There is evidence that patients with irritable bowel syndrome (IBS) have a low degree of inflammation in the intestinal mucosa. The aim of the study was to evaluate theprofile of pro- and anti-inflammatory cytokinesin plasmain Mexican pediatric patients with IBS.
Patients and methodsFifteen patients with IBS according to Rome III criteria for childhood and 15 healthy children, matched by age and sex, were included in the study. Plasma levels of tumoral necrosis factor alpha (TNF-α), interleukins 10 and 12 (IL-10, IL-12) and transforming growth factor beta (TGF-β) were quantified and compared between groups.
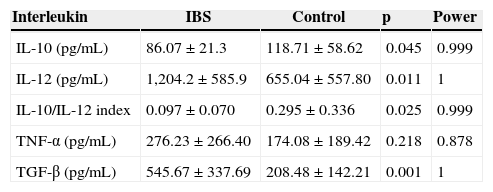
ResultsPlasma levels of IL-10 were lower in patients with IBS (86.07+21.3 pg/mL vs. 118.71+58.62 pg/mL: P=.045) and IL-12 levels were higher in patients with IBS compared to the control group of healthy children (1,204.2±585.9 pg/mL vs. 655.04±557.80 pg/mL; P=.011). The IL-10/IL-12 index was lower in patients with IBS (0.097±0.07 vs. 0.295±0.336; P=.025). Plasma concentration of TGF-β was higher in patients with IBS (545.67±337.69 pg/mL vs. 208.48±142.21 pg/mL; P=.001). There was no difference in plasma levels of TNF-α between groups.
ConclusionsThis study suggests that children with IBS have a state of altered immune regulation. This is consistent with the theory of low-grade inflammatory state in these patients. Further studies are needed to elucidate the role played by these cytokines, specifically TGF-β in the pathogenesis of IBS.
Existe evidencia de un grado bajo de inflamación a nivel de la mucosa intestinal en pacientes con síndrome de intestino irritable (SII). El objetivo del estudio fue evaluar el perfil de citocinas proinflamatorias y antinflamatorias en plasma en pacientes pediátricos mexicanos con SII.
Pacientes y métodosQuince pacientes con SII de acuerdo con los criterios de Roma III para pacientes pediátricos y 15 niños sanos, pareados por edad y sexo fueron incluidos en el estudio. Se cuantificaron en plasma y se compararon el factor de necrosis tumoral alfa (TNF-α), las interleucinas 10 y 12 (IL-10, IL-12) y el factor de crecimiento transformante beta (TGF-β).
ResultadosLos niveles plasmáticos de IL-10 fueron menores en los pacientes con SII (86.07+21.3 pg/mL vs. 118.71+58.62 pg/mL; p=0.045) y los niveles de IL-12 mayores en los pacientes con SII en comparación con el grupo control de niños sanos (1,204.2±585.9 pg/mL vs. 655.04±557.80 pg/mL; p=0.011). El índice IL-10/IL-12 fue menor en los pacientes con SII (0.097±0.07 vs. 0.295±0.336; p=0.025). La concentración en plasma de TGF-β fue mayor en los pacientes con SII (545.67±337.69 pg/mL vs. 208.48±142.21 pg/mL; p=0.001). No hubo diferencia en los niveles plasmáticos de TNF-α entre ambos grupos.
ConclusionesEste estudio indica que los niños con SII presentan un estado de alteración de la regulación inmune. Aún está pendiente por dilucidar el papel que juegan estas citocinas, específicamente la TGF-β, en la patogénesis del SII.
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder that is defined by the Rome III criteria for pediatric patients as the sensation of abdominal discomfort or pain that has presented at least once a week in the 2 months prior to diagnosis and is associated with 2 of the following characteristics at least 25% of the time: a) it improves with a bowel movement, b) onset is associated with changes in bowel movement frequency, and c) onset is associated with change in the form (appearance) of the bowel movements. There is no evidence of an inflammatory, anatomic, metabolic, or neoplastic process accompanying these characteristics and they should also be present at least once a week in the 2 months before diagnosis.1 Between 22-45% of the pediatric patients from 4 to 18 years of age that are seen at tertiary care clinics are diagnosed with IBS. Multiple mechanisms are involved in its pathophysiology. One of the first studied is visceral hypersensitivity, a consequence of an alteration in the brain-bowel axis that most likely is modulated by genetic factors that regulate the local inflammatory and immunologic responses to different processes such as infections, intestinal trauma, or allergy. These, in turn, cause intestinal motility disorders that clinically manifest as diarrhea or constipation, associated or not, with abdominal pain.2–5 However, psychologic and inflammatory factors, as well as motility alterations also come into play in relation to its pathophysiology.
