Liver transplantation is a lifesaving treatment that improves survival and quality of life. The procedure requires adequate transplant candidate selection carried out by a multidisciplinary team. Psychosocial evaluation is a necessary part of recipient selection and its primary aims are to identify problems and psychosocial needs of the patient and his/her family, to improve transplantation outcomes. Different psychosocial conditions are considered risk factors for morbidity and mortality after transplantation. The presence of those factors per se is not an absolute contraindication, thus adequate evaluation promotes equal access to healthcare, improves results, and optimizes resources. The present review provides an overview of and guidelines for the most important psychosocial issues during the pretransplantation phase.
El trasplante hepático es un tratamiento que salva vidas, mejora la supervivencia y la calidad de vida. El procedimiento requiere de una adecuada selección de los receptores, llevada a cabo por un equipo multidisciplinario. La evaluación psicosocial es necesaria para la selección de los receptores y tiene como objetivos principales la identificación de problemas y necesidades psicosociales del paciente y su familia para mejorar los resultados del trasplante. Diferentes condiciones psicosociales son consideradas factores de riesgo para morbilidad y mortalidad posterior al trasplante. La presencia de estos factores por si solos no es considerada contraindicación absoluta, por lo que una adecuada evaluación, promueve la equidad en el acceso a la salud, mejora los resultados y optimiza los recursos. Esta revisión proporciona una visión general y una guía de los aspectos psicosociales más importantes durante la fase pre-trasplante.
Over the past 50 years, orthotopic liver transplantation (LT) has become a worldwide practice, and is currently a standard therapy for acute liver failure and end-stage liver disease (ESLD).1 In general, transplantation should be considered in all patients whose life expectancy would be shorter without a transplant, or when the procedure is likely to improve their quality of life (QoL).1,2 Thanks to advances in surgical techniques, anesthesia, infection treatment, critical care, immunosuppressants, and early and optimal management of complications, the LT survival rate has reached 96% at one year and 60% at 10 years.3,4
The involvement of a multidisciplinary team at all stages of the process has improved outcomes for patients with chronic liver disease or ESLD, and includes working closely with patients while they are on the waiting list, during the transplant, and during their early postoperative care, as well as their long-term postoperative care.1,5–7 Psychosocial evaluation is a significant part of that process and includes the identification of any potential psychosocial issues or psychiatric comorbidities that could influence results.7 Therefore, efforts to select candidates believed to have the best chance for optimal posttransplant outcomes have been complemented with the evaluation of mental health professionals.8,9 The aim of the present article was to provide an overview of both the psychosocial issues that gastroenterologists/hepatologists must be aware of during the pretransplant phase and the psychosocial evaluation of the liver transplantation candidate.
Psychosocial issues in liver transplant candidatesThe primary goal of a pretransplant psychosocial evaluation is to determine whether a patient has psychosocial characteristics that may negatively affect adjustment to ESLD, the transplantation process, or posttransplant outcomes.10 Early identification of those issues provides the liver transplant team with the opportunity to develop treatment plans in advance,11,12 and promotes fairness and equal access to transplantation and care.
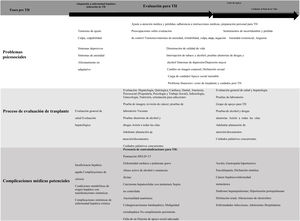
Liver transplant candidates encounter adaptation challenges at each phase of the illness and treatment (i.e., chronic illness/organ failure, pretransplant evaluation, and waiting list). Those phases can be stressful and can have psychiatric and/or psychosocial implications13 (Fig. 1).
The trajectory of functional decline in patients with ESLD can be unpredictable. Many patients are in a constant state of poor or declining health and often have acute hepatic decompensations and require hospitalization.14 Many liver disease-related factors interact to create a profound effect on the patient’s life, as well as that of his/her family. The most important of those factors are functional limitations, uncertain prognosis, emotional stress, financial problems, and the prolonged course of medical treatment. Thus, a psychosocial evaluation must assess stress, crises, type of coping with loss and grief, the impact on body image and QoL, and the presence of stigma.15
Acceptance of the role of patient is an important factor in facilitating patient participation in medical care, but the rate and level of acceptance may vary, based on the nature of his/her condition. For example, acceptance may develop gradually, in the case of chronic diseases, whereas patients with sudden, unexpected onset of liver failure may be particularly vulnerable to acute maladaptive denial, which can result in failure to adhere to best practices (i.e., noncompliance with medical treatment or the consumption of tobacco, alcohol, and other substances).16
Pretransplant evaluation phaseA patient during LT evaluation often requires adjustment to different losses (i.e., health, professional/occupational, financial, physical, and social). As a result, they can experience a range of emotions: distress, anxiety, irritability, guilt, anger, denial, a feeling of uncertainty, and/or loss of control due to declining functionality.17,18 Such a situation is particularly relevant in patients with a history of mental disorder10,19,20 because there can be a relapse or an exacerbation of symptoms. The patient and his or her family/social support network should keep in mind that medical care will continue after the transplant and that different complications can occur. Thus, it is imperative for the transplant team to engage in a cooperative, integrated effort to present a precise, timely, and consistent message to the patient and his/her family, when educating them about the transplantation process, medical care, and follow-up.16
Waiting list phaseIn many cases, patients are often required to wait longer for a liver due to organ shortages, thus risking serious health deterioration before the transplant or even the development of an absolute contraindication that can lead to death. That stage is the most stressful phase of the transplant process for most candidates.21 Comorbid mental disorder has been described to present in 40% of the patients during the waiting list period.22 Consequently, the relationship with the transplant team can become vulnerable at that time.
