The 5-aminosalicylates, especially mesalazine, are the first option in the treatment of mild-to-moderate ulcerative colitis (UC). High rates of remission induction and maintenance have been observed with the new multimatrix (MMX) mesalazine formulation, mainly in patients with distal disease. Our aim was to describe the real-world experience with MMX mesalazine in patients with UC at two tertiary care centers.
Materials and methodsA retrospective cohort study was conducted that included 142 patients with confirmed UC diagnosis, analyzed in three study groups: 1) oral MMX mesalazine as monotherapy for remission induction, 2) oral MMX mesalazine as monotherapy for remission maintenance, and 3) oral MMX mesalazine plus topical therapy for remission induction.
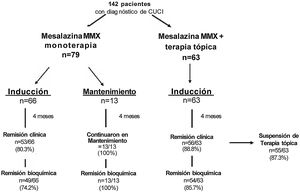
ResultsThe frequency of clinical remission induction in group 1 was 80.3%, with biochemical remission of 74.2%. Group 2 had 100% clinical and biochemical remission maintenance. The frequency of clinical remission induction in group 3 was 88.6%, biochemical remission was 85.7%, and topical therapy was suspended in 87.3% at the end of follow-up. No adverse events were documented.
ConclusionsThere were high percentages of clinical and biochemical remission in the two corresponding study groups and topical therapy was suspended in the majority of patients in ashort follow-up period.
Los 5-aminosalicilatos, principalmente la mesalazina, es la primera opción de tratamiento para la Colitis Ulcerosa Crónica Idiopática (CUCI) con actividad de leve a moderada. Con la nueva formulación de mesalazina multimatrix (MMX) se observan altas tasas de inducción y mantenimiento de la remisión clínica, principalmente en pacientes con enfermedad distal. Nuestro objetivo es describir la experiencia de la vida real en pacientes con CUCI y mesalazina MMX en dos hospitales de tercer nivel.
Material y métodosEs un estudio de cohorte retrospectivo donde participaron un total de 142 pacientes con diagnóstico confirmado de CUCI los cuales se analizaron en tres grupos de estudio: 1) mesalazina MMX vía oral en monoterapia para inducción a la remisión 2) mesalazina MMX vía oral en monoterapia para mantenimiento de la remisión y grupo 3) Inducción a la remisión con mesalazina MMX vía oral y terapia tópica.
ResultadosEn el grupo 1 se presentó una frecuencia de inducción a la remisión clínica del 80.3% con 74.2% de remisión bioquímica. En el grupo 2 se mantuvo la remisión clínica y bioquímica en el100%. En el grupo 3 la frecuencia de inducción a la remisión clínica fue del 88.6% y 85.7% de remisión bioquímica, con un 87.3% de suspensión de terapia tópica al final del seguimiento. No se documentó ningún efecto adverso.
ConclusionesSe presentaron altos porcentajes de remisión clínica y bioquímica en ambos grupos de estudio, con suspensión de terapia tópica en la mayoría de los pacientes en un corto tiempo de seguimiento.
Inflammatory bowel disease (IBD) is a chronic immune-mediated entity for which there is currently no cure, and treatment is for life. The primary treatment goal is to control the intestinal and extraintestinal symptoms, so the patient can have a better quality of life. Ulcerative colitis (UC) is limited to the rectum and colon1 and is the most frequent form of IBD in Mexico. For 2015, its incidence was 0.16 (95% CI: 0.14-0.18) per 100,000 persons and its prevalence was 1.45 cases per 100,000 persons. In the present study, extensive colitis was the most frequent, at 62.2%, followed by proctosigmoiditis, at 24.8%, and left-sided colitis, at 13%.2 Treatment of UC is based mainly on 5-aminosalicylates (5-ASAs) as the first option for the induction and maintenance of clinical and biochemical remission, and they are the most widely prescribed medications for UC.3 In addition, mesalazine suppositories in patients with proctitis and mesalazine enemas for patients with proctosigmoiditis and left-sided colitis are used for clinical remission induction.4 In recent years, the advances in IBD pathogenesis have led to the development of new treatment options. Multimatrix (MMX) mesalazine tablets are a new formulation of mesalazine, whose design with a polymer matrix enables prolonged continuous release throughout the colon. It differs from the other mesalazine presentations in that it contains a double matrix: a lipophilic matrix inside a hydrophilic matrix, preventing its alteration in the stomach. The tablets begin to dissolve only in the final portion of the ileum,5 which favors a larger quantity of treatment reaching the zones of greater inflammation in the colon and rectum. It has the advantage of being administered once a day, which is sufficient for achieving clinical and endoscopic remission. Consequently, there is no need for administering the medication in multiple doses throughout the day, as with other formulations,6 thus improving treatment adherence in patients with UC with mild-to-moderate activity.7 It has a good safety profile, with minimal adverse effects8 and adequate tolerance, and it does not interfere with the metabolism of other medications frequently used in IBD.9 Patients that have been treated with MMX mesalazine have substantial improvements in quality of life.10 The aim of the present study was to describe the real-world experience in patients with UC treated with MMX mesalazine at two tertiary care hospitals.
