Gadolinium-enhanced magnetic resonance for the evaluation of hepatic lesions is increasingly being used in clinical practice, especially in patients with suspicious focal lesions, whether benign or malignant. In regard to hepatocellular carcinoma, the diagnostic performance of magnetic resonance through the «conventional» protocols and multi-detector computerized tomography consisting of multiphase evaluation with intravenous contrast, largely depends on the size of the lesion. They are more reliable in lesions>2cm. However, in lesions measuring 1-2cm, establishing the definitive diagnosis is a real challenge, with sensitivity values of 45-65%, but generally with excellent specificity (>95%). Furthermore, if the lesion has a diameter<1cm, diagnosis is usually unreliable. In these last 2 settings, the complementary use of liver-specific contrast agents can be advantageous. The aim of our article was to review the current evidence on the usefulness of this new non-invasive diagnostic method in hepatic lesions.
La resonancia magnética con gadolinio para la evaluación de lesiones hepáticas es un método cada vez más utilizado en la práctica clínica, particularmente para pacientes con lesiones focales sospechosas, ya sean benignas o malignas. En el caso del carcinoma hepatocelular, el rendimiento diagnóstico de la resonancia magnética a través de protocolos «convencionales» y mediante la tomografía computarizada multidetector, que consiste en la evaluación de múltiples fases con contraste intravenoso, depende en gran medida del tamaño de la lesión, considerándose más certero en lesiones>2cm. Sin embargo, para aquellas lesiones de 1-2cm, el establecimiento de un diagnóstico definitivo es un verdadero reto, con valores de sensibilidad del 45-65%, aunque por lo general con una excelente especificidad (>95%). Además, si la lesión tiene un diámetro<1cm, el diagnóstico es generalmente poco fiable. En estos 2 últimos escenarios, el uso complementario de medios de contraste hepatoespecíficos puede ser útil. El objetivo de este artículo es revisar la evidencia actual de la utilidad de este nuevo método de diagnóstico no invasivo en las lesiones hepáticas.
Gadolinium-enhanced magnetic resonance (MR) for the evaluation of hepatic lesions is being increasingly used in clinical practice, particularly in those patients suspected of having focal lesions, either benign or malignant.
With respect to hepatocellular carcinoma (HCC), the epithelial tumor whose incidence is on the rise and that currently holds 5th place in frequency, the diagnostic performance of the so-called “conventional” MR and multidetector computed tomography (MDCT) protocols that consist of multiphase evaluations with intravenous contrast, largely depends on the size of the lesion. Both imaging modalities are considered reliable and they have a sensitivity of 100% if the lesions are larger than 2cm.1–4
However, establishing an accurate diagnosis for those lesions between 1-2cm is a real challenge, particularly in patients with cirrhosis, 80% of whom present with HCC and whose annual incidence of this type of lesion is 2-8%.5–7 The sensitivity values are 44-47% for lesions of 1-2cm1-4 and 29-43% for those that measure less than 1cm.2–4 In tumors with a diameter under 1cm, diagnosis is generally unreliable. This scenario is increasingly more frequent due to the use of ultrasound as the initial primary screening method recommended by the majority of the guidelines.6,8–10 In lesions measuring 1-2cm and under 1cm and in lesions regarded as undetermined after analysis with conventional strategies, the complementary use of liver-specific (LS) contrast agents in MR could be useful.