Mental disorders during the evaluationMental health disorders are common in patients with ESLD and LT candidates,23 but data related to other mental health disorders are scarce, given that most of the existing literature focuses on mood disorders, particularly depression.9
Mood disorderDepression is the most prevalent mood disorder discussed in the medical literature. It is characterized by a pervasive and persistent low mood that is accompanied by low self-esteem and a loss of interest or pleasure in normally enjoyable activities, for at least two weeks. Patients with ESLD, such as hepatitis B virus (HBV), hepatitis C virus (HCV), nonalcoholic fatty liver disease (NAFLD), and alcoholic liver disease (ALD), may present with different degrees of depression, which can unfavorably affect medical care, adherence, and outcomes.24 A past study reported a depression prevalence of 60% for patients on the waiting list and noted it was associated with poor QoL, poorer adaptive coping, lower functional status, and increased risk of dying during the waiting period, even after controlling the severity of the illness, and despite the fact that the survival rate after LT was no different between depressed and nondepressed patients.25 In a retrospective cohort study, 28% of the sample had depression and 8% had depression and anxiety. After depressed and nondepressed transplant recipients were compared, depressed patients had a higher prevalence of ALD (37% vs. 25%), of illicit drug use (35% vs. 25%), and of psychiatric morbidity one year after LT (HR 2.28; 95% CI: 1.27-4.11).26 However, there were no differences at year one in rates of hospital readmission, graft failure, infections, acute rejection, or death between the depressed and nondepressed recipients. Likewise, another study found that the presence of depression during the waiting period did not increase the risk of death in the posttransplant period (HR 2.43, 95% CI 0.88-6.70, p=0.09).27 Nevertheless, a prospective cohort study on 167 patients transplanted for ALD showed that appropriate treatment was necessary to reach those outcomes. Depression prevalence was 29% and recipients that were not treated for depression had a lower survival rate, compared with depressed patients that received treatment (HR 2.44, 95% CI: 1.45-4.11),28 suggesting that effective antidepressant treatment could improve transplant outcomes.
We must remember that de novo depression or a reactivation of depression can appear in times of stress. Notable triggers include undergoing the LT process, prolonged waiting list time, the presence of complications during the evaluation, and/or the presence of acute-on-chronic liver failure.28 Of course, depression per se is not an absolute contraindication for LT, but its early recognition and adequate treatment are important for optimizing postoperative outcomes.
Anxiety disordersDifferent studies report a prevalence of 21-50% for anxiety disorders or anxiety symptoms in patients during the wait list period.8,29 One study showed that anxiety was associated with poorer QoL, compared with LT recipients with no anxiety.30 In that study, the author suggested that greater levels of anxiety directly and indirectly affected QoL (e.g., by exacerbating symptoms, as previously suggested).31 Another possible explanation is that anxiety increases the tendency of patients to fail to adhere to a course of treatment. Such nonadherence impairs QoL because it is a major risk factor for graft rejection and other complications.32 In a prospective, multicenter cohort study on 153LT recipients,33 the trajectories of anxiety symptoms after LT were described, and 23% of the patients were found to have persistent anxiety. Several clinical variables were associated: the adverse effect of immunosuppressive medication, a lower level of personal control, a non-adaptive coping style, and stressful life events. That group of patients also had lower adherence and impaired QoL. Those results emphasize the relevance of psychosocial care, before and after transplantation. Further studies are needed to identify the early and continued treatment interventions that can improve QoL in those patients.
Substance use disorder and its importance during evaluationSubstance use disorders are linked to patients with ESLD.24
Alcohol use disorderIn the early days, ALD was considered a poor indication for liver transplantation and several patients were denied that lifesaving therapy. More recently, knowledge regarding the implications of ALD for LT has increased considerably, and with it, the suggestion that selected patients with ALD can become very good candidates for LT. Currently, ALD alone, or in combination with HCV infection, is one of the most common indications for LT worldwide.1,7 The amount of alcohol consumed over time is considered the main risk factor for ALD.34 Other identified risk factors are poor nutritional status, female sex, genetic predisposition, and the presence of an additional chronic liver disease.35 The American Society of Transplantation and the American Association for the Study of Liver Disease (AASLD) recommend the early referral of ALD patients to an LT center, facilitating psychosocial assessment and addiction treatment (if the patient is too sick to attend a rehabilitation program, posttransplant counseling and treatment may be appropriate), to obtain the best outcomes.2,7 Despite recent efforts to implement this updated perspective, 95% of patients with ALD are estimated to not be referred for LT evaluation, even when they meet the AASLD criteria.7,36 Current drinking is one of the reasons for a late referral. Historically, a 6-month minimum period of abstinence has been commonly enforced (although that requirement may vary between programs).1,35,37
Different cohort reports have shown that survival rates in patients that underwent LT for ALD were comparable to those of patients that underwent LT for other indications.38,39 Importantly, only 6 months of having achieved abstinence, without appropriate assessment or treatment, does not therapeutically address a potential addictive disorder.2 Apart from the pretransplant period of abstinence, evaluating the severity of the patient’s drinking, as outlined in the Diagnostic and Statistical Manual of Mental Disorders, version 5 (DSM-5), is crucial.40 The Alcohol Use Disorder Identification Test (AUDIT) and CAGE Questions for Alcohol Use can be used to screen for consequences of drinking and hazardous alcohol consumption.41 A detailed history of alcohol consumption patterns and other substance use should be obtained. For each substance, the duration of use, quantities used, problems that arose due to consumption (individual, family, work, health, legal, and financial), periods and duration of sobriety, treatment received, factors associated with relapses, and support for maintaining sobriety should be assessed.
