Autoimmune hepatitis (AIH) is a chronic inflammatory disease of the liver with nonspecific clinical manifestations that causes greater liver damage in children than in adults.
AimsTo analyze the clinical progression, biochemical profiles, histopathologic changes, and treatment response in 20 children with AIH.
Material and methodsA retrospective study was carried out on the variables associated with clinical progression, diagnosis, and treatment response in children seen at the the Unidad Médica de Alta Especialidad (UMAE) No. 71 IMSS in Torreón, Coahuila, Mexico, from 1992 to 2012.
ResultsTwenty patients were analyzed, 75% with type 1 AIH (AIH-1) and 25% with type 2 AIH (AIH-2). Girls predominated with a 3:1 ratio of girls to boys. The mean age was 10.07 ± 6.53 years for the AIH-1 cases and 6.75 ± 3.77 years for the AIH-2 cases. There was an association with immunologic diseases in 40% of the patients.
The patients in the AIH-2 group had greater biochemical profile alterations and IgA deficiency. Anti-nuclear antibody and anti-smooth muscle antibody were positive in 100% of the patients with AIH-1, and anti-liver kidney microsomal type 1 antibody was positive in 100% of the AIH-2 patients. Liver biopsy revealed interface hepatitis in both groups. The AIH-2 group responded more quickly to treatment, but had a higher recurrence rate.
ConclusionsAutoimmune hepatitis in the pediatric patient should be suspected in order to make an early diagnosis and thereby establish opportune treatment. Determining the type of AIH is necessary for making adequate diagnosis and for achieving a better outcome in relation to recurrence and complication rates.
La hepatitis autoinmune (HAI) es una enfermedad inflamatoria crónica del hígado con manifestaciones clínicas inespecíficas y mayor daño hepático en niños que en adultos.
ObjetivoAnalizar la evolución clínica, los perfiles bioquímicos, los cambios histopatológicos y la respuesta al tratamiento de 20 niños con HAI.
Material y métodosEstudio retrospectivo de las variables asociadas a evolución clínica, diagnóstico y respuesta al tratamiento en niños atendidos en la UMAE n.° 71 IMSS Torreón, Coahuila, México, en el período comprendido entre 1992 y 2012.
ResultadosSe analizó a 20 pacientes, el 75% con HAI-1 y el 25% con HA1-2. Se observó predominio del sexo femenino 3:1. La edad promedio ± desviación estándar fue de 10.07 ± 6.53 años para HAI-1 y de 6.75 ± 3.77 para HAI-2. La asociación a enfermedades inmunológicas fue del 40%.
Los pacientes del grupo HAI-2 mostraron mayores alteraciones en su perfil bioquímico y deficiencia de IgA. La positividad para anticuerpos antinucleares y anticuerpos antimúsculo liso fue del 100% en los pacientes con HAI-1, anticuerpos antimicrosomales para hígado y riñón tipo 1 en el 100% de HAI-2. Las biopsias hepáticas mostraron hepatitis de interfase en ambos grupos. Los pacientes del grupo HAI-2 respondieron más rápidamente al tratamiento y tuvieron mayor tasa de recaídas.
ConclusionesEs necesario sospechar la HAI en pediatría para poder realizar un diagnóstico temprano y así establecer el tratamiento oportuno. Determinar el tipo de HAI nos permitirá establecer el diagnóstico adecuado y elaborar un mejor pronóstico respecto a tasas de recaídas y complicaciones.
Autoimmune hepatitis (AIH) is a chronic inflammatory process involving cell destruction and fibrosis. It predominantly affects women and is characterized by an increase in transaminases and immunoglobulins, in particular immunoglobulin G (IgG).
AIH has 2 main variants: type 1 (AIH-1) and type 2 (AIH-2). AIH-1 is the most common form, affecting children and adults, and is characterized by the presence of anti-nuclear antibody (ANA) and anti-smooth muscle antibody (SMA). AIH-2 mainly affects children and young adults and is characterized by the presence of anti-liver/kidney microsomal type 1 antibody (LKM-1). The diagnostic criteria for AIH were published by the International Autoimmune Hepatitis Group in 1993,1 in 1999,2 and simplified in 2008.3,4
These criteria include: the exclusion of infectious viral processes, antibody profiles (ANA, SMA, LKM-1), IgG levels 1.10-fold above the normal limits, and a histologic pattern of interface hepatitis with plasma cells and rosette formation. Acute AIH presents in children and young adults and is more aggressive than in older adults.5,6
AimsThe aim of this study was to analyze the clinical progression, biochemical profiles, histopathologic changes, and treatment response in 20 children presenting with AIH.
