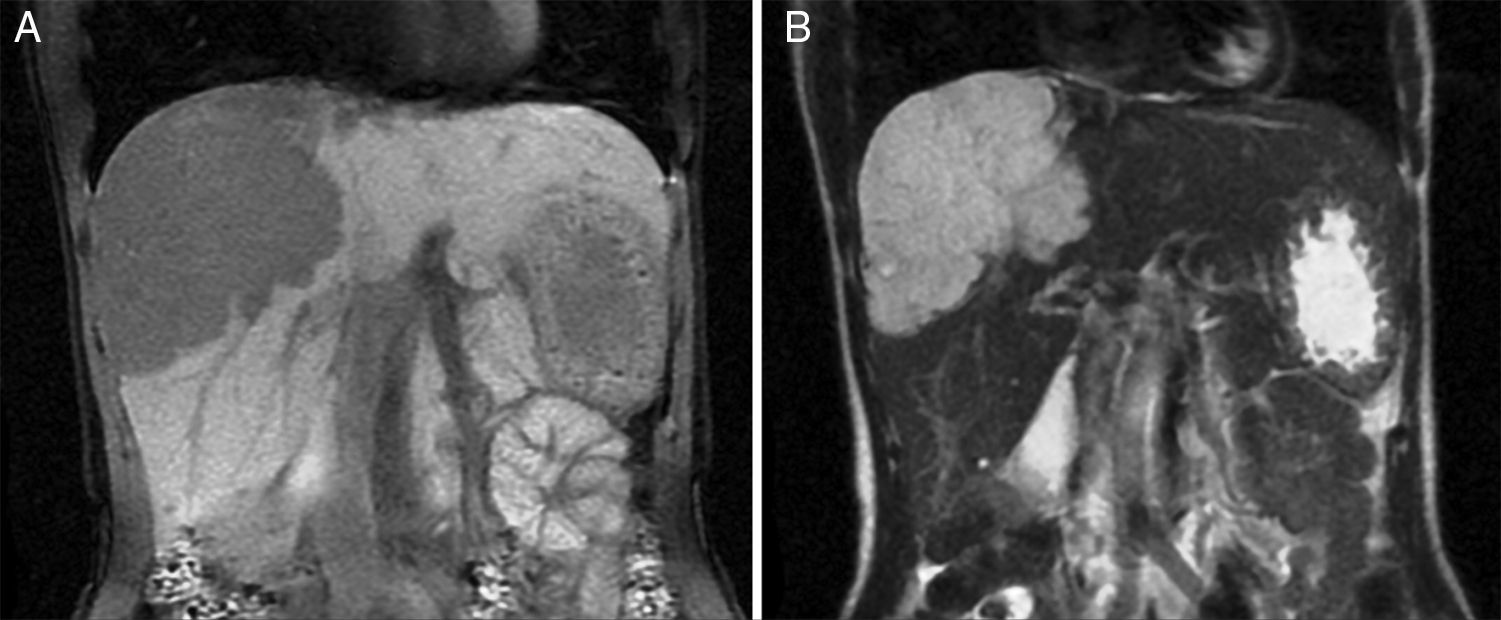
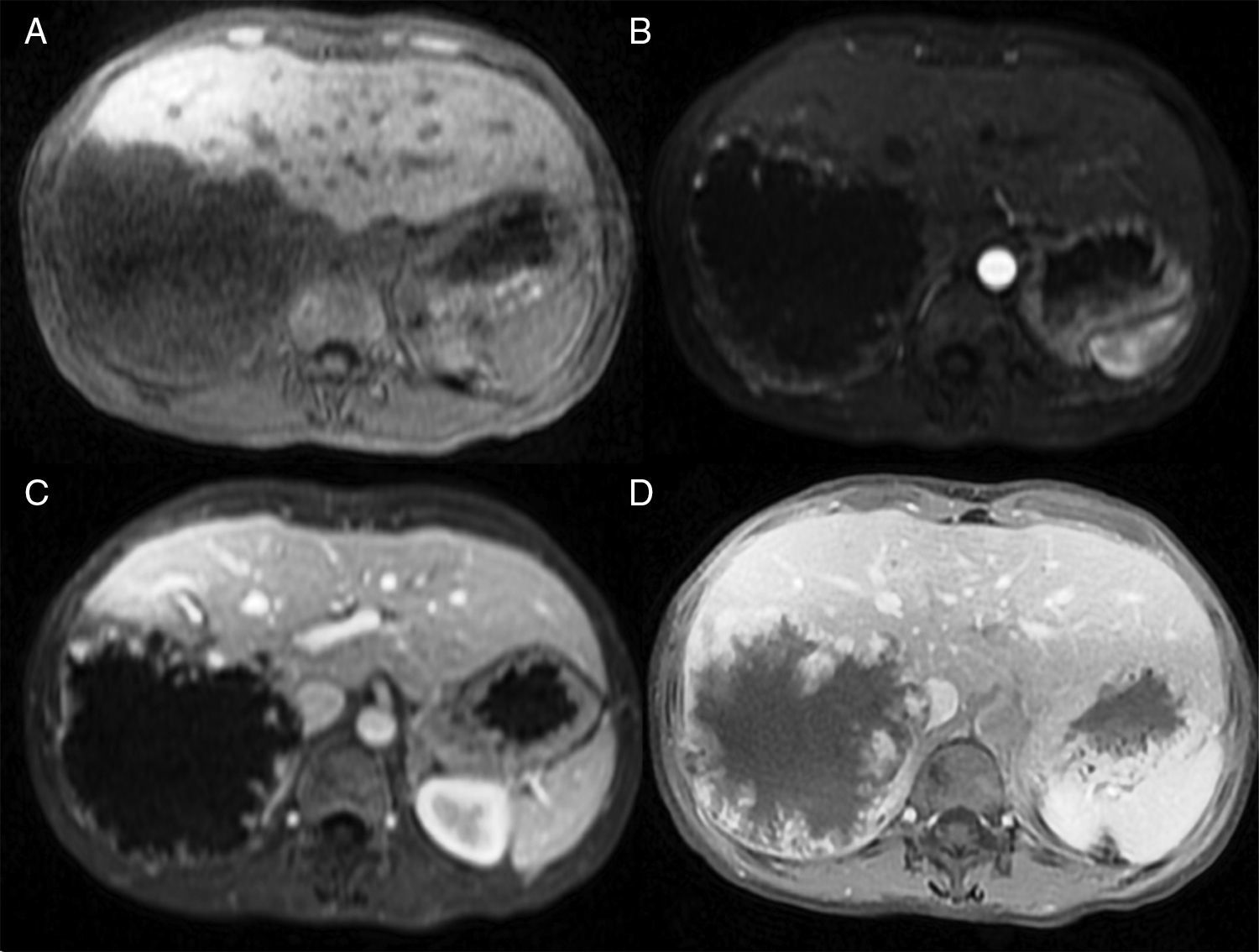