Nevertheless, alcohol relapse on the waiting list is detected in up to 25% of cases.42 Therefore, different strategies have been recommended to identify alcohol consumption in LT recipients, such as indirect biomarkers: i) gamma-glutamyl transferase, ii) aspartate aminotransferase, iii) alanine aminotransferase, iv) mean corpuscular volume, and v) carbohydrate-deficient transferrin percentage and direct biomarkers: vi) methanol, ethanol measured in serum, and even vii) ethyl glucuronide, as measured in urine and hair (for an extensive review, see Staufer et al.43).
The prevalence of alcohol consumption after LT is nearly 20%, especially for the amount considered harmful for the graft.39 The 6-month abstinence period has been shown to improve LT outcomes and several risk factors have been identified. They include younger age, a failure to adhere to medical treatment, an inability to remain abstinent before transplant, severe alcohol use disorder, longer durations of heavy drinking, a greater number of drinks per day, repeated alcohol treatment failures, social instability (work and home environment), absence of social support, first-degree relatives with an alcohol use disorder, current polydrug use, and comorbidity with severe mental disorders (i.e., personality disorder),8,44–46 and they should be evaluated in each patient.47 The objective of such an evaluation is to improve patient selection before LT and optimize surveillance and early detection of alcohol consumption during the protocol evaluation.43
It is important to consider the high prevalence of other mental disorders in those patients (dual disorders). In a study on 71 patients, 66% with ALD and 32% with non-ALD were found to also meet the criteria for mood disorders.48 A diagnosis of depression has been associated with an increased risk of alcohol consumption relapse after transplant but it does not appear to be associated with greater morbidity or mortality.11
Psychosocial interventions, such as addiction counseling before LT, appear to reduce drinking in the pretransplant period.49 Motivational enhancement therapy combined with contingency management can limit the quantity and frequency of alcohol consumption during the wait list time.50 Interestingly, psychosocial treatment, before and after LT, reduces relapse rates, compared with treatment only before LT or no treatment at all.35 Moreover, different prognostic tools to assess the risk of alcohol relapse after LT have been proposed (Table 1).
Prognostic instruments used to predict alcohol relapse after liver transplantation.
| Instrument | Risk factors assessed | Score | Interpretation |
|---|---|---|---|
| University of Michigan Alcoholism Prognosis Score (MAPS)74 | 1. Insight into alcoholism | ||
| 1. Patient and family | 4 | Total score range:5-20 | |
| 2. Patient only | 3 | ||
| 3. Family only | 2 | ||
| 4. Neither | 1 | ||
| 2. Prognostic indices | |||
| 1. Substitute activities | Yes=3 No=1 | A higher score indicates a reduced risk for relapse | |
| 2. Behavioral consequences | Yes=3 No=1 | ||
| 3. Hope/self-esteem | Yes=3 No=1 | ||
| 4. Social relationship | Yes=3 No=1 | ||
| 3. Social stability | |||
| 1. Steady job | Yes=1 No=0 | ||
| 2. Stable residence | Yes=1 No=0 | ||
| 3. Does not live alone | Yes=1 No=0 | ||
| 4. Stable marriage | Yes=1 No=0 | ||
| Alcohol Relapse Risk Assessment (ARRA)75 | 1. Absence of HCC | Present: 1 point | |
| 2. Tobacco dependence | Absent: 0 point | ||
| 3. Continued alcohol use after liver disease diagnosis | Total score range:0-9 | ||
| 4. Low motivation for alcohol treatment | Categories: | ||
| 5. Poor stress management skills | ARRA I: 0 pts | ARRA I: 0% | |
| 6. No rehabilitation relationship | ARRA II: 1-3 pts | ARRA II: 8% | |
| 7. Limited social support | ARRA III: 4-6 pts | ARRA III: 57% | |
| 8. Lack of nonmedical behavioral consequences | ARRA IV: 7-9 pts | ARRA IV: 75% | |
| 9. Continued engagement in social activities with alcohol present | |||
| High-Risk Alcoholism Relapse (HRAR)76 | 1. Duration of heavy drinking (years) | ||
| < 11 | 0 pts | Total score range:0-6 | |
| 11-25 | 1pt | ||
| > 25 | 2pts | ||
| 2. Usual number of daily drinks | |||
| < 9 | 0pts | <4 Low-risk alcoholism | |
| 9-17 | 1pt | ||
| > 17 | 2pts | ||
| 3. Number of prior alcoholism inpatient treatment experiences | 1pt | ||
| 0 | 1pt | ≥ 4 high-risk alcoholism | |
| 1 | |||
| >1 | |||
| Sustained Alcohol use post-Liver Transplantation (SALT)77 | 1. >10 drinks/day at presentation | + 4 pts | Total score range:0-11 |
| 2. ≥2 prior failed rehabilitation attempts | + 4 pts | A cutoff of ≥5, SALT had a c-statistic estimate of 0.76 to predict sustained alcohol use post-LT | |
| 3. Any history of prior alcohol-related legal issues | + 1pt | ||
| 4. History of non-THC illicit + 1pt substance abuse | + 1pt | ||
Different pharmacologic treatments have been approved for alcohol use disorder and the prevention of relapses, such as disulfiram, naltrexone, and acamprosate (Table 2). Of those, acamprosate undergoes minimal hepatic metabolism, has mild side effects, and can be safe for patients with ESLD.35,51
Medications used to treat alcohol use disorder.