The aim of the present manuscript was to review the current evidence on the emerging role of LS contrast agents in the evaluation of focal lesions, especially in HCC, and to describe their main differential diagnoses. According to the literature, these agents appear to be the most sensitive method for HCC detection, increasing sensitivity values by 6-15%.11–13
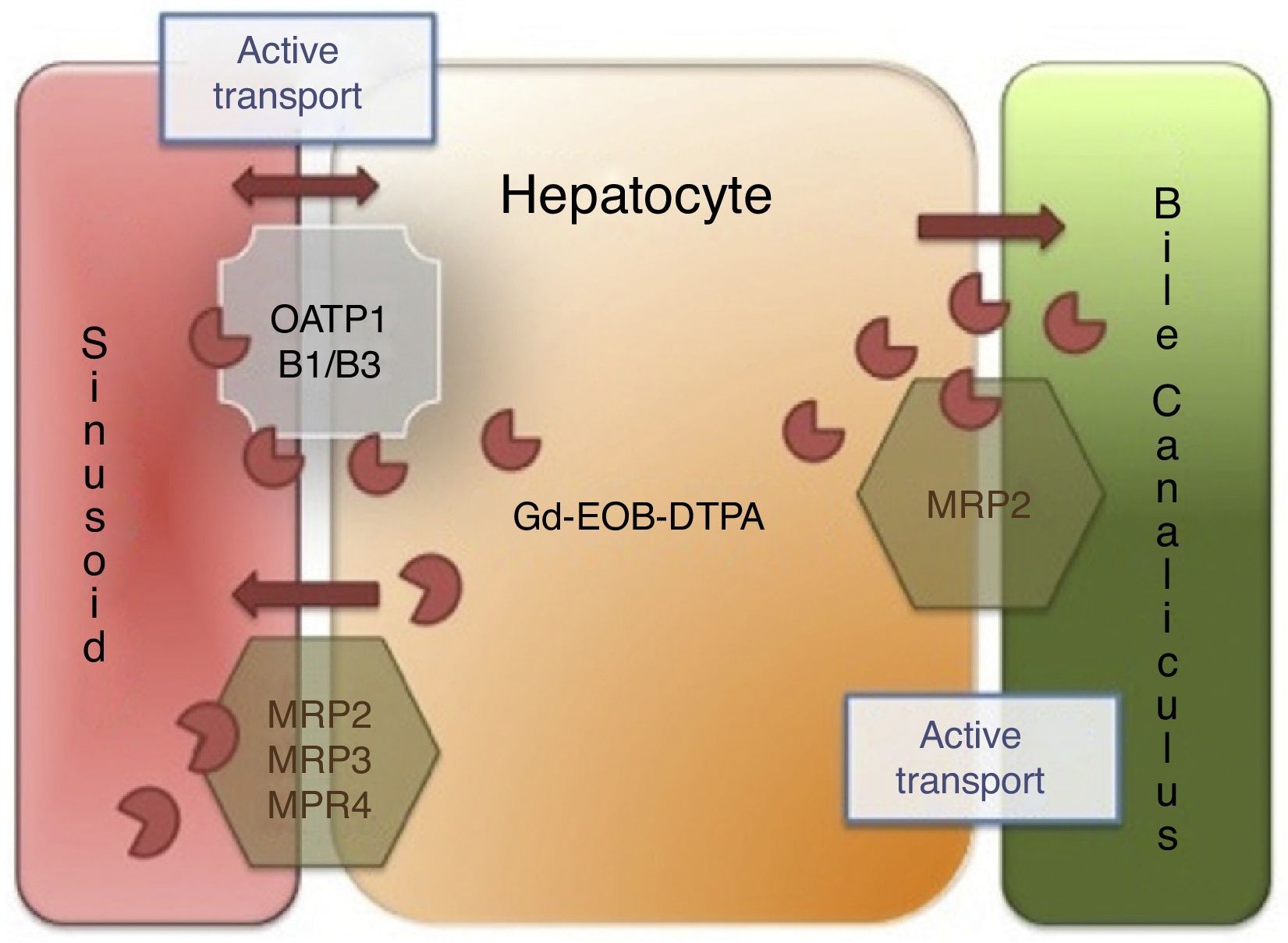
Liver-specific contrast agents: types and propertiesA simple way to classify the contrast agents employed in MR is by dividing them into 2 large groups: 1) non-LS and 2) LS. The “traditional” media belong to the first group, based on chelating agents of gadolinium, whose typical behavior is intravascular and extracellular. On the other hand, the liver-specific contrast media, which consist of superparamagnetic iron oxide particles (ferumoxides), are subdivided into those that are taken up by Kupffer cells and those that are taken up by functional hepatocytes. The latter are compounds that are also based on gadolinium, called gadobenate dimeglumine (Gd-BOPTA) and gadoxetic acid (Gd-EOB-DTPA). They are considered intravascular, extracellular, and intrahepatic contrast agents and are the topic of the present work.
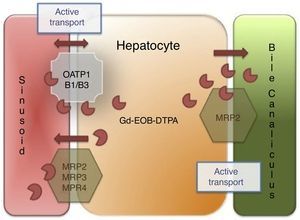
Hepatocytes take up less Gd-BOPTA (3-5%) than gadoxetic acid (50%) and both agents are excreted without changes into the biliary tract.14 In the case of biliary tract obstruction, there is an increase in their renal excretion. The intracellular step depends on the expression of the membrane receptors called multidrug-resistant protein 3 (MRP 3) and MRP 4 and the organic anion transporter polypeptide 8 (OATP8), also known as 1B1/B3, located in the basolateral sinusoidal membrane that borders the sinusoid and the space of Disse, whereas the MRP2 transporter, located in the canalicular membrane, is in charge of excreting the contrast medium into the bile canaliculus14 (Fig. 1).
Liver-specific contrast medium action mechanism. The liver-specific agent is introduced into the hepatocyte through the organic anion transporter polypeptide (OATP1, B1/B3) and exits through the ATP-dependent multidrug-resistant proteins (MRP2, MRP3, MRP4) located in the sinusoidal membrane. MRP2 is regulated through membrane recovery (reduced bile outlet flow) or introduction (increased outlet flow), and finally, the liver-specific agent is not metabolized by the hepatocyte and is excreted, unchanged, into the bile.
These types of LS agents behave similarly to the “traditional” contrast agents during the arterial, portal, and venous phases. In other words, they allow the perfusion of the lesion to be evaluated, which is an indispensible imaging element for establishing the differential diagnosis. However, the real additional diagnostic effect that these contrast agents offer, requires images in a delayed phase (15-25min), called the hepatobiliary phase. It is the time that is needed for the medication to become concentrated inside the functional hepatocyte.15–18 That is to say, a classic MR protocol for evaluating the focal lesion would include the T1 dual echo conventional sequences (in-phase and out-of-phase), T2 with and without fat saturation, 3D cholangiography, multiphase contrasted T1 (plain, arterial, portal, venous, and delayed for 2-3min), diffusion, and in addition, a T1 in the hepatobiliary phase,15 a protocol lasting approximately 50min.