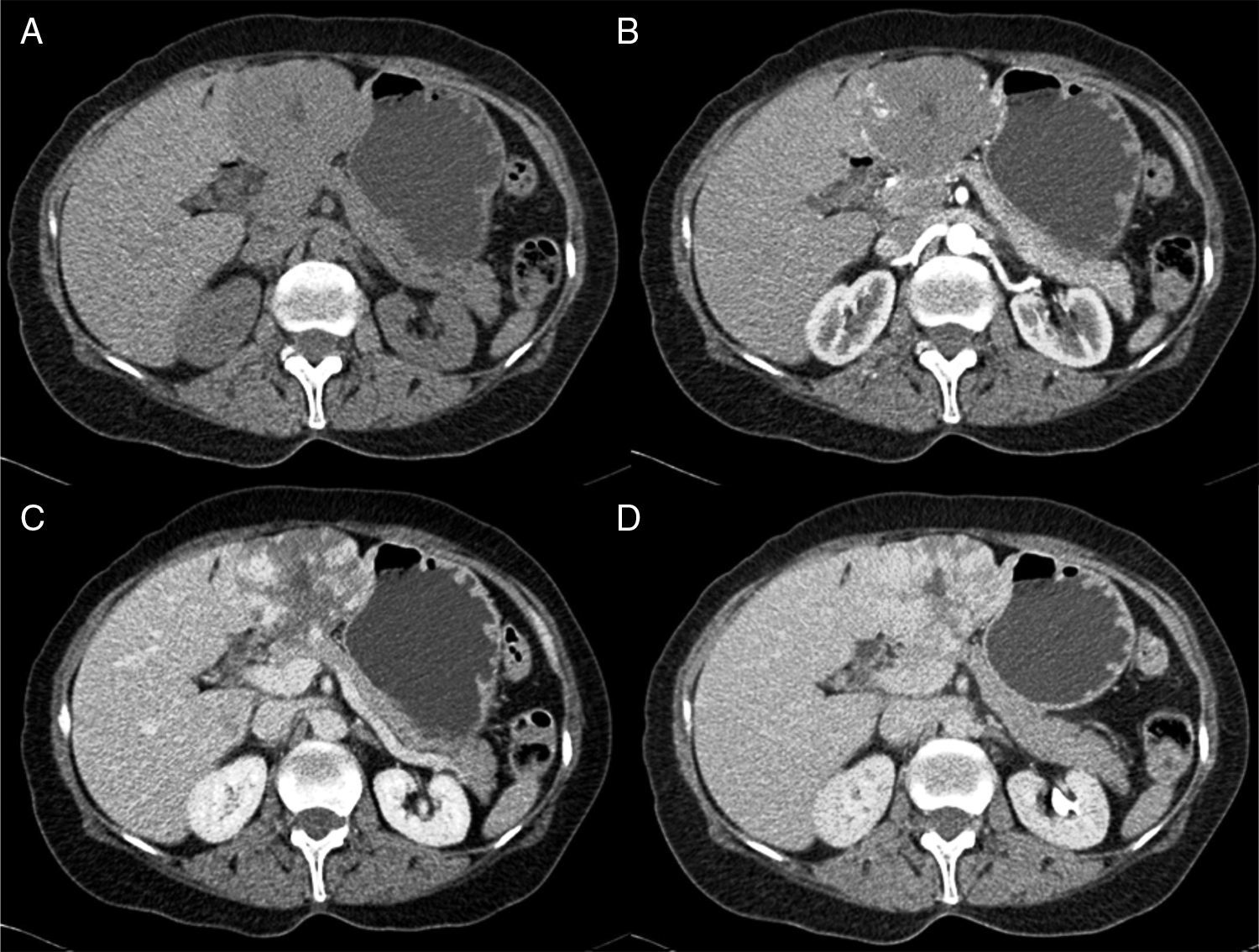
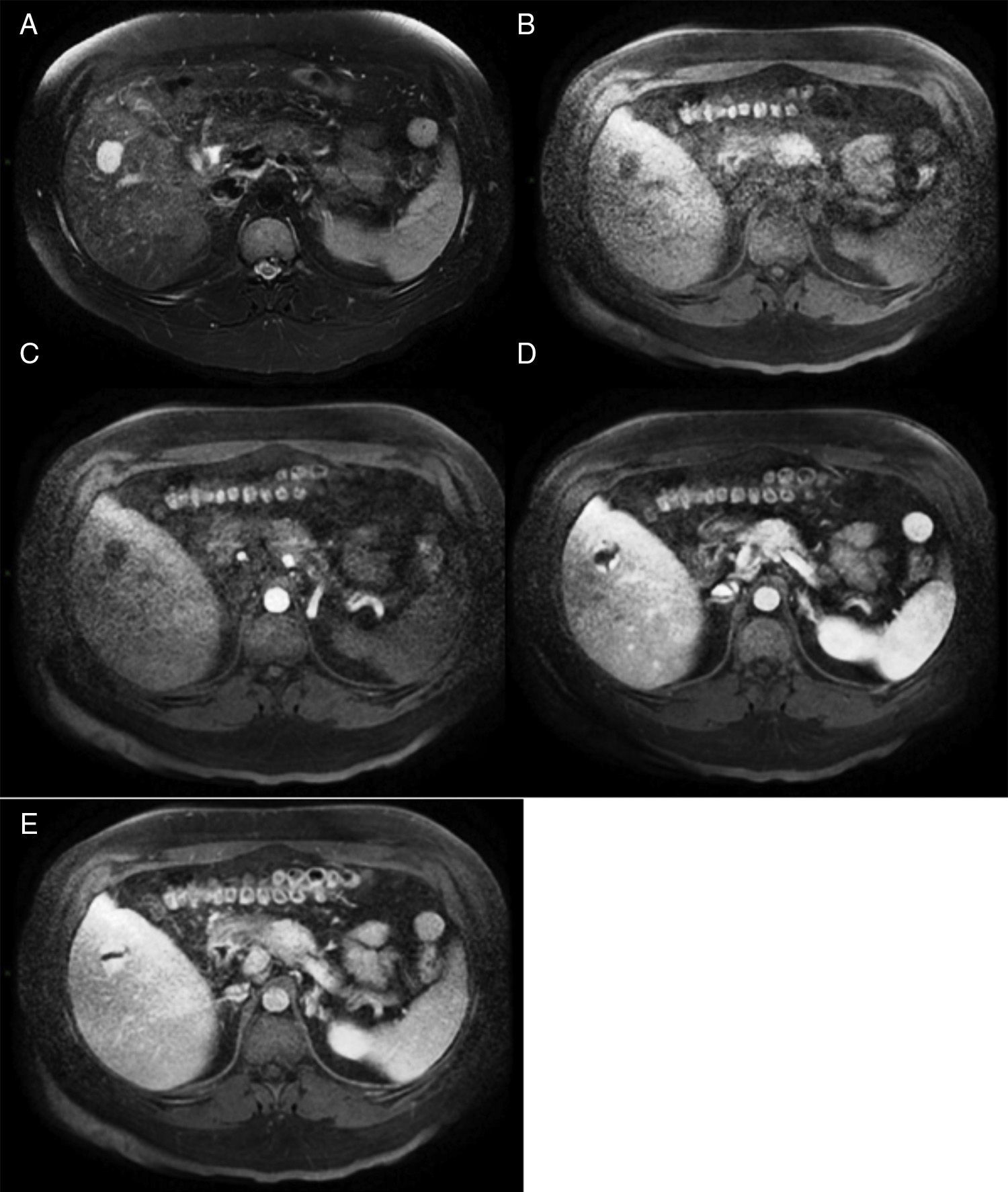
Giant hepatic hemangiomas (GHHs) are those that are larger than 4cm in size.