| FDA approved | |
| Disulfiram | Selected chronic alcohol patients that wish to remain in a state of enforced sobriety. Moderate or severe drowsiness. Severe adverse events (hepatitis, neuropathy, optic neuritis, psychosis, and confusional states) are rare. Hepatoxicity risk. |
| Naltrexone | Treatment of alcohol dependence. Can precipitate withdrawal symptoms in a patient physically dependent on opioids. Hepatoxicity risk. |
| Long-Acting Naltrexone | Treatment of alcohol dependence in patients that can abstain from alcohol in an outpatient setting. Monthly intramuscular application. Can precipitate withdrawal symptoms in a patient physically dependent on opioids. Hepatoxicity risk. |
| Acamprosate | Maintenance of abstinence from alcohol in patients with alcohol dependence that are abstinent. Lack of side effects. Not metabolized; can be used in patients with hepatic disease. |
| Non-FDA approved | |
| Topiramate | Contraindicated in patients with a predisposition to or history of metabolic acidosis, renal calculi, and secondary angle-closure glaucoma. Hepatoxicity risk. |
| Nalmefene | European Union approved: Helps reduce alcohol consumption in adults with alcohol dependence that consume > 60 g (≈4 drinks) per day (men) or > 40 g (≈3 drinks/day) (women) |
| Baclofen | Recommended in France for use in the management of alcohol dependence at a maximum recommended dosage of 80 mg/d |
FDA: Food and Drug Administration.
Up to 60% of LT candidates have a history of smoking.52 That subgroup is characterized by different adverse outcomes, with respect to LT.53 They include increased morbidity (i.e., hepatic artery thrombosis,54 oropharyngeal neoplasm,55 and gastrointestinal neoplasm56) and mortality. Therefore, some LT programs consider cigarette consumption cessation an important condition before permitting candidates to enlist, and may require negative serial nicotine screens to document cessation.2 Stopping smoking obliges an individual to overcome nicotine dependence and abandon a deeply ingrained rewarding behavior. Even brief advice from a gastroenterologist/hepatologist can increase the likelihood of successful smoking cessation. Evidence-based smoking cessation treatments include pharmacotherapies (including nicotine replacement therapy, varenicline, and bupropion) and behavioral treatment (e.g., brief advice and counseling). Both behavioral therapies and medications are effective for helping people stop smoking. The existing research suggests that a combination of the two types of strategies is the most effective approach.57,58
Intravenous drug dependenceThe use of intravenous drugs is importantly related to chronic liver diseases because it is an important risk factor for HCV infection.59 Thus, substance use disorder should be carefully screened for, alongside alcohol and tobacco use. Patients with comorbid HCV/HBV and alcohol use disorder tend to be younger, perhaps because the combined effect of alcohol and hepatotropic viruses accelerated their path toward ESLD.29 That situation is particularly relevant in women because they are more susceptible to the hepatotoxic effect of alcohol.60 Many LT programs require abstinence from all illegal drug use and utilize random screening to verify abstinence. In the case of opioid use, only 1.6% of LT programs considered chronic opioid use or opioid substitution therapy an absolute contraindication, whereas nearly 64% and 38% considered one of the two a relative contraindication, respectively,61 most likely because patients with ESLD have an increased burden of conditions associated with chronic pain. However, it is important to mention that opioid-related complications have been reported. For instance, Randall et al.62 found that opioid use in the first year after LT had prognostic implications and bore a graded association with subsequent death and graft loss. Patients that chronically use opioids should be evaluated extensively before transplantation and attempts to wean them off of the medications should be implemented. With respect to LT, the literature suggests that the current abuse of opioids is a contraindication and patients with addiction need to be involved in a rehabilitation program.
