cagA+; in 2009, Torres et al. found a frequency of 54.9% for the vacAs1m1 genotype and 70.6% for the cagA+ genotype in Cuban patients with functional dyspepsia. In our study, vacA and cagA genotype detection was carried out with the same starters used by Torres et al., and in both studies the H. pylori DNA and its genotypes were determined from the total DNA from the gastric biopsy. Thus, the discrepancies in the frequencies can probably be explained by the differences in the population origins, the number of patients, and the diagnostic criteria employed, and perhaps by the differences in methodology for collecting and processing the biopsies, as well.28-31 The probability of detecting H. pylori DNA is influenced by the number of bacteria in the tissue used as the genomic DNA source. The oligonucleotides employed in the PCR used in the present study for revealing the H. pylori 16S rRNA gene enabled the detection of 2.5 ng of the H. pylori DNA in 50 or 150 ng of human DNA and the distinction between the H. pylori 16S rRNA gene and Campylobacter spp. and the other isolated bacteria in the gastric mucosa.29 The use of multiplex PCR enabled the collection of the bacterial genotype in a shorter period of time, a reduced reagent expense, and more opportune delivery of the results to the patients.
In this study, we compared IFN-γ expression in patients with chronic gastritis and patients with gastric cancer that were either infected or not with H. pylori; we also compared the patients by different age groups and by groups infected with different bacterial genotypes. We found that 86.1% of the samples were IFN-γ-positive and despite the fact that there were no differences between IFN-γ expression and H. pylori infection in the two study groups, our analysis revealed lower IFN-γ expression in the gastric cancer group than in the chronic gastritis group, regardless of H. pylori infection. In contrast, in 2005 Lopes et al., in samples from Portuguese children and adolescents, and in 1998, Lindholm et al., in Swedish patients, reported that IFN-γ expression was higher in the samples of patients that were H. pylori-positive.9,13 Taking into consideration that chronic gastritis is an inflammatory process of varying magnitude that can give rise to gastric adenocarcinoma within a 10 to 15-year period of progression and that the intensity of the immune response undergoes changes as individuals get older, the number of cells with positive IFN-γ staining was analyzed with respect to patient age. The lack of statistically significant differences in regard to age may be related to the diversity and intensity of the inflammatory stimuli deriving from H. pylori, along with other factors related to patient lifestyle and the presence of other infectious agents such as the Epstein-Barr virus.
Through our analysis of IFN-γ expression, in accordance with the H. pylori vacA/cagA genotype, we found that the percentage of cells expressing the cytokine was lower in both groups when the subjects were infected with the less virulent genotype (vacAs2m2/cagA+), 69% in the gastritis patients and 70.5% in the gastric cancer patients; in the gastritis group there was higher IFN-γ expression (86.5%) in the H. pylori-positive vacAs1m2/cagA+ and vacAs1m1/cagA- patients; in the gastric cancer group there was a decrease in expression to 79 and 78% in H. pylori-positive vacAs1m1/cagA- and vacAs1m1/cagA+ patients, respectively. Similar findings were reported by Wang et al. in 2007 in a Chinese population; they found that there was a reduction in IFN-γ expression in patients with chronic gastritis and gastric cancer, infected with H. pylori cagA+, that occurred as the gastric lesion became more severe.16 In patients with chronic gastritis, the increased IFN-γ levels could contribute to gastric inflammation due to mononuclear phagocytic activation and to over-regulation of MHC-class I and class II molecular expression.13 In addition to playing an important role in the antitumor response, IFN-γ has also been reported to possibly have tumorigenic effects.36 In 2009, Sayi et al. reported that, in a murine model, IFN-γ produced by CD4+ T cells played an important role in H. pylori infection control but, on the other hand, induced pre-neoplastic changes in the gastric mucosa.11 High IFN-γ expression in the group of patients with gastric cancer can have different significations: a) it could be a good outcome factor, in accordance with the reports that IFN-γ can promote the elimination of neoplastic cells through its angiostatic action that restricts tumor growth by interfering with the blood supply,15 and b) it could be exerting a pro-tumor effect through proliferative and anti-apoptotic signals, and facilitating the escape of tumor cells from the cytolytic action of the NK cells and cytotoxic T lymphocytes.36 Patient follow-up is necessary in order to verify the significance of our findings.
In conclusion, IFN-γ expression varies depending on the H. pylori vacA and cagA genotype, but not in accordance with the presence of chronic gastritis or gastric cancer. Further studies are needed in order to determine whether IFN-γ expression could be a useful biomarker in gastric cancer prognosis.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that the procedures followed conformed to the ethical guidelines of the responsible committee on human experimentation and was in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors declare that they have followed the patient data publication protocols of their workplace and that all the patients included in the study received adequate information and signed statements of informed consent in order to participate in this study.
Right to privacy and informed consentThe authors have obtained statements of informed consent from the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Financial disclosureThis study received financial support from the Universidad Autónoma de Guerrero, 2013 Call for Proposals; from the SEP through the PIFI-2011, and from the High Quality Postgraduate Study Academic Strengthening Program, code I010/455/2013 C-677/2013.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the Laboratorio de Biología Celular del Cáncer of the Universidad Autónoma de Guerrero for their permission to photograph the immunohistochemistry, Dr. Miguel Ángel Mendoza Catalán for his aid in taking the images, and the personnel of the Endoscopic Service of the Hospital General «Raymundo Abarca Alarcón», the Unidad Especializada en Gastroenterología Endoscopia, and the Instituto Estatal de Cancerología «Arturo Beltrán Ortega».
Please cite this article as: Martínez-Carrillo DN, Atrisco-Morales J, Hernández-Pando R, Reyes-Navarrete S, Betancourt-Linares R, Cruz-del Carmen I, et al. Diversidad de los genotipos vacA y cagA de Helicobacter pylori y expresión de interferón gamma en pacientes con gastritis crónica y cáncer gástrico. Revista de Gastroenterología de México. 2014;79:220–228.