Materials and methodsA retrospective cohort study was conducted at the Inflammatory Bowel Disease Clinic of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán and the Gastroenterology and Obesity Service of the Hospital Médica Sur in Mexico City, within the time frame of May 2019 and July 2020. A total of 142 patients were included that were above 18 years of age, had a diagnosis of UC confirmed by histopathology, agreed to participate in the study, and signed a privacy notice for the protection of their personal data. All the patients from both hospitals that had UC and were in remission or had clinical activity, that at some time had been under treatment with other presentations of 5-ASAs (sulfasalazine or mesalazine), oral or rectal (suppositories and/or enemas), with adequate tolerance and no documented signs of adverse effects, were included in the study. Patients exclusively taking MMX mesalazine at different doses, according to their status of clinical and biochemical activity, were analyzed. The established induction dose was 3.6 to 4.8 g per day P.O. for mild and moderate activity, respectively. The remission maintenance dose was 2.4 g per day P.O. Three groups were formed: 1) clinical remission induction with oral MMX mesalazine monotherapy, as the only therapy, and with concomitant therapy (steroids and immunomodulators) but no topical mesalazine therapy, 2) clinical remission maintenance with oral MMX mesalazine monotherapy, as the only therapy, and concomitant therapy (immunomodulators) but no topical therapy, and 3) clinical remission induction based on the combined therapy of oral MMX mesalazine and topical therapy (mesalazine suppositories and/or enemas), with or without another type of concomitant therapy.
All the study patients had laboratory studies that included fecal calprotectin levels at the beginning of and during treatment with MMX mesalazine.
The following variables were collected via personal interview and clinical record review: sex, current age, age at diagnosis, years of disease progression, disease extension according to the Montreal classification,11 clinical course, extraintestinal manifestations, prior concomitant treatment, and adverse effects.
Operational definitions:
- •
Clinical activity: mild activity was considered 7-10 points, moderate activity 11-14 points, and severe activity >14 points, in accordance with the Truelove and Witts severity index.12
- •
Clinical remission: defined as a score of 6 points on the Truelove and Witts activity scale12 and/or the absence of blood in stools and normal bowel habit frequency.4
- •
Biochemical activity: patients with a fecal calprotectin value > 250 µg/g.13–14
- •
Biochemical remission: patients with a fecal calprotectin value < 250 µg/g.13–14
- •
Topical therapy suspension: absence of the use of therapy with mesalazine suppositories or enemas.
- •
Adverse event: any lesion caused by the use of a drug.15
The quantitative variables were described through mean and standard deviation and the qualitative variables through frequency and percentage. The Student’s t test was employed to compare the numerical variables between groups and the chi-square test for the categorical variables. The SPSS v24 program was utilized and statistical significance was set at a p < 0.05.
Ethical considerationsOnly patient data were used in the present study and all the participants signed a personal data privacy notice, as well as a statement of informed consent. Likewise, because the medication taken is approved for the treatment of the disease, the study is considered low risk and was approved by the local research and ethics committee. In addition, the analysis meets the current research regulations, and the confidentiality of identification and personal data, as well as patient anonymity (volunteers), was guaranteed. Neither the patients nor the physicians were remunerated for their participation. The present article contains no personal information that could identify the participants.
ResultsClinical and demographic variablesTable 1 summarizes the clinical and demographic variables, demonstrating homogeneous characteristics in the two induction groups. Group 1) MMX mesalazine monotherapy, with 66 patients, for clinical remission induction; group 2) MMX mesalazine monotherapy, with 13 patients, for clinical remission maintenance; and group 3) MMX mesalazine + topical therapy, with 63 patients, for clinical remission induction. Female sex predominated in group 1, at 54.5%, in group 2, at 69.2%, and in group 3 at 54.0%. Extensive colitis was the most frequent extension in the three groups, at 86.4%, 76.9%, and 52.4%, respectively, and the mean follow-up time was 4 months. One hundred percent of the patients were under previous 5-ASA-based treatment, mainly with mesalazine in 95.5%, 100%, and 95.2%, respectively. The rest of the patients in groups 1 and 3 were under treatment with sulfasalazine.