Marijuana consumptionMarijuana is the most commonly used illegal drug worldwide. At the same time, recreational use and abuse of marijuana have become an increasingly important topic in LT because of its legalization in an increasing number of states or countries, for both recreational and medical use.63,64 As a result, among the psychosocial issues involved in the LT candidate’s evaluation, the use of marijuana is one of the most controversial aspects. That was made manifest in a survey carried out in the United States that examined LT providers’ views regarding the manner in which controversial psychosocial characteristics influence candidate eligibility for LT. The most controversial issues were found to be i) incarceration, ii) marijuana use, and iii) psychiatric diagnoses.65 Likewise, a survey of liver transplant programs in the United States, intended to evaluate policies regarding marijuana use (including reactional use) in LT candidates, revealed that 33 programs (72%) would not accept patients that used marijuana recreationally. Another 13 programs stated that they would accept patients that used recreational marijuana, but 5 of them said they would permit such patients on a case-by-case basis. One of the programs required that patients quit marijuana use, at least 3 months before LT, and undergo a 12-step program.66
Ranney et al.67 found that marijuana users were more likely to have benzodiazepines, amphetamines, and other narcotics in their evaluation reports. Nevertheless, no significant differences in survival (HR 1.09; 95% CI 0.78-1.54) or racial and psychiatric comorbidity were identified between the groups. Serrano Rodriguez et al.68 conducted a retrospective analysis of adult LT recipients, evaluating morbidity and mortality after LT in marijuana users. They identified a prevalence of marijuana users (current/former) of 26% and a prevalence of tobacco/marijuana smokers of 20%. The 1-year and 3-year survival rates were 96% and 91% for former marijuana users, respectively, and 100% and 85% for current marijuana users, respectively. The overall 5-year survival was 75% and no significant difference in 5-year survival was seen between never users, current users, and former users. Furthermore, no significant interaction between marijuana and tobacco use was seen in all-cause 5-year mortality (p=0.79).
When evaluating those patients, different aspects should be considered, such as type of use, psychiatric comorbidities, other drug consumption, and the impact those issues have on healthcare. According to the available literature, marijuana use does not appear to adversely affect outcomes in LT recipients.69 However, it is important to note that the evidence regarding marijuana and liver transplantation is scarce, and so, in the absence of clear data, more definitive treatment, guidelines, and policies regarding marijuana use in LT candidates are needed.66,69
Summary of substance abuse disorders and their importance during evaluationIn summary, the detection of substance use disorders in patients with liver disease is of major relevance in patients that could become LT candidates. Information about history, current usage, and corroborated reports of abstinence from collateral sources is recommended. That can clarify the nature and severity of the disorder, enabling the team to intervene with strategies for treatment or relapse prevention.
Social, family, and financial issuesLT is a stressful procedure and the patient requires emotional and financial support, as well as regular follow-up.70 Therefore, social and family support are required for the overall success of the procedure. At the same time, the patient’s family/social support system often faces many challenges and adjustments, which can significantly impact family function/roles and the relationship the members of the support system have with the patient.71 Including the family members and/or caregivers in the evaluation consultations (medical and psychosocial) is recommended, to provide education and support, to identify mental health problems, and if necessary, refer the patient to a mental health center to receive specialized care. Inclusion of patients and family into educational and support groups has been found to improve adherence, facilitate the education process, offer social support, and increase a sense of control.16 From an economic perspective, LT is extraordinarily expensive in low-income and middle-income countries, and most patients cannot afford the procedure without medical insurance or some form of assistance (i.e., foundations). Therefore, financial counseling is highly recommended.
Pretransplant psychosocial/psychiatric assessmentPsychosocial evaluations are frequently used to assist in determining a candidate’s eligibility for transplantation and to identify psychiatric and/or psychosocial problems and needs that must be addressed to prepare the candidate and the family/social support network for LT.2,13,72,73 Comprehensive assessment of potential liver recipients is best accomplished within the framework of a multidisciplinary approach.16,20 The psychosocial assessment criteria and procedures may vary by center/program, but the relevant components of the psychosocial evaluation are shown in Table 3.
Elements of psychosocial evaluation for liver transplantation candidates.
| Mental health | Diagnoses and treatment of mental disorders (e.g., major depression, anxiety disorder) |
| Personality | Coping skills and personality characteristics |
| Substance consumption | Diagnoses and treatment of substance use/disorder (legal and illegal) and risk factors for relapse after LT |
| Cognitive function | Establish baseline cognitive functioning to be able to monitor postoperative changes |
| Adherence | Evaluate the candidate’s ability to collaborate with the transplant team and adhere to treatment (immunosuppressives, clinic appointments, laboratory tests, exercise, and self-monitoring) and identify barrier to adherence |
| Liver disease/LT indication | Knowledge and understanding of the clinical liver condition, disease course, treatment options |
| LT process | Knowledge and understanding of LT processes (including the different phases), risks, benefits, medical care, and potential complications |
| Decision-making | Determine the decision-making capacity to provide informed consent for LT: |
| 1. Autonomy | |
| 2. Information | |
| 3. Capacity: | |
| a) Understanding | |
| b) Appreciation | |
| c) Reasoning | |
| d) Choice | |
| 4. Informed consent document | |
| Social support | Evaluate the level and stability of social support available to the candidate for pretransplant and posttransplant phases (including stable family/others committed to assisting, adequate insurance and financial resources, and logistical support) |
| Determine the psychosocial needs of the patient and family for services during the waiting list, recovery, and rehabilitation periods | |
| LT expectations | Delineate the specific transplant-related expectations and establish a meaningful dialogue with the patient and family, establishing a therapeutic alliance |
LT: liver transplantation.