MethodsTwenty children diagnosed with AIH that were seen at the outpatient service of the Department of Gastroenterology and Pediatric Nutrition of the Advanced Speciality Medical Unit No. 71 of the Instituto Mexicano del Seguro Social within the time frame of 1992 and 2012 were studied. In each case, the disease was classified in accordance with the criteria of the International Autoimmune Hepatitis Group (IAIHG) and reclassified with the 2008 simplified diagnostic criteria, and the data of the following variables were collected: age, sex, type of clinical presentation, association with immunologic diseases, clinical manifestations, liver function tests, serum albumin, INR, serum immunoglobulins, liver biopsy findings, progression time, treatment, recurrences, and complication frequency.
Statistical analysis: the results were analyzed using the Student's t test and the Wilcoxon test for determining the mean differences. Statistical significance was set at a value of p ≤ 0.05 (SPSS version 17.0 statistical software, Chicago, IL, USA).
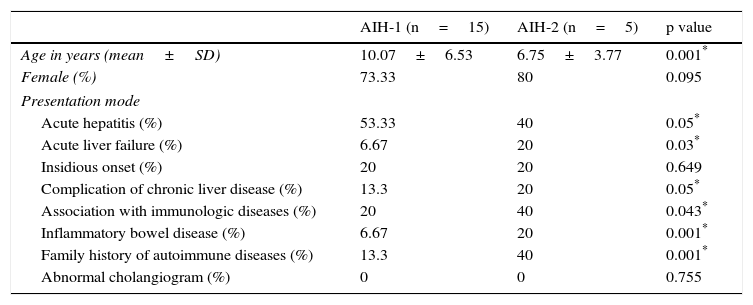
ResultsOf the 20 patients analyzed, 15 (75%) presented with AIH-1 and 5 presented with AIH-2. The population was made up of 15 girls and 5 boys, with a 3:1 proportion. The mean age was 10.07±6.53 in the patients with AIH-1 and 6.75±3.77 in the patients with AIH-2. The main presentation mode was acute hepatitis in the two groups, although the frequency was slightly higher in the AIH-1 group. There was a greater frequency of other immunologic diseases in the AIH-2 group, such as thyroiditis and ulcerative colitis (UC) (Table 1).
Clinical findings in the presentation of AIH-1 and AIH-2.
| AIH-1 (n=15) | AIH-2 (n=5) | p value | |
|---|---|---|---|
| Age in years (mean±SD) | 10.07±6.53 | 6.75±3.77 | 0.001* |
| Female (%) | 73.33 | 80 | 0.095 |
| Presentation mode | |||
| Acute hepatitis (%) | 53.33 | 40 | 0.05* |
| Acute liver failure (%) | 6.67 | 20 | 0.03* |
| Insidious onset (%) | 20 | 20 | 0.649 |
| Complication of chronic liver disease (%) | 13.3 | 20 | 0.05* |
| Association with immunologic diseases (%) | 20 | 40 | 0.043* |
| Inflammatory bowel disease (%) | 6.67 | 20 | 0.001* |
| Family history of autoimmune diseases (%) | 13.3 | 40 | 0.001* |
| Abnormal cholangiogram (%) | 0 | 0 | 0.755 |
Chi-square test, Fisher exact test.
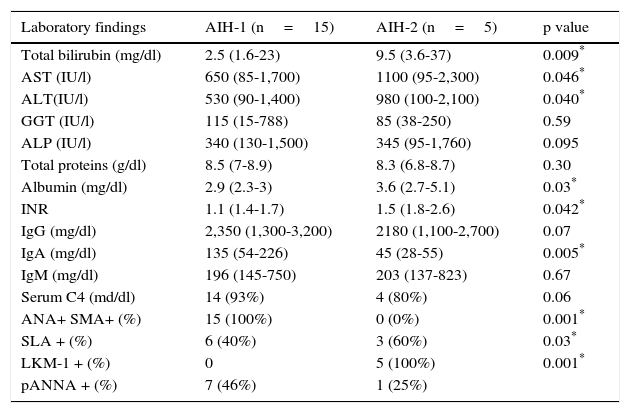
The biochemical profile included the determination of total bilirubin (TB), direct bilirubin (DB), indirect bilirubin (IB), aminotransferases (AST, ALT, GGT), alkaline phosphatase (ALP), total proteins, albumin and globulin, the prothrombin time (PT) and international normalized ratio (INR) coagulation tests. The immunologic profile was carried out and IgG, IgM, IgA, complement component 4 (C4), anti-nuclear antibodies (ANA), anti-smooth muscle antibodies (SMA), anti-liver/kidney microsomal type 1 antibodies (LKM-1) were also determined.