AimsThe aim of this study was to describe GHH clinical findings, their risk factors, diagnostic approach and management, and to compare these data with those of conventional hemangiomas.
MethodsWe performed a retrospective analysis of patients diagnosed with hemangiomas, whether by imaging studies or histopathology, at our hospital within the time frame of 1990-2008. The medical records of each patient were reviewed to obtain clinical and surgical data.
ResultsOf the 57 patients with liver hemangioma, 41 (72%) were women and 32 (56%) had GHH. Liver hemangioma median size was 4.49cm. In regard to the patients with GHH, 31.2% were asymptomatic and when symptoms presented, pain was the most common. Both symptoms and oral contraceptive exposure were more common in the GHH patients. Nine patients with GHH underwent surgery: 2 open biopsies due to diagnostic uncertainty, one enucleation, and 6 resections.
ConclusionsGHHs are more prevalent in women and when symptomatic, pain is the most frequent complaint. Diagnosis is usually made through imaging studies, but when there is diagnostic doubt, surgical exploration is sometimes needed. Oral contraceptive use is most likely more of a risk factor for GHH than for conventional hemangioma, but this association needs to be studied further.
Los hemangiomas hepáticos gigantes (HHG) son aquellos con un tamaño superior a 4cm.
ObjetivoEl objetivo de este estudio fue describir los hallazgos clínicos del HHG, sus factores de riesgo, el abordaje diagnóstico y el manejo, y comparar esta información con la del hemangioma convencional.
MétodosSe realizó un análisis retrospectivo del periodo 1990-2008 de pacientes con diagnóstico, sea por imagen o histopatología, de hemangioma hepático atendidos en nuestro hospital. De cada paciente se revisó su expediente para extraer información médica y/o quirúrgica.
ResultadosDe 57 pacientes con hemangioma hepático, 41 (72%) eran mujeres y 32 (56%) tenían un HHG. La mediana de tamaño fue de 4.49cm. Con respecto a los HHG, el 31.2% estaban asintomáticos; el síntoma más común fue dolor. Tanto los síntomas como el uso de anticonceptivos orales fueron más comunes en pacientes con HHG. Nueve pacientes con HHC fueron sometidos a cirugía: 2 biopsias abiertas por duda diagnóstica, una enucleación y 6 resecciones.
ConclusionesLos HHG son más prevalentes en mujeres y, cuando son sintomáticos, el dolor es la manifestación más frecuente. El diagnóstico habitualmente se hace por imagen, pero a veces se requiere abordaje quirúrgico por duda diagnóstica. El uso de anticonceptivos orales es probablemente un factor de riesgo de más peso para el HHG que para el hemangioma convencional; esta asociación necesita ser estudiada más a fondo.
Hepatic hemangiomas (HHs) are the most common benign hepatic tumors, with an estimated prevalence of 7% in autopsies and 1-20% in the general population.1–4 They are more common in women, probably as a result of an influence of female sex hormones on their growth.5 Giant hepatic hemangiomas (GHHs), defined as those HHs greater than 4cm in size, represent 10% of all HHs.
HHs are usually asymptomatic and found incidentally.6 Symptoms have been reported in up to 40% of patients with a GHH in some case series, mainly abdominal pain, but also symptoms related to a mass effect such as early satiety, nausea, vomiting, cholestasis, or even cough.7–10 Less common manifestations are chylous ascites, hemoperitoneum due to spontaneous rupture, and Kasabach-Merritt syndrome, in which the HH is associated with thrombocytopenia and intravascular coagulation.6,11–13
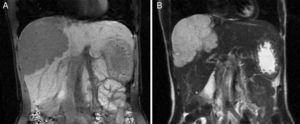
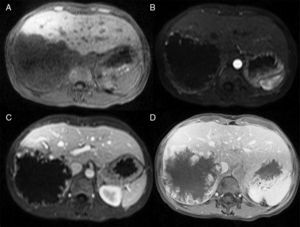
HHs are suspected when ultrasonography shows nodules with homogeneous hyperechogenicity, but additional imaging tests, usually triphasic computed tomography (TCT) or magnetic resonance imaging (MRI), are required to confirm the diagnosis due to the lack of specificity of ultrasonographic findings. TCT typically reveals progressive enhancement during the arterial phase in a centripetal fashion. MRI shows a low signal intensity on T1-weighted images, high signal intensity on T2-weighted sequences, and an inward enhancement after gadolinium administration can be seen.14 Tc-99m-labeled red blood cell single emission photon computed tomography may be helpful when there is diagnostic uncertainty; it has sensitivity, specificity and accuracy of 97%, 83%, and 96%, respectively.15 Positron emission tomography is useful to differentiate heterogeneous HH from angiosarcomas.16 The differential diagnosis includes angiomatosis, metastases, hematic cyst, hepatic peliosis, and hepatocellular carcinoma, among others.