Due to the complex nature of the issues that must be analyzed during the psychosocial evaluation, different clinical evaluations are often necessary. Those evaluations aim to clarify relevant issues, construct a working relationship with the patient and family, and resolve problems.13 Collateral information may be required from medical records, other medical specialties (i.e., transplant surgeons, hepatologists, nutritionists), psychosocial healthcare providers (i.e., social workers, psychologists, psychiatrists, and addiction specialists), and family members (always with the patient’s authorization). Interviews with family members offer an excellent opportunity to assess the nature and quality of the patient’s social/family support network and can facilitate the expression of family members’ concerns and fears about the transplantation and their perceived role in the process.74
Different instruments have been developed to assess the reliability and validity of the selection of transplant candidates. Nevertheless, none of them are meant to substitute the psychosocial/psychiatric clinical evaluation and they are generally used in conjunction with instruments to evaluate psychiatric and psychologic characteristics. The most widely used recipient selection instruments are75:
Stanford Integrated Psychosocial Assessment for TransplantationThe Stanford Integrated Psychosocial Assessment for Transplantation evaluates 18 psychosocial risk factors and provides an overall risk severity score, ranging from 0 to 115, with higher scores indicating greater risk of negative outcome. Risk factors are divided into four domains: 1) patient readiness level, illness knowledge, and understanding of illness management; 2) social support system level of readiness; 3) psychologic stability and psychopathology; and 4) lifestyle and effect of substance use.
Within each domain, criteria are specifically defined with an associated score. Risk factors that are more predictive of clinical outcomes are more heavily weighted.76 This instrument aids the transplant team in knowing as much as necessary about the psychosocial factors that could influence transplant outcome.
Psychosocial Assessment of Candidates for TransplantationThe Psychosocial Assessment of Candidates for Transplantation (PACT) is an 8-item clinician-rated semi-structured scale that evaluates social support, lifestyle factors including substance use, consistency with medications, psychologic health, and understanding of transplant and follow-up, in addition to the rater’s overall impressions. Each item is evaluated on a scale from 0 (the lowest score) to 4 (the highest score). The final global assessment integrates all of the previous items into one final score and assigns candidates an overall 0-4 score. A score of 0 denotes a poor candidate, 1 is a borderline candidate, 2 an acceptable candidate, 3 a good candidate, and 4 an excellent candidate. Thus, the PACT is a subjective determination of whether the severity of a single factor (i.e., absence of a support system or active addiction) or, alternately, multiple factors combined, could place the patient at high risk.16,77
Transplant evaluation rating scaleThe Transplant Evaluation Rating Scale (TERS) consists of 10 items which index aspects of psychosocial functioning thought to be important in adjusting to transplantation. Each item is rated by the clinician on a three-point scale of relative severity, based on a clinical interview. Each item rating is multiplied by an a priori assigned weight (range 1-4), and the weighted ratings are added together to provide a total score (range 26.5-79.5), with higher scores indicative of more problematic presentations.78
In summary, the use of assessment instruments, such as those mentioned above, helps clinicians eliminate the emotional and subjective factors from the decision-making process, preventing their influence on the selection process.
Management of psychiatric and psychosocial issuesThe psychiatrist/mental health professional plays an important role in the prompt identification and treatment of psychiatric disorders. Many of those conditions can be treated effectively with pharmacologic and/or psychologic treatments, and general recommendations are shown in Table 4. The clinician prescribing psychotropic medications for a patient with liver disease should consider the severity of the disease, comorbidities, renal function, electrolyte levels, complications of the liver disease (e.g., ascites, variceal bleeding, and/or hyponatremia risk), drug-drug interactions, potential side effects, and the risk of hepatic encephalopathy. The safest treatment recommendation early on is to lower initial dosages, lengthen the dosing intervals, and then gradually titrate the dose, so that the drug level reaches a steady state more slowly. In many cases, cirrhotic patients will require an adjustment (in most cases, dosage reduction), as their liver function deteriorates over time.
Psychopharmacologic drug use recommendations in end-stage liver disease.