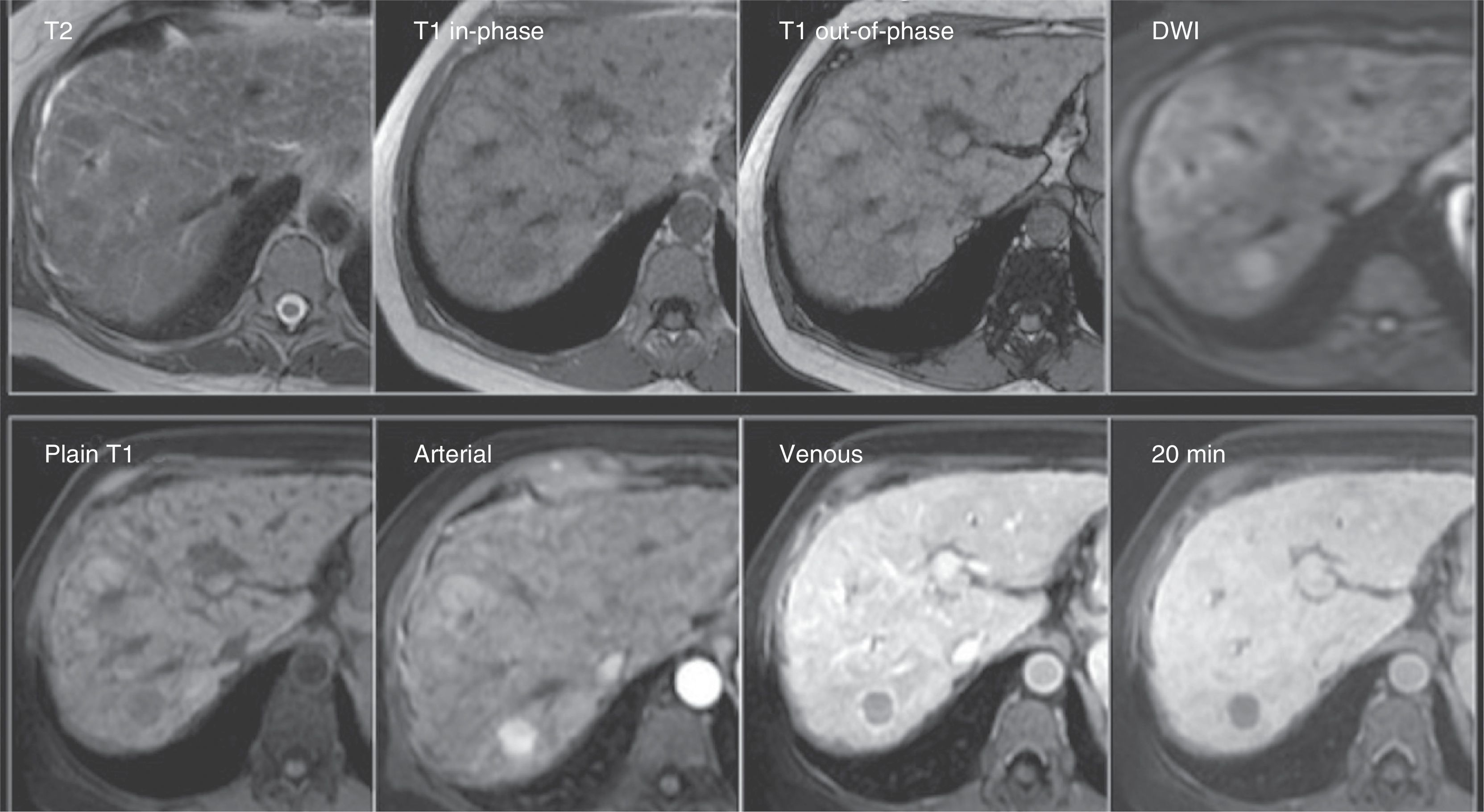
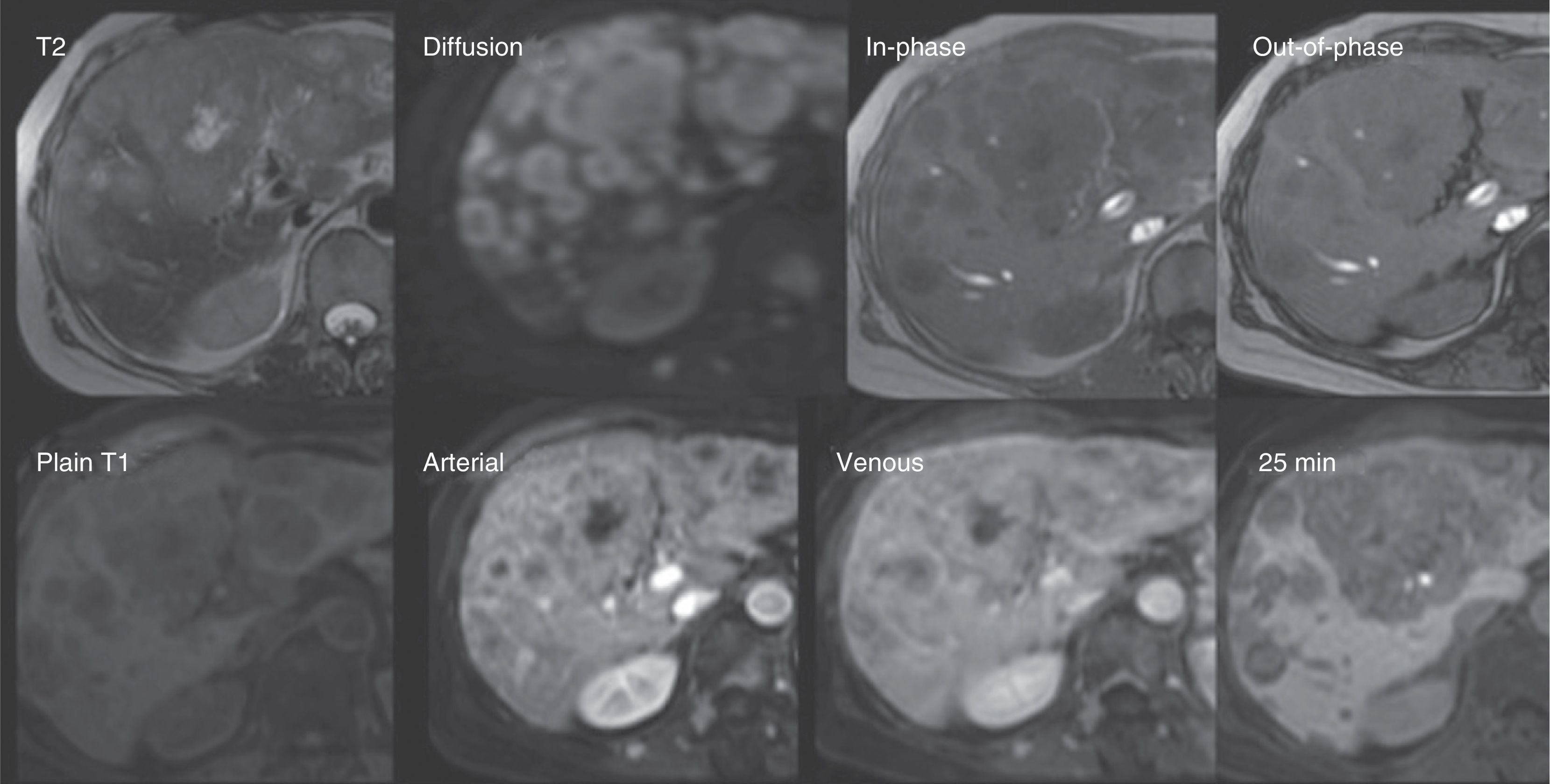
Gadoxetic acid usefulness in the evaluation of high-grade dysplastic nodules and hepatocellular carcinomaThe use of this type of contrast agent enables lesions that contain hepatocytes to be distinguished from those that do not. As a result, malignant lesions composed of non-functioning hepatocytes (high-grade dysplastic nodules and HCC) or that have no hepatocytes (metastasis) do not show uptake of this type of contrast in the hepatobiliary phase (Fig. 2). Likewise, these agents also have been used to distinguish focal nodular hyperplasia from fibrolamellar carcinoma and from hepatic adenoma, 2 benign lesions with different treatment and follow-up strategies.19
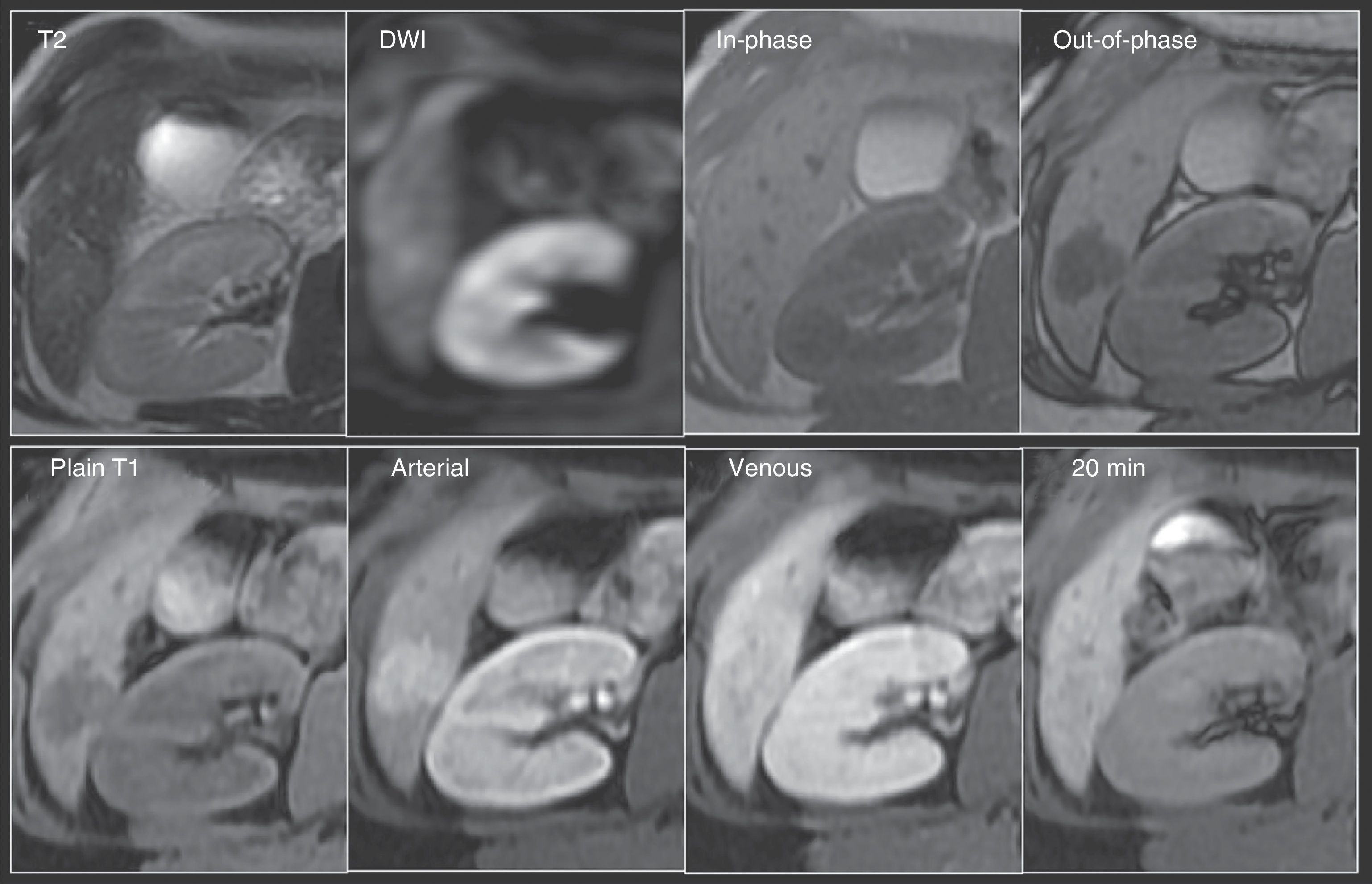
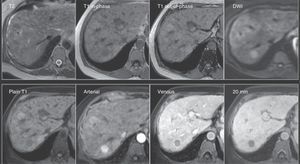
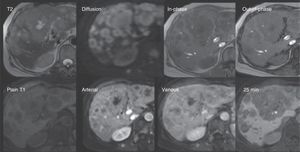
Small-size hepatocellular carcinoma. A 60-year-old woman whose liver has changes from cirrhosis with important contour lobulation and nodularity, as well as heterogeneous signal intensity of the parenchyma, and a lesion that was undetermined through MDCT (not shown). Top row: Note the rounded lesion in segment 7, isointense on T2 and hypointense on T1 both in-phase and out-of-phase, and with restricted diffusion (DWI). Bottom row: In the LAVA sequence dynamic phases there is important enhancement in the arterial phase with rapid washout in the portal venous phase -notice the enhancement of the rest of the parenchyma- and the hypointensity of the lesion in the 20min hepatobiliary phase, which are characteristic findings of hepatocellular carcinoma.