All the cases of AIH-1 were positive for ANA and/or SMA, and all the AIH-2 patients were positive for LKM-1. Other antibodies such as the anti-soluble liver antigen (SLA) antibody were positive in both groups, and predominated in the AIH-2 patients at 60% (Table 2).
Immunologic and biochemical findings in the presentation of AIH-1 and AIH-2.
| Laboratory findings | AIH-1 (n=15) | AIH-2 (n=5) | p value |
|---|---|---|---|
| Total bilirubin (mg/dl) | 2.5 (1.6-23) | 9.5 (3.6-37) | 0.009* |
| AST (IU/l) | 650 (85-1,700) | 1100 (95-2,300) | 0.046* |
| ALT(IU/l) | 530 (90-1,400) | 980 (100-2,100) | 0.040* |
| GGT (IU/l) | 115 (15-788) | 85 (38-250) | 0.59 |
| ALP (IU/l) | 340 (130-1,500) | 345 (95-1,760) | 0.095 |
| Total proteins (g/dl) | 8.5 (7-8.9) | 8.3 (6.8-8.7) | 0.30 |
| Albumin (mg/dl) | 2.9 (2.3-3) | 3.6 (2.7-5.1) | 0.03* |
| INR | 1.1 (1.4-1.7) | 1.5 (1.8-2.6) | 0.042* |
| IgG (mg/dl) | 2,350 (1,300-3,200) | 2180 (1,100-2,700) | 0.07 |
| IgA (mg/dl) | 135 (54-226) | 45 (28-55) | 0.005* |
| IgM (mg/dl) | 196 (145-750) | 203 (137-823) | 0.67 |
| Serum C4 (md/dl) | 14 (93%) | 4 (80%) | 0.06 |
| ANA+ SMA+ (%) | 15 (100%) | 0 (0%) | 0.001* |
| SLA + (%) | 6 (40%) | 3 (60%) | 0.03* |
| LKM-1 + (%) | 0 | 5 (100%) | 0.001* |
| pANNA + (%) | 7 (46%) | 1 (25%) |
Chi-square test, Fisher exact test.
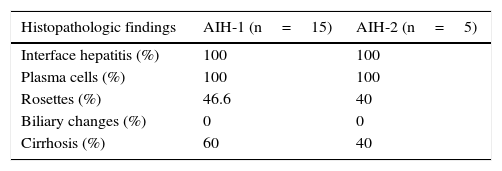
A liver biopsy was taken and interface hepatitis with plasma cells was observed in all the cases. Rosette formation and cirrhosis were more frequent in the AIH-1 group. No changes in the biliary ducts were observed in any of the cases (Table 3).
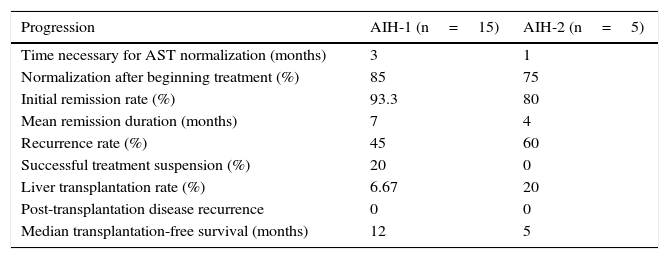
The patients in the AIH-2 group responded to treatment more quickly, but the recurrence rate was higher. The patients in the AIH-1 group had a higher remission rate and for a longer period of time than the AIH-2 patients (Table 4).