Treatment options of HH, including GHH, are observation, enucleation, hepatic resection, and transcatheter arterial embolization; recently, enhanced radiofrequency ablation has been used to treat GHH, with good results.17 Clinical observation is recommended for most patients, active treatment should be reserved for patients with severe symptoms or disease-associated complications.18 Surgical indications include abdominal pain, mass effect, Kasabach-Merritt syndrome, increased hemangioma size during follow-up, and equivocal results during radiological evaluation leading to uncertainty of diagnosis. Regarding surgery, there are 2 different strategies: enucleation and hepatic resection. The most important factors associated with surgical complications are tumor size and the presence of symptoms.19 Enucleation is a better option because resection is associated with a longer hospital stay, greater blood loss, and transfusion requirements.20–23 Preoperative percutaneous transcatheter arterial embolization is useful in reducing the risk of intraoperative bleeding.24 Embolization with bleomycin and lipiodol is a useful alternative when HHs are unfit for surgery.25
The aim of this study was to describe the experience regarding diagnosis and management of giant hemangiomas at our hospital, and compare these data with those of conventional hemangiomas.
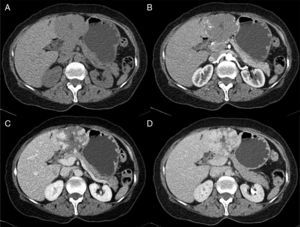
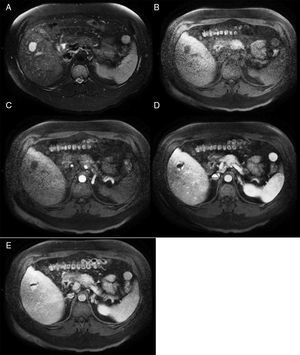
MethodsWe performed a retrospective analysis of patients with HH at our hospital within the time frame of January 1, 1990 and November 31, 2008. We made an electronic search regarding outpatient and inpatient hospital records looking for the diagnosis of hemangioma and/or benign hepatic tumor, which corresponded to the D 18.0 and D 13.4 categories of the CIE 10, respectively. We included all patients with GHH and HH, and diagnosis was based on the presence of conclusive imaging findings on TCT or MRI (peripheral nodular enhancement in the early phase followed by centripetal enhancement during the late phase) (figs. 1-4) or on the histopathology report. Medical records were reviewed for each patient to obtain demographic, clinical, radiological, and laboratory information, evolution, and treatment; if surgery was performed, data regarding the type of surgery, duration, complications, and transfusion requirements were recorded. Pain due to HH was defined as persistent pain in the upper right abdomen that could not be better explained by an alternative diagnosis, including irritable bowel syndrome.
A Siemens Sonoline Antares, Phillips, or General Electric Logiq 9 US device was used, with convex probes from 1 to 7MHz. The exploration protocol at our institution begins with the left hepatic lobe and then the right hepatic lobe, looking for focal lesions; if a focal lesion is found, it has to be demonstrated in 2 planes, measured, and then color Doppler is applied to assess vascularity.
Triphasic Computed TomographyA 16 or a 64-slice multidetector CT (Somatom, Sensation 16 or 64; Siemens Munich, Germany) was used; images were obtained with a section thickness of 3-5mm and a reconstruction interval of 2-2.5mm. All cases were analyzed on a workstation with the capacity to produce coronal reformatted images. All patients received both intravenous and oral contrast. For intravenous contrast, 120ml of Conray (Mallinckrodt Baker Inc., St Louis Missouri, USA) was given 45 s prior to performing the scan; for oral contrast, 40ml of Ioditrast M60 (Justesa Imagen Mexicana) were diluted in 1,000ml of water and given to all patients orally one hour prior to computed tomography. All TCT images were analyzed by at least 2 certified radiologists.
Magnetic resonance imagingMagnetic resonance imaging (MRI) was performed on a 1.5 T system (Signa Excite HD, GE Healthcare, Milwaukee, USA), using a variety of software upgrades that evolved during the study period. Standard liver imaging sequences included T1-weighted In-phase and Opposed-phase gradient echo and T2-weighted fast spin echo sequences. T1-weighted imaging was repeated after contrast material administration during hepatic arterial (19-25 s), portal (40-45 s), venous (60-65 s) and delayed (3-5min) phases. Patients received Gadopentate Dimeglumine (Gd-DTPA [Magnevist, Bayer Schering Pharma]) at a mean dose of 0.1 mmol/kg of body weight, a unique bolus at a 1.5 - 2ml/s rate, followed by a saline flush (mean volume 20ml).