An infectious event can apparently precipitate IBS development in individuals with a psychosocial and genetic susceptibility, probably by conditioning a mild grade of intestinal mucosa inflammation that leads to immune system activation.4,6–8 Recent studies support the hypothesis of immune activation in adults with this syndrome: infiltration of immune cells into the intestinal mucosa of IBS patients9–11 and a greater expression of proinflammatory cytokines, such as interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, and IL-12, with low levels of IL-10.12–16 Polymorphisms of a single nucleotide in the genes that encode for TNF-α and IL-10 have been reported in adult IBS patients, compared with healthy controls.12,17,18 Hua et al. recently found that IL-10 levels were lower in pediatric IBS patients when compared with healthy controls; they found no differences in the levels of TNFα and IL-6, both proinflammatory cytokines, but these have not been evaluated in the Latin population.19 The aim of our study was to analyze the proinflammatory and anti-inflammatory cytokine profile in pediatric patients presenting with IBS at a tertiary care center. The study hypothesis was that Mexican children with IBS would present with lower plasma levels of IL-10 and higher plasma levels of IL-12 and TNF-α.
MethodsStudy populationTwo researchers recruited the IBS patients from the outpatient consultation service of the Department of Gastroenterology and Nutrition of the Hospital Infantil de México Federico Gómez and the healthy controls from schools in Mexico City and its suburbs (RVF and ACS). The non-probability convenience sampling technique was used to create the study group and the controls. All the participants were residents of Mexico City and its Metropolitan Area.
The IBS patients were diagnosed according to the Rome III criteria for pediatric patients: sensation of abdominal discomfort or pain that presented at least once a week in the 2 months prior to diagnosis, associated with 2 of the following characteristics at least 25% of the time: a) it improves with a bowel movement, b) onset is associated with changes in bowel movement frequency, and c) onset is associated with change in the form (appearance) of the bowel movements. There is no evidence of an inflammatory, anatomic, metabolic, or neoplastic process accompanying these characteristics and they should also be present at least once a week in the 2 months before diagnosis.1
No patients or controls were included in the study if they presented with malnutrition or overweight according to the World Health Organization and Centers for Disease Control tables, with any infectious gastrointestinal process, if they were carriers of any known allergic or immune alteration, or if they had received antibiotics, probiotics, analgesics, or anti-inflammatory agents in the month prior to their recruitment. A hemogram, blood chemistry test, 3-sample stool ova and parasite exam, fecal Giardia intestinalis antigen test, and a urinalysis were ordered for all the participants, none of which showed any alterations.
Evaluation of symptomatologySymptomatology was present, through questioning, for at least 4 months prior to the study (4-37 month interval). To demonstrate the characteristics of their symptoms and corroborate the IBS diagnosis for pediatric patients according to the Rome III criteria, all the IBS patients or their relatives filled out a symptoms diary at least 2 months prior to their enrollment. They kept a daily record of the presence or absence of symptoms such as abdominal discomfort or pain, abdominal bloating, and bowel movement frequency and characteristics.
Sample collection and the enzymatic immunoassay processA 10ml venous blood sample was collected in tubes with anticoagulant. The samples were centrifuged at 400xg for 20min to obtain plasma, which was then stored at −70°C until its use. The plasma levels of the TNFα, IL-12, IL-10, and TGF-β cytokines were quantified in duplicate utilizing commercial kits (EIA) (Assay Designs TiterZyme®EIA, Inc. Ann Arbor, MI, USA), following the manufacturer's instructions. Optic density was measured at a 450nm wavelength with a 620nm reference wavelength, utilizing an ELISA microplate reader (Multiskan FC, Thermo Fisher Scientific Oy Microplate Instrumentation Ratastie 2, P.O. Box 100 Vantaa, Finland). The values obtained were correlated linearly with the standard concentrations, forming the calibration curve for each cytokine. The sensitivity limit for each assay was the following: TNF-α: 8.43 pg/mL, IL-10: 3.75 pg/mL, TGF-β: 3.3 pg/mL, and IL-12: 0.9 pg/mL. The samples were run in duplicate.