These contrast media could be especially useful in those patients with hepatic lesions that do not have the “classic” vascular behavior of HCC. The typical characteristics that define HCC in a dynamic or multiphase MR study after intravenous contrast-enhancement according to the guidelines of the American Association for the Study of Liver Diseases (AASLD) are: 1) a hypervascular lesion during the arterial phase, and 2) washout of the contrast agent during the venous phase or the appearance of capsule if there is no washout; the latter is not contemplated in the guidelines despite its having great diagnostic specificity (Fig. 2).19–21 This behavior is highly suggestive of HCC with specificity values above 95%, but with sensitivity of 45-65% in lesions measuring 1-2cm in diameter.8,22 Nevertheless, the absence of “classic” vascular characteristics occurs relatively frequently due to the fact that during hepatocarcinogenesis–a complex and gradual process of epigenetic and genetic alterations in the transformation of malignant hepatic cells to HCC–it is recognized that the vascular support of the lesion varies. It encompasses a spectrum in which first, there is a predominance of portal vascular flow due to the greater number of portal triads and a lower grade of “normal” arterial flow, as occurs with the regeneration nodules. Then, there ends up being an output that is 100% arterial from the “abnormal” vessels, due to the null or lower density of the portal triads and the greater density of both the sinusoids with capillarization and the unpaired arteries; in other words, those arteries that are not associated with the portal triads, as occurs in poorly differentiated HCC, that show a typically arterial enhancement and a portal and venous washout.20,23 In addition, within this spectrum there are lesions with scant portal flow, as well as a mixture of normal and abnormal arterial output, and it is here where some lesions are found that are considered high-grade dysplastic nodules and early stage HCC (microinvasive), also called in situ carcinoma. The majority are under 2cm in diameter and both are difficult to detect through conventional methods.20,23 Therefore, the high-grade dysplastic nodules, as well as early stage HCC, can appear atypical, being hypervascular in the arterial phase, although without washout in the portal or venous phases, or with an absence of arterial vascularity and its presence in later phases. Hence, the general sensitivity of MR with conventional contrast agents is only 62%.19 In regard to these types of lesions, if LS contrast agents were to be used and if the lesion were hypointense in the 20min phase, strict surveillance of the lesion, or biopsy if HCC were highly suspected, would be the steps to follow. In other words, in the hepatobiliary phase, the contrast medium is not concentrated in these lesions because they lack functioning hepatocytes, differentiating them from the perilesional liver that has greater signaling intensity due to the intracellular presence of the LS contrast agent.
LS contrast agents are being employed in the diagnosis of high-grade dysplastic nodules and early stage HCC because there is recent evidence suggesting that the expression of the transporters in charge of introducing these contrast media into the cell diminishes during the process of hepatocarcinogenesis, before the typical sequential changes in the abovementioned tumor vascularity occur. Reduced OATP8 transporter expression can be partly attributed to the expression of the 3 beta nuclear factor of the hepatocyte, a transcription factor that is overexpressed in HCC and that represses OATP8 transcription.15
Therefore, the potential indications for the use of LS contrast agents would be in those patients with lesions larger than 2cm that are considered undetermined after conventional analysis through tomography or magnetic resonance with non-LS contrast media, and initially in lesions smaller than 2cm that are frequently undetermined after following said strategies.
Evidence of gadoxetic acid usefulness in the evaluation of focal lesions and hepatocellular carcinomaIn accordance with initial series, the diagnostic performance of LS contrast-enhanced MR for establishing the diagnosis of high-grade dysplastic nodules or well differentiated HCC in lesions previously considered undetermined significantly improves, compared with conventional strategies, with sensitivity and specificity values of 82-99% and 70-95%, respectively.19,24–26 The hypointense behavior of a lesion in the hepatobiliary phase is regarded as a sign that, together with the rest of the conventionally evaluated characteristics, improves the overall test performance. In a study by Kim et al. on 96 patients with 116 nodules of 1-2cm in diameter, in which LS contrast-enhanced MR was compared with a pathologic reference standard and/or 18-month follow-up, the sensitivity of the radiologic signs considered diagnostic was 67% (CI: 51-79%), according to the guidelines of the AASLD. It improved up to 84% (CI: 70-92%) when the hypointensity on T1 in the hepatobiliary phase and/or the hyperintensity of the lesion on the T2 sequences were added to these radiologic signs.19 In another study on 62 patients with 83 HCCs that compared the diagnostic performance of multiphase MDCT and LS contrast-enhanced MR vs pathology (surgical resection), no overall significant difference was observed between the imaging methods.24 However, in the subgroup of lesions < 1cm, a significant difference in sensitivity was observed (70% vs 30-50%, p < 0.05).