Demographic and clinical characteristics in the three study groups.
| Remission induction with MMX mesalazine monotherapy | Remission maintenance with MMX mesalazine monotherapy | Remission induction with MMX mesalazine + topical therapy | |
|---|---|---|---|
| n = 66 (%) | n = 13 (%) | n = 63 (%) | |
| Sex | |||
| Women | 36 (54.5) | 9 (69.2) | 34 (54.0) |
| Men | 30 (45.5) | 4 (30.8) | 29 (46.0) |
| Current age (years) mean ± SD | 43.9 ± 14.2 | 46.5 ± 8.2 | 47.1 ± 14.95 |
| Age at diagnosis (years) mean ± SD | 38.7 ± 14.1 | 39.6 ± 15.3 | 44.2 ± 15.24 |
| Median years of progression (range) | 42 (1-36) | 41 (1-20) | 4 (1-29) |
| UC extension | |||
| Proctitis (E1) | 3 (4.5) | 0 | 9 (14.3) |
| Left-sided colitis (E2) | 6 (9.1) | 3 (23.1) | 21 (33.3) |
| Extensive colitis (E3) | 57 (86.4) | 10 (76.9) | 33 (52.4) |
| Clinical course | |||
| Initially active and then inactive | 40 (60.6) | 8 (61.5) | 22 (34.9) |
| Two or fewer relapses per year | 22 (33.3) | 5 (38.5) | 32 (50.8) |
| More than two relapses per year | 4 (6.1) | 0 (0) | 9 (14.3) |
| Extraintestinal manifestations | 19 (28.8) | 1 (7.7) | 8 (12.7) |
| Arthralgias | 11 (16.7) | 0 | 6 (9.5) |
| Ankylosing spondylitis | 2 (3.0) | 1 (7.7) | 3 (4.8) |
| Primary sclerosing cholangitis | 3 (4.5) | 0 | 2 (3.2) |
| Previous medical treatment | |||
| Mesalazine | 63 (95.5) | 13 (100) | 60 (95.2) |
| Sulfasalazine | 3 (4.5) | 0 | 3 (4.8) |
| Steroids | 17 (25.8) | 0 | 18 (28.6) |
| Azathioprine | 25 (37.9) | 4 (30.8) | 27 (42.9) |
| Follow-up time in months mean ± SD | 4.4 ± .65 | 4.2 ± .72 | 4.39 ± .87 |
Clinical remission was induced in 80.3% (53/66) of the group 1 patients, clinical remission was maintained in 100% of the group 2 patients (13/13), and clinical remission was induced in 88.8% (56/63) of the group 3 patients, as shown in Fig. 1. The overall rate of the three groups was 85.9%.
The grade of clinical activity was evaluated at 0 and 4 months of treatment in the two induction groups, in which clinical remission rose to 86.6%, compared with 18.3% at the beginning of treatment. There was a decrease in frequency of mild-to-moderate activity, below 10% for groups 1 and 3, at the end of the analysis. None of the patients presented with severe clinical activity, as shown in Table 2.
Clinical activity of the patients with MMX mesalazine monotherapy and combined therapy.
| Group 1) MMX mesalazine monotherapy n = 66 | ||
|---|---|---|
| Clinical activity | 0 months n (%) | 4 months n (%) |
| Remission | 13 (19.7) | 53 (80.3) |
| Mild activity | 43 (65.2) | 9 (13.6) |
| Moderate activity | 10 (15.2) | 4 (6.1) |
| Severe activity | 0 | 0 |
| Group 3) MMX mesalazine + topical therapy n = 63 | ||
|---|---|---|
| Remission | 0 | 57 (90.5) |
| Mild activity | 38 (60.3) | 3 (4.8) |
| Moderate activity | 25 (39.7) | 3 (4.8) |
| Severe activity | 0 | 0 |
Biochemical remission based on fecal calprotectin levels was 74.2% for group 1 with MMX mesalazine monotherapy and 85.7% for group 3 with MMX mesalazine + topical therapy at the end of the mean follow-up, as shown in Table 3. None of the clinical remission maintenance group 2 patients presented with biochemical activity at the end of the study period. A substantial reduction regarding the mean baseline and final fecal calprotectin values of the responder patients and nonresponder patients was achieved, with significant differences between both groups (p < 0.000001), as shown in Table 4.