Statistical analysisTaking into account the difference found between the levels of the IL-10 (exemplified) and IL-12 cytokines in a previous study on adults by O¿Mahony et al.,16 we made a sample calculation using the mean of the 2 groups with an alpha value of 0.05 and a power of 95%. We applied the formula for comparing the means of the 2 samples, and using a two-tailed sample, obtained 5 cases per group; considering the differences in the IL-10/IL-12 index, an n of one per group was figured; and allowing for the differences found for TNF-α with the same alpha and power resulted in 8 patients per group. The decision was made to include 15 patients in each group.
The plasma levels of the interleukins (pg/mL) of both groups were analyzed through the Shapiro-Wilk test to determine if they had a normal distribution. Upon finding that the normality criteria were not met, the nonparametric Mann-Whitney U test was applied for independent group evaluation. A power calculation of the differences was carried out to establish the probabilities of committing a type II error. The statistical calculations were made using the Windows SPSS 16.0 program (SPSS Inc, Chicago, IL, USA).
Our study was approved by the Research Commission and the Ethics Committee of the Hospital Infantil de México Federico Gómez. The parents of all the study participants gave their informed consent and the participants above 8 years of age signed statements of informed consent.
ResultsDemographic data and clinical characteristicsFifteen patients with IBS were included in the study; 8 were girls (53%). The mean age was 10.5±3.9 years, with a median age of 10.5 years (6-17 year interval). According to IBS subtype, 8 patients (53%) presented with the mixed subtype (IBS-M), 4 (26%) with diarrhea (IBS-D), and 3 (20%) with constipation (IBS-C).
In regard to the healthy controls, 15 children and adolescents, that included 8 girls (53%), had a mean age of 11.4±3.2 years and a median age of 10.75 years (6.1-17.3 year interval). There were no significant basal differences in relation to sex and age between the groups.
Interleukin plasma levelsIL-10 levels were lower (p=0.045) and IL-12 levels were higher (p=0.011) in the IBS patients than in the controls (p=0.011). The IL-10/IL-12 index was lower in the patients with IBS (p=0.025). Concerning the plasma levels of TNF-α, there was no difference between the groups (p=0.218). The mean TGF-β concentration in plasma was higher in the patients with IBS (p=0.001). These results are shown in Table 1.
Cytokine plasma levels in IBS patients and controls.
| Interleukin | IBS | Control | p | Power |
|---|---|---|---|---|
| IL-10 (pg/mL) | 86.07±21.3 | 118.71±58.62 | 0.045 | 0.999 |
| IL-12 (pg/mL) | 1,204.2±585.9 | 655.04±557.80 | 0.011 | 1 |
| IL-10/IL-12 index | 0.097±0.070 | 0.295±0.336 | 0.025 | 0.999 |
| TNF-α (pg/mL) | 276.23±266.40 | 174.08±189.42 | 0.218 | 0.878 |
| TGF-β (pg/mL) | 545.67±337.69 | 208.48±142.21 | 0.001 | 1 |
IBS is a functional gastrointestinal disorder that presents in both the adult and pediatric populations. IBS prevalence in the pediatric population ranges from 14 to 21%, depending on the criteria used for defining its presence.1 In Mexico the estimated prevalence is between 16 and 35.5% in adult populations.20,21 The study by Schmulson et al. with adult volunteers from Mexico City showed a prevalence of 35.5%, almost 2-fold greater than that reported in an open population (16%), with a woman:man (W:M) ratio of 4:1.27.20 There are no available data on prevalence in pediatric patients in Mexico.
The causes of IBS are not completely understood, but there is evidence of the participation of multiple pathophysiologic mechanisms, all of which are interrelated. They include genetic and psychosocial factors, visceral hypersensitivity, motility alterations, intestinal permeability, and inflammation.1,22–24
A potential explanation of IBS pathophysiology is the low-grade inflammation status of the intestinal mucosa.25 Intestinal inflammatory states, regardless of the specific events responsible for their onset, share common immunologic pathways that mediate tissue damage and repair. There is evidence that lends support to this theory of low-grade inflammation, as demonstrated in a recent systematic review on the participation of the inflammatory processes at the level of the intestinal mucosa.26 This review describes the numerous studies reporting that inflammatory cells, such as activated mast cells and B and T lymphocytes, are higher than normal in number in the mucosa of a group of IBS patients. Data also suggest that there is a state of immunologic activation manifested by increased cytokine production at the level of the mucosa, blood, and feces, which can be a product of the activation of mast cells and other immune system cells.25 However, it is very unlikely that these are the only alterations responsible for IBS etiology.