In a study on 34 explanted patients with 102 hepatic nodules, none of the 32 low-grade dysplastic nodules showed hypointense behavior in the hepatocellular phase, unlike the HCCs in which only 1 tumor out of 40 showed no typical hypointense behavior in that phase.27 The results of this study coincide with those observed in several studies during follow-up of non-hypervascular nodules in the arterial phase and with hypointensity in the hepatocellular phase, in which the accumulative rate of typical HCC development varied from 15-35% at one year and up to 46% at 2 years, mainly in lesions greater than 1cm.28–30 These results have motivated some medical associations to incorporate the use of LS contrast agents in their diagnostic algorithms as evidence to be followed, once any abnormality is detected in the initial studies (ultrasound or high tumor marker level).31,32
However, it should be taken into account that 5-12% of HCCs and close to 30% of high-grade dysplastic nodules are not hypointense in the LS contrast-enhanced hepatic phases, and there can even be a greater accumulation of contrast medium in the hepatobiliary phase.14,16,24,30 This finding is possibly due to the paradoxical overexpression of the OATP8 genes and to the absence of functional bile ducts or the transporter outlet membrane in some tumors, in which case a different cellular origin or genetic alterations during hepatocarcinogenesis are contemplated.14,33 Histologically, the majority of these tumors are moderately or well differentiated.34,35 In this setting, the recommendation is to look for other signs that support HCC diagnosis, such as: absence of a central scar, a nodule-in-nodule image, presence of a hypointense ring, hyperintensity in the diffusion sequences because of its restriction and/or signal loss (hypointensity) in the out-of-phase sequences due to the presence of intralesional fat.36
The fact that the presence of certain factors can limit the efficacy of the hepatobiliary phase in lesion detection and characterization should also be considered. Those patients with important hepatic dysfunction or cholestasis may show a limited concentration of the hepatobiliary agents.37 Recent studies report that there is a decrease in the diagnostic performance of LS contrast media when cirrhosis becomes more severe, due to reduced enhancement in the hepatocellular phase.38
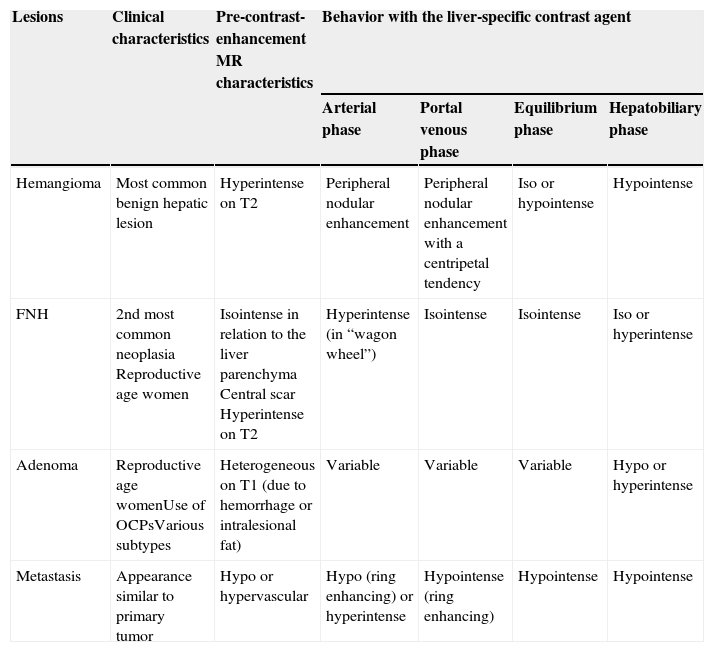
Differential diagnosisThe main usefulness of the studies with LS contrast media is the improved characterization of focal lesions, particularly the distinction between benign and malignant tumors. Nevertheless, it is not recommended that a hepatic focal lesion study protocol only include the hepatobiliary phase, given that all the lesions that are not composed of hepatocytes will behave as hypointense ones. Therefore, in order to avoid incorrect diagnoses, it is important to be familiar with the lesions that will have hypotensive behavior in the hepatobiliary phase with these types of media due to the histologic lack of functioning hepatocytes. The distinction between them will depend on the analysis in conjunction with all the sequences, behavior on T1 and T2, fat saturation, dual echo, diffusion, and perfusion characteristics; in other words, if there is arterial or venous enhancement, the presence or not of washout, the type of enhancement (central or peripheral), and the presence or not of capsule. The MR characteristics of the different focal lesions are summarized in Table 1 and Figures 3–6.