Treatment response and progression in the presentation of AIH-1 and AIH-2.
| Progression | AIH-1 (n=15) | AIH-2 (n=5) |
|---|---|---|
| Time necessary for AST normalization (months) | 3 | 1 |
| Normalization after beginning treatment (%) | 85 | 75 |
| Initial remission rate (%) | 93.3 | 80 |
| Mean remission duration (months) | 7 | 4 |
| Recurrence rate (%) | 45 | 60 |
| Successful treatment suspension (%) | 20 | 0 |
| Liver transplantation rate (%) | 6.67 | 20 |
| Post-transplantation disease recurrence | 0 | 0 |
| Median transplantation-free survival (months) | 12 | 5 |
The initial established treatment was prednisone (PDN) at a dose of 2mg/kg/day, with a maximum dose of 60mg per day. This treatment lasted a mean of 8 weeks and was given until achieving a decrease of at least 80% in the serum levels of AST and ALT from their initial values.
The steroids were then progressively reduced until the aminotransferases were normalized. When this goal was achieved, a maintenance dose of PDN of 5mg daily was established. If the goal was not reached, azathioprine (AZA) at 0.5-2.5mg/kg/day was given until AST was normalized. A maintenance dose of AZA at 0.5mg/kg/day was continued. If there was response failure or signs of toxicity, mycophenolate mofetil at 20mg/kg was started. Cyclosporine or tacrolimus was used when there was a lack of response or when there was toxicity, which occurred in 6.67% (n=1) of the AIH-1 cases and in 20% (n=1) of the AIH-2 cases. These 2 patients underwent liver transplantation.
DiscussionThe definitive diagnosis for AIH is made based on clinical, laboratory, and histopathologic criteria that have been defined by the IAIHG.
Some of these criteria are directly related to the diagnosis and some, such as partial IgA deficiency observed in AIH-2 patients, have been associated with worse outcome.7
Even though autoantibodies are an essential part of the AIH diagnosis, ANA, SMA, and LKM-1 are not detected at the time of clinical presentation in 10 to 15% of the patients, but they become detectable later; they remain negative in only 5% of the patients.8–10 In these cases the presence of the other criteria, such as an increased IgG level and histopathologic changes observed in the liver biopsies,11 are diagnostic determinants.
In the diagnostic criteria, titers of 1:40 for ANA, SMA, and LKM-1 have been established as positive; this is applicable to adults, but for children a titer of 1:20 for ANA and SMA and of 1:10 for LKM-1 are sufficient.12 We found SLA positivity in both AIH groups, with a predominance in the AIH-2 group. This is related to a more severe disease course and a greater recurrence tendency,13 as was seen in the AIH-2 patients and in the two patients that required liver transplantation. In order to make the definitive diagnosis, a liver biopsy is required, along with taking into account the abovementioned histopathologic criteria with a high specificity of 81-99%, but a low sensitivity of 36-57%, and a positive predictive value (PPV) of 62-91%.14 In a blinded study for pathologists, Kumari N et al.15 compared liver biopsies of autoimmune and non-autoimmune liver disease. They found: interface hepatitis, lymphoplasmacytic portal infiltrate, emperipolesis, and rosette formation in 56% of the cases of autoimmune liver disease. When 3 out of 4 changes were considered, the diagnostic accuracy for AIH was 76.9%, with a PPV of 93.3% and a negative predictive value (NPV) of 70.7%. Cirrhosis of the liver was present in a high proportion of cases, and predominated in the AIH-1 group (60%). Fortes et al.16 reported that patients that had the HLADRB1-1301 allele had a higher risk for presenting with cirrhosis. Cirrhosis presents in 44 to 80% of the cases in children at the time of diagnosis and they remain clinically stable with a low mortality rate and good quality of life with long-term treatment.17
Treatment goals are to improve symptoms, induce biochemical remission, subdue hepatic inflammation, and prolong survival.18 Clinical and biochemical remission do not necessarily reflect histologic resolution, given that the histopathologic changes take longer.19 It is stated that after 4 years of treatment, improvement in fibrosis and the intensity of portal inflammation is observed in more than 95% of the cases.