Statistical AnalysisExcept for age, all variables had a non-normal distribution and non-parametric statistical analyses were employed. Categorical variables were described using frequencies and percentages and continuous variables were summarized using the median and interquartile range. Comparisons between groups were made with the chi-square test or Fisher's exact test. Data analysis was done with SPSS v17.
ResultsA total of 57 patients with HH were evaluated and 72% were women. The mean age was 46.7±11.5 years and it was not significantly different in patients with or without GHH. Thirty-two patients had a GHH. Of the 96 lesions included in the analysis, 32 were GHH and 64 were conventional hemangiomas. Thirteen patients with GHH had an additional one or more conventional hemangiomas, and 7 patients without GHH had more than one lesion. The median size of the hemangiomas was 4.49cm, ranging from 1-11cm. The median number of lesions was 1.68 hemangiomas for each patient, ranging from 1 to 10. GHH distribution was as follows: right lobe in 18 patients (56%), left lobe in 7 patients (22%), and both lobes in 7 patients (22%). Absence of alcohol consumption was more common in patients without GHH (p=0.003, OR 3.8 CI 2.7-5.4), while previous or present contraceptive use was more common in patients with GHH (p=0.001, OR 1.45 CI 1.29-1.7).
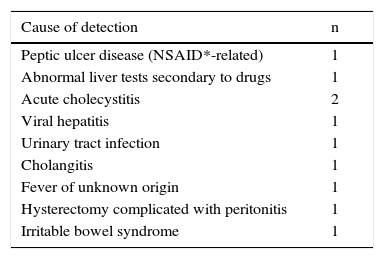
Ten patients with GHH (31.2%) were asymptomatic and it was an incidental finding; in the rest of them, pain was the most common symptom and was found in 11 patients (34.4%), followed by 3 patients (9.4%) with an increase in abdominal perimeter. One patient developed cholangitis because the GHH compressed the common bile duct. Table 1 shows the diagnosis that led to the discovery of the GHH in the cases in which it was an incidental finding. Symptoms were more common in patients with a GHH (p=00.003). When we divided the HHs into those bigger and smaller than 3cm, rather than 4cm, there was no significant difference in relation to the presence of symptoms between the 2 groups (p=0.09).
Diagnosis which led to the incidental finding of giant hepatic hemangiomas.
| Cause of detection | n |
|---|---|
| Peptic ulcer disease (NSAID*-related) | 1 |
| Abnormal liver tests secondary to drugs | 1 |
| Acute cholecystitis | 2 |
| Viral hepatitis | 1 |
| Urinary tract infection | 1 |
| Cholangitis | 1 |
| Fever of unknown origin | 1 |
| Hysterectomy complicated with peritonitis | 1 |
| Irritable bowel syndrome | 1 |
NSAID: nonsteroidal anti-inflammatory drug.
Diagnosis of GHH was initially made with ultrasound in 29 patients and with TCT in the other 3. Diagnosis was confirmed with TCT or MRI in all cases except for one patient in whom hepatic resection was performed after the diagnostic ultrasound was made. Liver function tests were normal in all patients.
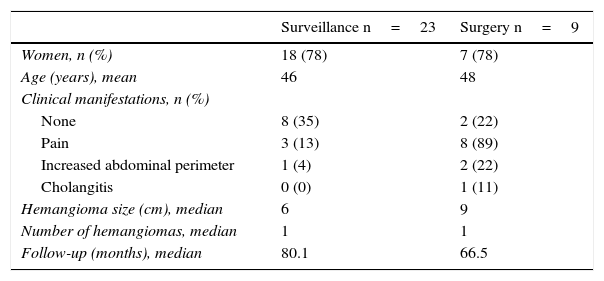
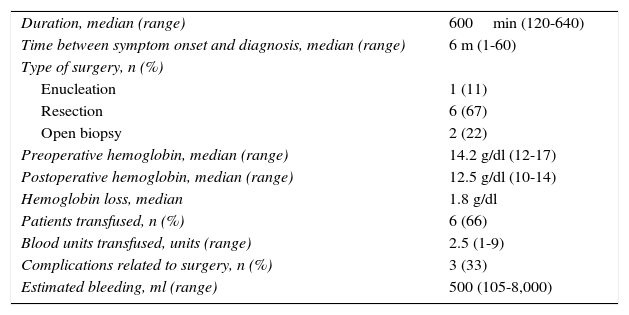
Surveillance was indicated in 23 (72%) of the patients with GHH. Three of them had pain, but did not undergo surgery; the pain was consistent with a functional disorder and disappeared with antispasmodics in 2 of those patients, and one did not accept the surgical procedure. Surgery was performed in the other 9 patients (28%). Two of them were asymptomatic and the indication was to perform an open biopsy because of diagnostic uncertainty after a thorough radiological evaluation; in both cases the radiologists could not exclude a metastasis of an unknown primary. Table 2 shows the characteristics of the patients assigned to surveillance and those treated with surgery. Three patients had surgery-related complications. One of them developed a hepatic hematoma leading to hypovolemic shock, and the other 2 had right pleural effusion, both of which were resolved with a chest tube and negative pressure. No mortality related to surgery was observed in the long-term follow-up (66.5±58 months). Table 3 shows important information regarding the surgical procedures.