Important characteristics for differentiating the most frequent hepatic lesions through the use of liver-specific contrast agents.
| Lesions | Clinical characteristics | Pre-contrast-enhancement MR characteristics | Behavior with the liver-specific contrast agent | |||
|---|---|---|---|---|---|---|
| Arterial phase | Portal venous phase | Equilibrium phase | Hepatobiliary phase | |||
| Hemangioma | Most common benign hepatic lesion | Hyperintense on T2 | Peripheral nodular enhancement | Peripheral nodular enhancement with a centripetal tendency | Iso or hypointense | Hypointense |
| FNH | 2nd most common neoplasia Reproductive age women | Isointense in relation to the liver parenchyma Central scar Hyperintense on T2 | Hyperintense (in “wagon wheel”) | Isointense | Isointense | Iso or hyperintense |
| Adenoma | Reproductive age womenUse of OCPsVarious subtypes | Heterogeneous on T1 (due to hemorrhage or intralesional fat) | Variable | Variable | Variable | Hypo or hyperintense |
| Metastasis | Appearance similar to primary tumor | Hypo or hypervascular | Hypo (ring enhancing) or hyperintense | Hypointense (ring enhancing) | Hypointense | Hypointense |
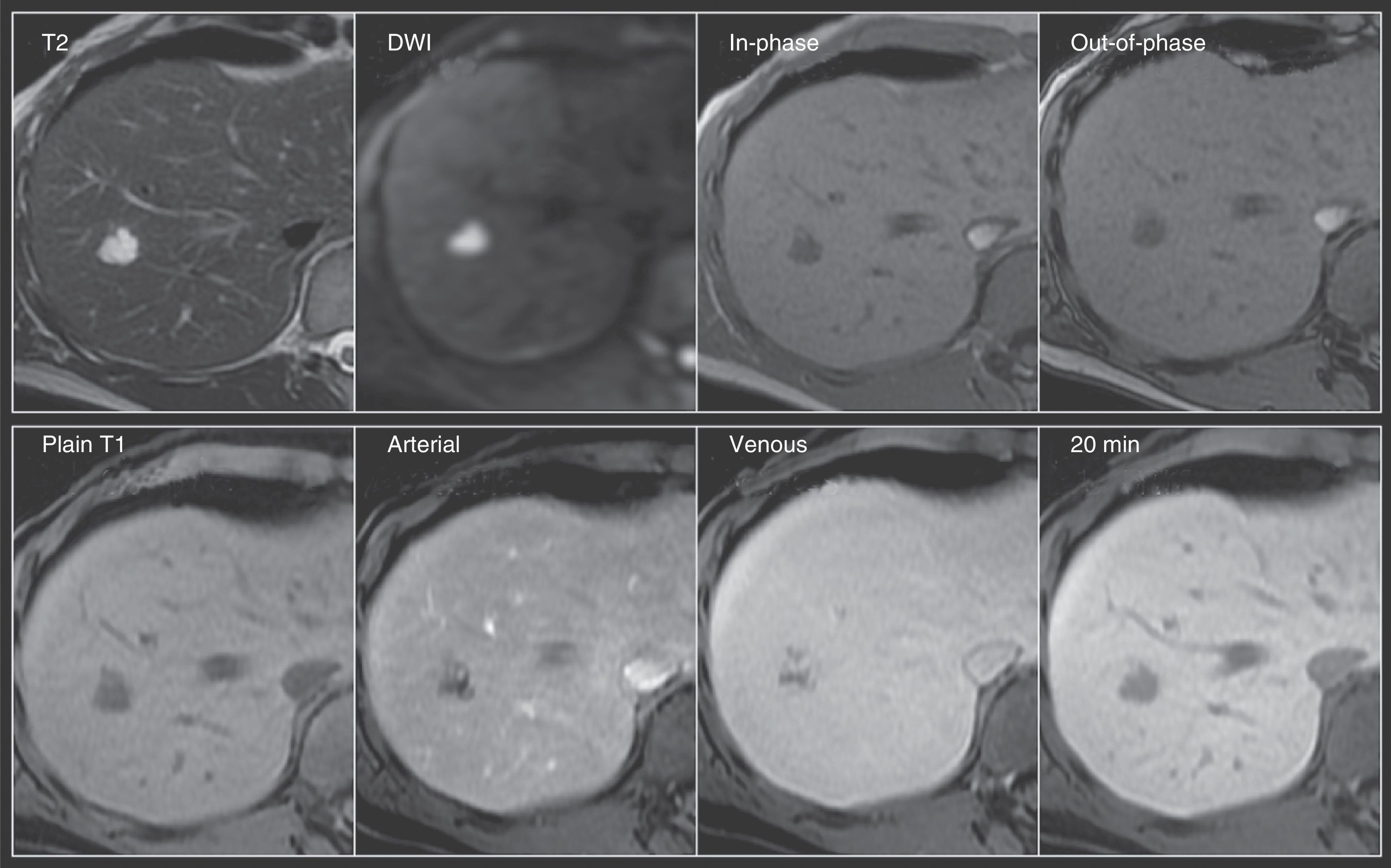
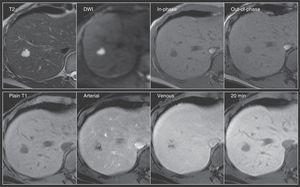
Hemangioma. A 56-year-old woman with an ultrasound finding of a hepatic focal lesion in segment 8. Top row: Nodular lesion with lobulated contours, hyperintense on T2, with important restricted diffusion, and hypointense on T1 in the images both in-phase and out-of-phase. Bottom row: note the typical peripheral nodular enhancement from the arterial phase with progressive centripetal fill-in on the venous phase -signal intensity similar to the rest of the parenchyma- and hypointensity on the 20min delayed phase, characteristics of cavernous hemangioma.
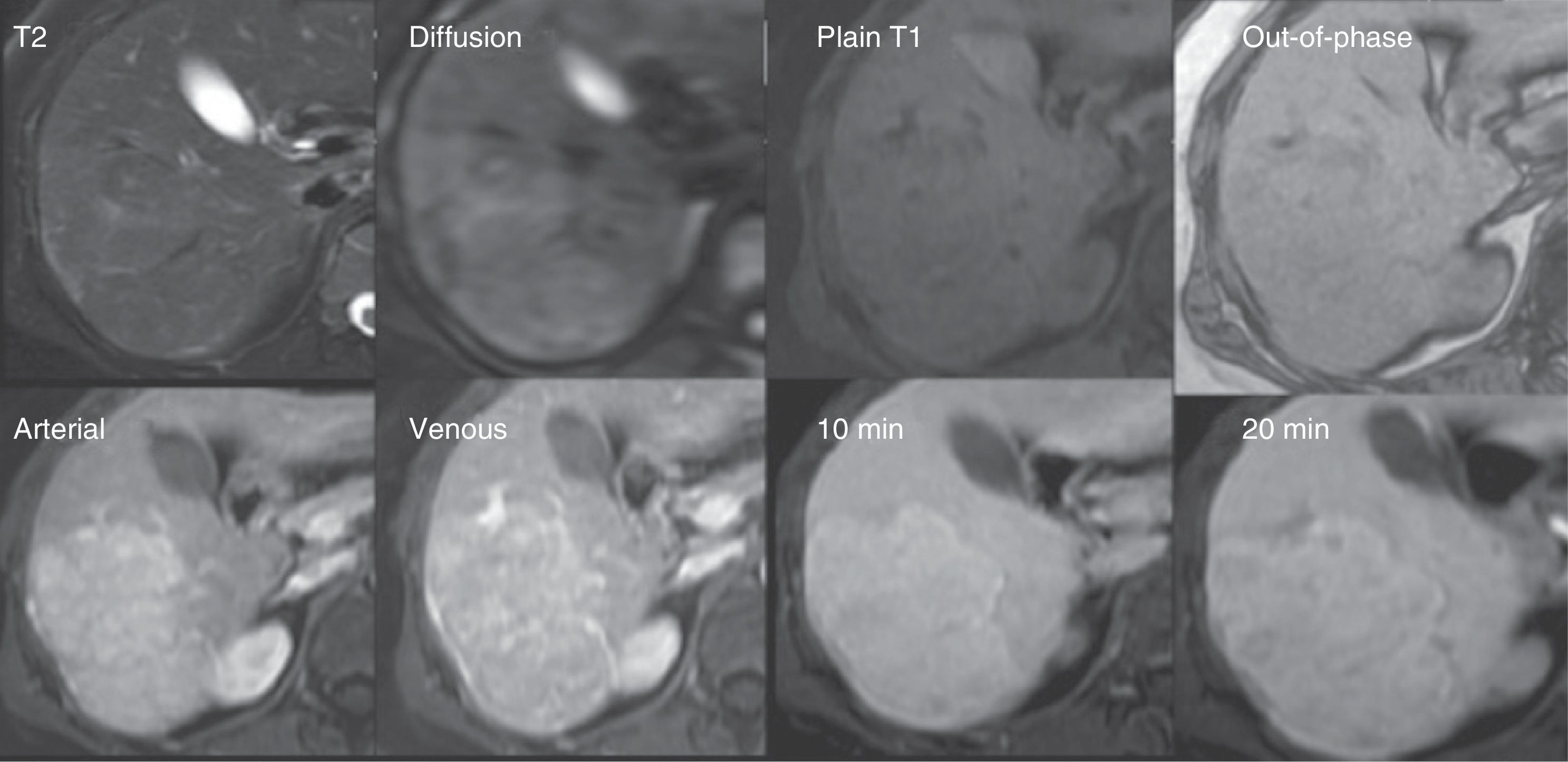
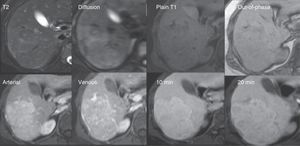
Focal nodular hyperplasia. A 42-year-old woman with focal lesions in segments 6 and 7 undetermined through ultrasound and computed tomography. The distinction between adenoma vs focal nodular hyperplasia is relevant due to their different management. Top row: Isointense nodular lesion on T2 with hyperintense central scar in the diffusion (DWI) and isointense to the rest of the parenchyma on T1 with no signal decay in the out-of-phase sequence. Bottom row: In the dynamic sequences with gadoxetic acid, the lesion presents enhancement starting from the arterial phase. The 20min equilibrium and hepatobiliary phases show behavior similar to the rest of the parenchyma, calling attention to the presence of a hypointense central scar that has no hepatocytes. These data are typical of focal nodular hyperplasia.
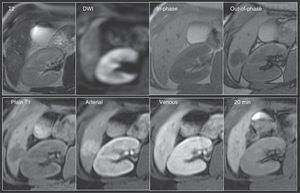
Adenoma. A 40-year-old woman with a focal lesion in segment 6 observed through ultrasound. It was not possible through MDCT to distinguish between adenoma and focal nodular hyperplasia without central scar. Top row: Lesion hyperintense to the rest of the parenchyma on T2 with discrete restricted diffusion (DWI), isointense on the T1 in-phase images and markedly hypointense on the out-of-phase sequence. Bottom row: The lesion shows important enhancement in the arterial phase and is isointense on the venous and hepatobiliary phases. It is important to remember that the behavior with the LS contrast agent will depend on the adenoma subtype. In this case, the distinction between focal nodular hyperplasia was made due to the important signal decay observed in the out-of-phase images, characteristic of the presence of intralesional fat in adenomas.