Cytokines are proteins produced by the activation of immunologic cells that influence the activity, differentiation, and proliferation of other cells, modulating the innate and adaptive immune response.27 It is known that these proteins play an important role in the pathogenesis of inflammatory bowel disease, which is the most severe state of intestinal inflammation, but they also influence other states of tissular damage, such as infectious enterocolitis, celiac disease, eosinophilic gastroenteritis, and they have recently been described in IBS.26,28,29 Their presence is also found in many other diseases outside of the gastrointestinal tract that involve infectious, allergic, or autoimmune inflammatory phenomena. These cytokines are present in the gastrointestinal mucosa, blood (monocytes and plasma), and feces, and can be measured in these tissues or samples.16,19,24,29 Cytokines can influence the function of the epithelial cells, smooth muscle cells, and the enteric nervous system. Changes in the cytokine profile of patients with IBS can exacerbate changes in secretion, permeability, motility, and intestinal sensitivity, and produce symptoms of IBS.19,24,30,31
A clear cytokine profile in blood in patients with IBS does not yet exist. Results are not consistent in all the studies, which could be the result of measuring, methodology, and study population differences.32,33 A decrease in the levels of anti-inflammatory cytokines, such as IL-10, has been shown in adult IBS patients, as well as an increase in the proinflammatory cytokines, such as TNF-α, IL-6, IL-8, and IL-12, displaying a proinflammatory profile in these patients.16,33–35
In a study on adult patients, the cytokine level in the supernatant from a non-stimulated monocyte culture was measured and showed a lower IL-10 concentration and a higher IL-12 concentration in patients with IBS, compared with healthy subjects; and the IL-10/IL-12 index was lower in the IBS group.16 However, a recent study conducted on 39 adults with IBS and 34 healthy controls showed no differences with respect to IL-1 β, IL-6, IL-8, IL-10, IL-12, or TNF-α in serum levels or in the colonic mucosa; there was only a difference at the level of the messenger RNA of IL-10 in the colonic mucosa in the subgroup of female patients.36 In the Mexican adult population, low levels of serum IL-10 were shown in IBS patients compared with healthy controls.35
At the time our study was conducted there was no information on the profile of these cytokines in pediatric patients. A recently published study that measured the cytokines in the supernatant of a non-stimulated peripheral blood mononuclear cell (PBMC) culture with lipopolysaccharides from Escherichia coli showed that IL-10 levels were lower in pediatric patients with IBS (82.6±46.41 pg/mL), compared with healthy controls (214.65±76.23). The levels in both groups were very similar to those found in our study.19
The low IL-10 levels found in our population of children with IBS, compared with healthy controls, are consistent with those found in other studies on the adult population, including Mexican adults, as well as in another pediatric study.16,19,35 Likewise, IL-12 levels were also increased in our group with IBS, similar to that reported in the adult population.16
Unlike the studies on adults, including those on Mexican patients, our study did not show a difference in the plasma levels of TNF-α in the pediatric population.35,37 Nevertheless, it is striking that very high levels of TNF-α were found in both groups of children, compared with that reported in another study that also measured TNF-α plasma levels, even though the study was conducted on adult women that had other comorbidities.38 In a study by Hua et al. on a pediatric population, they also found very high levels of TNF-α in a supernatant of a PBMC culture, but unlike our study that measured plasma levels, they found differences between the 2 groups, with higher levels in the children with IBS.19 Another report on adult patients showed no evidence of a difference in TNF-α between the 2 groups, but it corresponded to measurements of a supernatant of a monocyte culture stimulated by lipopolysaccharides for 72h prior to measurement; the authors commented that their results were probably related to the limited number of patients in their study.39 It is known that TNF-α has a proinflammatory effect and it has been clearly implicated as a trigger of the septic phenomenon by increasing the plasma concentrations of small mediating molecules such as the platelet-activating factor, prostaglandins, and nitric oxide. In relation to the participation of TNF-α in IBS, in addition to its being found in higher levels in adults with IBS, compared with controls, both in serum and in the supernatant of non-activated polymorphonuclear cells, other studies have also reported that the combined possession of the high producer TNF-α genotype (G/A heterozygotes) and the low producer IL-10 genotype is a risk factor for IBS, given that this combination was significantly more prevalent in the subjects with IBS, mainly those with IBS-D.17,20 However, in a study on Mexican adult volunteers, no differences in the lL-10 (-1082G/A) or TNF-α (-308G/C) genotypes were found between the subjects with IBS and the controls.40