| Drug | Indications | Dosing information | Comments |
|---|---|---|---|
| Antidepressants | |||
| SSRIs | Major depression anxiety disorders, eating disorders, OCD | Extensively metabolized; decreased clearance and prolonged half-life. Target doses are typically substantially lower than usual | Potential drug interactions (fluoxetine and paroxetine), few side-effects, hyponatremia risk, QT prolongation risk (citalopram and escitalopram), high discontinuation syndrome risk (paroxetine), anticholinergic side effects (paroxetine), long half-life (fluoxetine) |
| TCAs | Major depression, anxiety disorders, pain | Extensively metabolized | Potential hepatoxicity, QT prolongation risk, anticholinergic side effects, hypotension risk, can precipitate hepatic encephalopathy |
| SNRIs | Major depression, anxiety disorders, pain | Duloxetine: do not use in patients with any hepatic insufficiency | Dose-dependent blood pressure elevation (venlafaxine), hyponatremia risk (venlafaxine), few drug interactions, and few side-effects (desvenlafaxine) |
| Desvenlafaxine: No adjustment required | |||
| Venlafaxine: Reduce dosage by 50% in Child-Pugh B or C | |||
| Other antidepressants | Major depression, anxiety disorders, nightmares in PTSD (trazodone), nausea (mirtazapine), fatigue, and smoking cessation (bupropion) | Extensively metabolized | Excessive sedation, priapism risk (trazodone) |
| No dosing guidelines | Few sexual side-effects (mirtazapine and bupropion). Low hyponatremia risk, low metabolic risk, potential hepatotoxicity, and seizure risk in high doses (bupropion) | ||
| Bupropion: do not exceed 100mg/day for sustained-release formulations in Child-Pugh C | High metabolic risk (mirtazapine) | ||
| Mood stabilizers | |||
| Lithium | BPD | Renally excreted, not metabolized. Adjust dosage based on renal function | Nephrotoxicity risk, intoxication risk, drug interaction with ACE inhibitors, calcium channel blockers, NSAIDs, loop diuretics, tramadol (serotoninergic syndrome risk), and thiazide diuretics. |
| Divalproex | BPD | Extensively metabolized; reduced clearance and increased half-life, reduce dosage, avoid in Child-Pugh C | Potential hepatoxicity, neutropenia, and thrombocytopenia |
| Lamotrigine | Bipolar depression | Initial, escalation, and maintenance dosages should be reduced (by 50% in Child-Pugh B and by 75% in Child-Pugh C) | Rash, Stevens-Johnson syndrome risk |
| Antipsychotics | |||
| Typical | Psychosis, delirium, agitation | Extensively metabolized | Risk of an extrapyramidal and neuroleptic syndrome, increased QT |
| No specific dosing recommendations | |||
| Atypical | Psychosis, delirium, BPD | Extensively metabolized | Risperidone: risk of extrapyramidal symptoms (dose-related) |
| Quetiapine: Start at 25mg/day; increase by 25-50mg/day. | Quetiapine and olanzapine: low risk of extrapyramidal symptoms, sedation risk, high metabolic risk | ||
| Risperidone: starting dosage and dose increment not to exceed 0.5mg twice daily | Aripiprazole: low risk of metabolic and extrapyramidal effects | ||
| Benzodiazepines | Anxiety | Alprazolam and clonazepam: decreased metabolism and increased half-life | Sedation falls risk, cognitive impairment (long time use), abuse and dependence risk, can precipitate hepatic encephalopathy |
| Lorazepam, oxazepam, temazepam: metabolized by conjugation; clearance not affected. No dosage adjustment is needed. | |||
| Zolpidem | Insomnia | Extensively metabolized, not recommended in Child-Pugh C | Abuse and dependence risk |
| Gabapentin and Pregabalin | Anxiety, pain | Renally excreted, not metabolized, adjust dosage based on renal function | Sedation falls risk, can precipitate hepatic encephalopathy |
BPD: bipolar disorder; OCD: obsessive compulsive disorder; PTSD: posttraumatic stress disorder; SNRIs: serotonin norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants.
Patients that present with fulminant hepatic failure require emergent evaluation. Those patients are often unable to undergo a lengthy evaluation process and, in some cases, may not even be conscious. Under such conditions, informed consent cannot be obtained, and interviewing family and/or social support members to determine a patient’s eligibility for transplant is critical, particularly if there are concerns about the patient’s ability to cope with the responsibilities of becoming a transplant recipient.16 In the case of fulminant hepatic failure due to acetaminophen or other toxic ingestion/overdose, it is necessary to determine whether the overdose was accidental or intentional (suicide attempt or self-inflicted harm). Details regarding the ingestion, mental health status, personality traits, prior history of suicide attempts or self-inflicted harm, substance use, current stressors, social support, and other risk factors for future suicide attempts must be obtained.21 One suicide attempt with a favorable history and other good prognostic factors is not a contraindication for LT. Emphasis should be placed on obtaining information from past medical records, especially that related to adherence, substance use, social support, and psychiatric illness.
Alcoholic hepatitis (AH)Patients with AH, with poor recovery indicators, may need an LT as a lifesaving procedure. In such a situation, healthcare providers cannot follow the patient longer or evaluate his/her capacity to remain sober. The authors of a multicenter study found that early LT improved the survival of patients with a first episode of severe AH that had not responded to other medical therapy.79 With those data, other transplant programs in Europe and the United States are beginning to consider patients with acute AH (even with <6 months of abstinence). Therefore, the transplantation team should be prepared to evaluate and provide treatment recommendations for shorter-term abstinence patients.80 In a recent meta-analysis, the author analyzed the alcohol relapse and 6-month survival rate of LT recipients due to AH.81 In the overall analysis, the pooled estimate for alcohol relapse was 0.22 (95% CI: 0.12-0.36) and the risk of alcohol relapse was no different between AH recipients and ALD recipients. Six-month survival in the AH group was similar to that of patients with ALD that underwent elective LT (OR=2.00: 95% CI: 0.95-4.23, p=0.07, I2=0%).
Those findings represent a paradigm shift in the treatment of highly selected patients with AH that are not responding to medical treatment. Nevertheless, more prospective studies are needed to resolve the controversies that still exist.