Adequate treatment response was faster in the AIH-2 group and on average it was seen within the first month of treatment. However, there was a poor response in 20% of the cases in that group. Outcome and treatment response in children have been associated with the genetic and immunologic markers such as HLA-DRB1*1301 and DRB1*07.20–23 In a study by Czaja et al.,24 they concluded that the patients with HLA-DRB1*03 were younger at the time of disease onset and had a worse response to steroid treatment than the patients with HLA-DRB1*04; these latter patients were more frequently women that had an association with other immunologic diseases.25 The class II histocompatibility antigen (MHC II) HLA-DRB1*04 antigen was more common in ethnic groups in Mexico and Japan.26
Relapses are common during the course of treatment. In our study, 45% of the cases with AIH-1 and 60% of the cases with AIH-2 had disease recurrences, causing a temporary increase in steroid dose. In a study by Van Gerven et al.27 conducted on a cohort of 131 patients, they reported a 47% recurrence rate. The greatest relapse risk was observed in very young patients that also presented with a concomitant autoimmune disease.
Another factor associated with disease recurrence is the lack of treatment adherence. In our population, 20% of the cases in each group did not adhere to treatment, a figure similar to that reported by Sotelo y López28 in 2005 in a study on Mexican children from Hermosillo, Sonora, in which treatment abandonment was 22%.29
Successful treatment suspension was achieved in only 20% of the patients with AIH-1 and in none of the patients in the AIH-2 group. Therefore, treatment suspension is not recommended for AIH-2 patients because relapses are more frequent and remission failure is an almost certain condition.30 In a population-based retrospective cohort study, Deneau et al.31 found a sustained immunosuppressant therapy-free remission in 41.6% of the pediatric patients within the first 5 years of diagnosis.
With respect to the type of treatment, it is important to underline the favorable response to steroids and immunosuppressant drugs in AIH.32–34 Response is achieved through the exclusive use of PDN or in combination with AZA. Another option is budenoside, which has fewer side effects and more rapid hepatic elimination. However, it cannot be used in patients with cirrhosis, a complication that limits its use in a large proportion of patients with AIH. In a cohort of pediatric patients, Mieli-Vergani et al.35 compared the use of budenoside with AZA versus PDN with AZA and found no difference in the remission rate at 6 and 12 months. When there is treatment failure, the medications employed are cyclosporine, tacrolimus, and mycophenolate mofetil.
Standard therapy with PDN-AZA is effective in 85% of the cases,36 but there are other alternatives that should be considered. The infusion of antigen-specific regulatory T cells for restituting the damaged immunologic regulation and restoring the lost peripheral tolerance37–42 has been suggested for AIH-2 management, but its availability is limited.
Optimum duration of immunosuppressant treatment is controversial and it is only considered successful if inflammation is histologically resolved after one or two years of treatment in which there are normal transaminase and immunoglobulin levels, as well as autoantibody negativity. Note: in order for optimum treatment duration to be considered, there must be remission; this is defined as the absence of symptoms and the normalization of serum immunoglobulins, ALT/AST aminotransferases, and autoantibodies. It must also be demonstrated histopathologically, with minimum inflammation and no necrosis. It is suggested not to suspend treatment within the first 3 years of the diagnosed disease, or immediately before puberty, due to the possibility of frequent relapses.
Unlike the patients with autoimmune sclerosing cholangitis, AIH patients have better treatment response, with a better survival rate, and they require a low dose of maintenance medication.43
Ngu et al.44 analyzed the poor treatment response predictors that included incomplete ALT normalization at 6 months of management, low albumin concentration, and age ≤ 20 years and ≥ 60 years. These variables were independent predictors for the necessity of liver transplantation and/or the possibility of death. However, cirrhosis was not associated with poor outcome and did not influence the initial immunosuppressant treatment response.
ConclusionsAutoimmune hepatitis must be suspected in pediatric patients in order to make early diagnosis and thus establish opportune treatment. Diagnostic delay results in cirrhosis and liver failure.
AIH in children presents in acute form and has a more aggressive disease course than in adults.
The presence of cirrhosis does not modify treatment response.
Optimum treatment response is achieved more rapidly in patients with AIH-2, but they also present with higher recurrence rates within a shorter period of time.
The presence of SLA autoantibodies is associated with an inferior treatment response and higher recurrence frequency.
Relapses are common in the course of treatment, presenting in 45-60% of the cases and they are observed more frequently in patients with AIH-2.
The combination of PDN + AZA is the initial treatment of choice.
Further genetic studies and analyses of immunologic markers are required that will aid in determining the susceptibility of presenting with AIH and in establishing outcome and treatment response factors.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Nares-Cisneros J, Jaramillo-Rodríguez Y. Hepatitis autoinmune en niños: evolución de 20 casos del norte de México. Revista de Gastroenterología de México. 2014;79:238–243.