To the best of our knowledge, no reports have measured the plasma levels of TGF-β in pediatric or adult patients with IBS; we decided to measure it because it is an important immune response modulator. Our study showed higher plasma levels of TGF-β in the patients with IBS, compared with the controls. The specific role that TGF-β has in the pathogenesis of IBS is not known. Even though a study on adult patients did not show any differences in the frequencies of genotypes for TGF-β between patients with IBS and healthy controls,6 it is thought that TGF-β could be involved in the pathophysiology of IBS. There is evidence that TGF-β participates in the pathophysiology of inflammatory bowel disease, specifically Crohn's disease. It acts together with IL-6 as an inducer of Th-17 cells, which produce IL-17 and IL-22 implicated in the production of inflammation at the tissular level; the Th-17 cells are then modulated by IL-23. There is also evidence that the differentiation of Th-17 cells in the absence of IL-23 leads to IL-10 production, which is why these cells are poor inducers of inflammation. Th17 cells require TGF-β for their differentiation. In addition, T cells produce large quantities of TGF-β and they can induce naive CD4+cells to produce IL-17 and can themselves be converted into Th17 cells.29 Furthermore, the data obtained from the study by Gebhardt et al. indicate that TGF-β1 acts as a potent inhibitor and modulator of mast cells, in such a way that the study confirms what has been referred to in other articles, about the fact that TGF-β1 is an anti-inflammatory cytokine that is constitutively expressed in the intestine.41 The plasma levels of TGF-β that we found in our study could be increased as a response to the proinflammatory state that IBS patients present. However, the role that TGF-β plays in the pathogenesis of IBS is still to be defined.
The cytokine levels shown in our study, in the IBS patients as well as the controls, are very high in comparison with other similar studies. However, this is probably due to the type of commercial kit utilized. One study conducted on pregnant women, specifically during their first trimester, in which TNF-α levels were determined, reported that one of the assays employed (Pierce®) showed levels using 1 to 2-digit figures (4.23 pg/mL; 1.34 –77.2 pg/mL interval), whereas the measurements with TiterZyme®, the same kit employed in our study, were expressed with 2 to 3-digit figures (176.96 pg/mL; 54.7-283.9 pg/mL), in ranges similar to those of our study.42 A Colombian study carried out on obese and eutrophic children, utilizing another commercial kit, showed TNF-α levels in three-digit figures, with a wide standard deviation (210.27+281.96 pg/mL vs 284.28+384.99, respectively).43 It is feasible that the very high results found in the 2 groups of our study are secondary to the type of commercial kit used, making the comparison with other studies difficult.
Our study has the following limitations: IBS subtype comparisons could not be made because of the small sample size; the study was carried out on patients that were seen at a tertiary care hospital, rather than on an open population, and therefore our results cannot be generalized; celiac disease was not ruled out appropriately in all the patients; and finally, cytokine measurement was not made directly from the colonic mucosa to determine if it was correlated with the serum levels.
ConclusionsOur results showed that children with IBS presented with an altered immune regulation state by showing plasma levels that were lower for IL-10 and higher for IL-12. No difference in TNF-α levels was found between the 2 groups. TGF-β levels were higher in the patients with IBS, but the role this plays in IBS pathogenesis remains to be determined. These results suggest that the alteration in immune system modulation may be participating in the development of IBS in children.
Financial disclosureFinancial support was received from the Mexican federal government in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Vázquez-Frias R, Gutiérrez-Reyes G, Urbán-Reyes M, Velázquez-Guadarrama N, Fortoul-van der Goes TI, Reyes-López A, et al. Perfil de citocinas proinflamatorias y antiinflamatorias en pacientes pediátricos con síndrome de intestino irritable. Revista de Gastroenterología de México. 2015;80:6–12
See related content at DOI: http://dx.doi.org/10.1016/j.rgmx.2015.01.001 Schmulson M, Saps M y Bashashati M. Regulación inmune anormal en niños con síndrome de intestino irritable. Revista de Gastroenterología de México. 2015;80(1):3–5.