Characteristics of patients according to treatment.
| Surveillance n=23 | Surgery n=9 | |
|---|---|---|
| Women, n (%) | 18 (78) | 7 (78) |
| Age (years), mean | 46 | 48 |
| Clinical manifestations, n (%) | ||
| None | 8 (35) | 2 (22) |
| Pain | 3 (13) | 8 (89) |
| Increased abdominal perimeter | 1 (4) | 2 (22) |
| Cholangitis | 0 (0) | 1 (11) |
| Hemangioma size (cm), median | 6 | 9 |
| Number of hemangiomas, median | 1 | 1 |
| Follow-up (months), median | 80.1 | 66.5 |
Characteristics of the surgical procedures (n=9).
| Duration, median (range) | 600min (120-640) |
| Time between symptom onset and diagnosis, median (range) | 6 m (1-60) |
| Type of surgery, n (%) | |
| Enucleation | 1 (11) |
| Resection | 6 (67) |
| Open biopsy | 2 (22) |
| Preoperative hemoglobin, median (range) | 14.2 g/dl (12-17) |
| Postoperative hemoglobin, median (range) | 12.5 g/dl (10-14) |
| Hemoglobin loss, median | 1.8 g/dl |
| Patients transfused, n (%) | 6 (66) |
| Blood units transfused, units (range) | 2.5 (1-9) |
| Complications related to surgery, n (%) | 3 (33) |
| Estimated bleeding, ml (range) | 500 (105-8,000) |
Thirty-two patients in our study presented with GHH. Twenty-three of them were candidates for surveillance strategy and the remaining 9 underwent surgical treatment. Most of our patients were women in their mid-forties, which concurred with previous studies. Ten patients were asymptomatic and GHH was diagnosed incidentally, as a result of an imaging study indicated for another reason. In symptomatic patients, the most frequent symptom was pain. It is important to mention that persistent pain in the upper right abdomen that could not be better explained by any other diagnosis was attributed to the GHH. However, the pain was nonspecific and because there is no gold standard to diagnose hemangioma-induced pain, there is a possibility that in some cases this symptom could be due to another pathology.
Most authors consider GHHs as those larger than 4cm, while others define them as larger than 5cm. When we analyzed symptom presentation of the HHs that were bigger and smaller than 3cm we did not find a significant difference, so we feel the best cutoff point is 4cm. In contrast to other case series, GHHs represented a greater proportion of the HHs at our institute. A possible explanation to this is that our hospital is a referral center and smaller hemangiomas are usually diagnosed and/or treated elsewhere.
Compared to conventional HH, GHHs were more common in female patients who had been previously exposed to oral contraceptives and in patients who said they had no prior or present alcohol consumption. Concerning these risk factors, neither alcohol consumption nor oral contraceptive use was quantified (for example, number of years using oral contraceptives or drinking pattern) in order to analyze this association more carefully. In some studies, oral contraceptive use has been found to be a risk factor for GHH, but in others this association has not been encountered, while alcohol abuse has been proposed as a risk factor for HH in another case series. We know that HHs are more common in women, suggesting an effect of female sex hormones, but this relation is unclear. For example, there are cases of hemangiomas that have grown during pregnancy and that were negative for estrogen and progesterone receptors.5,26–29
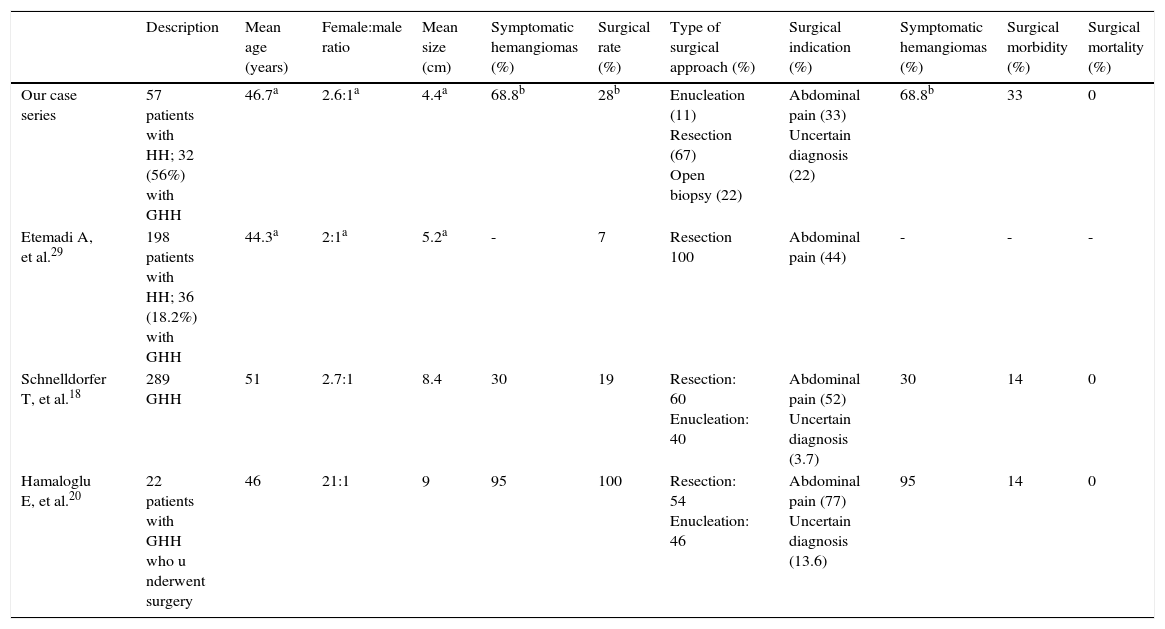