Metastasis. A 61-year-old woman with a history of cancer of the left breast. Follow-up ultrasound identified multiple dispersed focal lesions in the hepatic parenchyma. MR was done to improve characterization. Top row: The presence of multiple rounded images of diffuse distribution, hypointense on T1 and hyperintense on T2 and diffusion (DWI), with no signal decay in the out-of-phase sequence. Bottom row: In the dynamic sequences with gadoxetic acid, said lesions present with ring enhancement on the arterial phase and centripetal fill-in on the later phases. The lesions are observed to be hypointense on the hepatobiliary phase due to the absence of hepatocytes. In addition, other lesions are identified that have similar characteristics and enhancement of the vertebral bodies and paravertebral muscles. Data are related to metastasis.
There is still a group of patients in whom the use of this type of contrast medium is limited. This is particularly true in patients with liver dysfunction (hyperbilirubinemia > 3mg/dL), as well as in patients with focal lesions whose vascular permeability is to be evaluated, especially portal permeability, due to the low volume of contrast medium employed. It is also true for HCC patients in the post-ablation follow-up period, in the abovementioned individuals with well differentiated HCC that can accumulate the contrast medium in the late hepatobiliary phases, and in patients with rapid-filling hemangiomas.7 A joint overall analysis of the MR characteristics of the lesion in the diffusion sequences and its behavior on T2 can be useful in distinguishing between these entities.
Respiratory artifacts. Triple acquisition of the arterial phase with a single apnea has been shown to provide adequate images in the majority of patients that experience respiratory movement artifacts in the arterial phase with the use of a liver-specific agent.39
Other points to consider are cost (up to 3 times higher), the impact of examination time (study lasting 20min longer), and the potential lower enhancement in the arterial phase with the use of the approved doses (0.025 mmol/kg).3
ConclusionsThe recent introduction of LS contrast agents in the evaluation of hepatic focal lesions is an additional complement that is potentially useful in undetermined lesions, whether in tumors > 2cm with uncharacteristic behavior in conventional gadolinium-enhanced multiphase studies or computed tomography, or in lesions < 2cm in patients with underlying hepatopathy in whom early and accurate detection of HCC is essential.
Therefore, even though the role of this type of contrast medium must still be defined through clinical trials with larger numbers of patients, and especially in patients with advanced cirrhosis, its use can aid in improving diagnostic sensitivity and specificity, when it is compared with the performance of the conventional strategies in these groups of patients.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Cossio-Torrico PE, Ramírez-Carmona CR, Stoopen-Rometti M, Perochena-González A, Sosa-Lozano LA, Kimura-Hayama E. Resonancia magnética con ácido gadoxético —contraste hepatoespecífico— para la evaluación de lesiones focales. Revista de Gastroenterología de México. 2015;80:267–275.