Bariatric surgery and LTNAFLD affects 25% of the global adult population. It is associated with different metabolic comorbidities82 that increase the risk of progressive chronic liver disease. Currently, NAFLD is one of the most important indications for LT worldwide. NAFLD/NASH is also the fastest growing indicator for simultaneous liver-kidney transplantation. Bariatric surgery (BS) appears to be feasible and effective in the setting of LT, although it is associated with a high postoperative complication rate. Therefore, its recommendation should be based on stringent selection criteria.83 In addition, a multidisciplinary approach is highly recommended to establish a risk minimization plan in those patients.83 Patients with NAFLD, who are considering LT, should be assessed by a psychiatrist/psychologist in the pretransplant setting because of the high prevalence of psychiatric comorbidity in obese patients undergoing BS. The most prevalent mental disorders in that population are mood disorders, anxiety disorders, and eating disorders. Presurgical comorbidity stemming from those issues could negatively impact postsurgical outcomes. Importantly, the identification and treatment of those conditions (e.g., through the use of weight loss and metabolic control) improves the preoperative and postoperative outcomes.84
Psychiatric evaluation of BS candidatesA successful outcome for BS is largely dependent on the patient’s ability to adhere to postoperative behavioral/lifestyle changes.85 A psychiatric and psychologic evaluation is often required during the protocol evaluation.86 An effective preoperative psychiatric work-up includes different clinical and psychosocial factors.87,88 Contraindications for BS are active psychosis, severe major depression, uncontrolled eating disorder (e.g., bulimia nervosa, binge eating disorder, night eating disorder), severe personality disorder, current substance use disorder including alcohol use disorder, neurodevelopmental disorder (intellectual disability) with no adequate social support system, noncompliance with nutritional requirements, and high risk for suicide (ideation, behavior, and a history of suicide attempts).86,87
The continuation of psychiatric care in the postoperative period is highly recommended. Such care can include supportive psychotherapy, regular follow-ups, and the adjustment of psychotropic doses (depending on drug and BS type).89–91
Are there psychosocial contraindications?There is considerable debate about whether psychosocial factors should be considered absolute exclusion criteria for LT. Although different psychosocial conditions have been contemplated as possible exclusion criteria for LT (Table 5), those conditions must be interpreted with caution, given that their presence per se does not exclude a patient.2,8,41,92,93
Relative psychosocial contraindications for liver transplantation.
| • Inadequate social support system |
| • Active illicit substance use |
| • Active alcohol dependence/abuse |
| • Active nicotine abuse |
| • Current manic or psychotic symptoms that may impair treatment adherence |
| • Current suicidal ideation (in a patient with a history of multiple suicide attempts or self-harm behaviors) |
| • Severe neurocognitive disorder with no adequate social support system |
| • Treatment nonadherence or inability to collaborate effectively with the transplant treatment team |
| • History of recidivism of substance abuse |
Team discussions and consultation with other colleagues are the rule in complicated cases. In those instances, team discussions not only aid in resolving candidacy quandaries, but also can help alleviate team members’ anxiety and discomfort over declining a patient for transplantation. Transplant candidacy should always be a team decision.13
Palliative carePalliative care (PC) is an approach that improves the quality of life of patients and their families facing the problems associated with a life-threatening illness, by preventing and alleviating suffering through early identification, assessment, and treatment of pain and other problems of a physical, psychosocial, and spiritual nature.94 Defined as such, PC does not apply solely to those facing imminent death. The discussion with patients and families about end-of-life issues and PC should, ideally, take place relatively early in the course of the protocol evaluation and such discussions should be revisited as often as indicated by changes in the patient’s condition, wishes, or care options. Patients must be prepared for all outcomes, before the loss of decision-making capacity occurs. An ESLD patient will often have periods of exacerbation followed by stabilization, making prognostication and care-planning more difficult, particularly in patients that remain hopeful for undergoing transplantation. The transition from aiming for a potential lifesaving LT to PC is particularly difficult.95 Thus, patients with ESLD are prime candidates for a concurrent palliative care model. Such a model allows for palliative care support for patients, even while awaiting LT or undergoing active protocol evaluation.96
ConclusionsA multidisciplinary approach in the evaluation of LT candidates includes the harmonization of various objectives, points of view, and measurement methods, which is a challenge for the transplant team and requires a patient-centered approach. Mental health providers play an important role in helping patients and their families to understand the overall transplantation process and deal with the plethora of psychosocial issues that may be involved. Hence, the psychosocial pretransplant evaluation promotes equal access to care, maximizes optimal outcomes, and enables the wise use of scarce resources. At the same time, it can provide: 1) risk/benefit data, by identifying and treating potential risk factors that may increase morbidity and decrease survival, 2) a treatment plan for individuals at high risk, and 3) information for the transplant selection committee that is needed to make the best clinical decision, based on the currently available data.
Ethical considerationsThe present research did not require the informed consent of the patients or the authorization of the ethics committee because it is a narrative review article.
The authors declare that this narrative review does not contain personal information that can identify the patients.
Financial disclosureNo financial support was received in relation to this article.
Authorship/collaboratorsAll authors have made substantial contributors to all of the following: conception, data acquisition, critical manuscript revision for important intellectual content, and final approval of the version to be submitted.
The view expressed in this paper is that of the authors and does not necessarily reflect the position or the policy of the Department of Transplantation at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: García-Alanís M, Toapanta-Yanchapaxi L, Vilatobá M, Cruz-Martínez R, Contreras AG, López-Yáñez S, et al. Evaluación psicosocial para trasplante hepático: una guía breve para gastroenterólogos. Revista de Gastroenterología de México. 2021;86:172–187.