Even though enucleation is associated with less morbidity, hepatic resection was the procedure of choice in all the patients, except for one.20–22 This can be explained by the fact that treatment decisions often reflect the experience of the surgeon. Six patients required blood transfusions; one of them developed hypovolemic shock and had to be transferred to the intensive care unit. Mayor morbidity related to the surgical procedure and transfusion rate was slightly increased when compared with other case series.19,20,23,30 Patients in the surveillance group developed no symptoms during follow-up, supporting the benign course of this entity, regardless of hemangioma size.18Table 4 compares our results with other case series in relation to mean age, size, number of symptomatic hemangiomas, surgical rate, and type of approach, as well as morbidity and mortality associated with surgery.
Comparison with other case series.
| Description | Mean age (years) | Female:male ratio | Mean size (cm) | Symptomatic hemangiomas (%) | Surgical rate (%) | Type of surgical approach (%) | Surgical indication (%) | Symptomatic hemangiomas (%) | Surgical morbidity (%) | Surgical mortality (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Our case series | 57 patients with HH; 32 (56%) with GHH | 46.7a | 2.6:1a | 4.4a | 68.8b | 28b | Enucleation (11) Resection (67) Open biopsy (22) | Abdominal pain (33) Uncertain diagnosis (22) | 68.8b | 33 | 0 |
| Etemadi A, et al.29 | 198 patients with HH; 36 (18.2%) with GHH | 44.3a | 2:1a | 5.2a | - | 7 | Resection 100 | Abdominal pain (44) | - | - | - |
| Schnelldorfer T, et al.18 | 289 GHH | 51 | 2.7:1 | 8.4 | 30 | 19 | Resection: 60 Enucleation: 40 | Abdominal pain (52) Uncertain diagnosis (3.7) | 30 | 14 | 0 |
| Hamaloglu E, et al.20 | 22 patients with GHH who u nderwent surgery | 46 | 21:1 | 9 | 95 | 100 | Resection: 54 Enucleation: 46 | Abdominal pain (77) Uncertain diagnosis (13.6) | 95 | 14 | 0 |
GHH: Giant hepatic hemangioma; HH: Hepatic hemangioma.
Our study has several limitations and one of them is its retrospective design. Imaging tools for increasing sensitivity and specificity of the radiological evaluation of hepatic lesions have evolved since 1990; therefore, we have no way of knowing if surgery could have been spared in the 2 cases of diagnostic uncertainty after radiological evaluation, had they been reevaluated with these more recent tools.15 In addition, we could not compare enucleation vs hepatic resection because only one patient was assigned to the former. Moreover, as previously mentioned, oral contraceptive use and alcohol consumption were not objectively measured. Finally, we cannot exclude referral bias, the fact that the majority of HHs were GHHs and that most of them were symptomatic, could be the reason they were referred to our hospital.
In conclusion, GHHs are more frequent in women, many of them are asymptomatic, and when they are symptomatic, pain is the most frequent complaint. As imaging techniques have evolved, diagnosis is usually made in terms of radiological studies. However, in a minority of cases, diagnostic uncertainty remains and surgical exploration may be needed. Oral contraceptive use is probably more a risk factor for the development of GHH than for conventional HH, while alcohol consumption seems to be less frequent in GHH patients. Nevertheless, these associations need to be studied further.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Moctezuma-Velázquez C, López-Arce G, Martínez-Rodríguez LA, Escalona-Huerta C, Chapa-Ibargüengoitia M, Torre A. Hemangioma hepático gigante versus hemangioma hepático convencional: características clínicas, factores de riesgo y manejo. Revista de Gastroenterología de México. 2014;79:229–237.