Clinical and biochemical remission rates at the end of mean follow-up (month 4).
| Remission induction with MMX mesalazine monotherapy | Remission induction with MMX mesalazine + topical therapy | |
|---|---|---|
| n = 66 (%) | n = 63 (%) | |
| Clinical remission | 53 (80.3) | 57 (90.5) |
| Biochemical remission | 49 (74.2) | 54 (85.7) |
Biochemical remission based on fecal calprotectin values in patients with MMX mesalazine.
| Month 0 median (range) | Month 4 median (range) | p value | |
|---|---|---|---|
| Fecal calprotectin value in responder patients | 863.23 (10-6,903) | 62.77 (11-229.80) | < 0.000001 |
| Fecal calprotectin value in nonresponder patients | 1,995 (50-13,047) | 697 (251-2,264) | < 0.000001 |
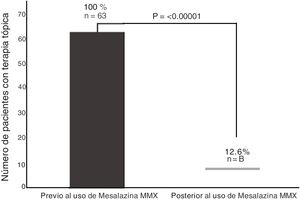
Of the 63 patients with topical therapy at the beginning of the study, 55 (87.3%) were able to suspend said therapy because they achieved clinical remission, and only 12.6% continued with topical therapy at the end of the study period (p < 0.00001, OR = 49.9, 95% CI: 7.14-349.3), as shown in Fig. 2.
Adverse eventsNo adverse effect related to the administration of MMX mesalazine, topical therapy with a 5-ASA, or any other concomitant treatment undergone during the period analyzed was documented.
Discussion and conclusionsMesalazine is the cornerstone of treatment for patients with UC. Previous studies have compared the efficacy of the more current MMX form with other presentations of mesalazine, and induction to remission of 68.0% and 65.9%, with MMX mesalazine vs another 5-ASA presentation, respectively, has been reported.16 In recent years, the prescription of MMX mesalazine by gastroenterologists has increased considerably because of its benefits over the other 5-ASA presentations. The present study is the first conducted in Latin America describing a real-world experience with the use of MMX mesalazine in patients with UC, and we found it to be efficacious and safe for clinical and biochemical remission induction in patients with mild-to-moderate UC, as well as for remission maintenance and the suspension of rectal topical therapy based on suppositories and/or enemas. The clinical induction rate in our study patients was similar to that previously reported in clinical trials that described induction percentages of 35.1% at 8 weeks7 and remission of up to 93.2% at 12 months.17 Those figures were higher than the percentages reported in a real-world study conducted in Spain, in which clinical remission was 55%.18 With respect to clinical remission maintenance, our group 2 patients had been using another mesalazine presentation and were changed to MMX mesalazine, and despite their small number, clinical and biochemical remission was maintained in all of them (13/13). In the clinical trial that analyzed maintenance up to 12 months in a larger group of patients, remission was maintained in 93.2%.17
For patients with distal activity, the combination of oral 5-ASA and topical therapy has been shown to achieve clinical and endoscopic improvement in a greater number of cases, and in less time, compared with the oral route, alone.19 Importantly, the high percentage of topical therapy suspension of 87.3% due to clinical improvement in our group 3 patients should be emphasized, given that there can be reduced adherence to any therapy based on suppositories and/or enemas, as well as the concomitant use of other therapies such as steroids, which could cause prolonged clinical activity. The main adverse effects of the 5-ASAs, including the MMX presentation, are gastrointestinal effects,17 in up to 4.3% of patients.16 The fact that no adverse effect was documented in our study could be because 100% of the patients included in the analysis had previous adequate tolerance of 5-ASAs, and therefore changing the matrix of the drug did not cause a reaction in those patients. Treatment adherence is an important problem in patients with IBD. More than half of patients have reported that they do not adequately carry out their treatment,20 stating that the main reasons for nonadherence are: the medication administration schedules are inadequate, in relation to their daily activities; concern about adverse effects; and the high cost of treatment.21–22 In the present study, treatment adherence was 100%, given that none of the patients suspended treatment on their own or because of an adverse effect during the study period. That could be attributed to the fact that another advantage of treatment with MMX mesalazine, in addition to clinical remission, is its oral administration once a day, resulting in a lower number of tablets, regardless of the induction and remission doses, when compared with other 5-ASA presentations.
Therefore, we conclude that treatment with MMX mesalazine is a good option for Mexican patients with UC, primarily in cases with left-side colitis (distal colitis) that may require topical therapy, reducing its use in a short period of time.
Financial disclosureThe medication was donated to the patients of the institution by the Laboratorio Alfasigma Mexico S.A. de C.V.
Conflict of interestDr. Jesús Kazuo Yamamoto Furusho is a member of the Advisory Board, an opinion leader, and speaker for Abbvie Laboratories de México, Abbvie International, Takeda International, Takeda México, Pfizer International and Regional, Janssen Cilag International and Janssen Cilag México. He is an opinion leader and speaker for Farmasa, Ferring, and Farmasa Schwabe, a research consultant for UCB México, and has received funding for research studies from the Shire, Bristol Myers Squib, Pfizer Takeda, and Celgene laboratories.
Dr. Norma Nathaly Parra-Holguín declares that she has no conflict of interest.
Please cite this article as: Yamamoto-Furusho JK, Parra-Holguín NN. Experiencia de la vida real con el uso de mesalazina MMX en pacientes mexicanos con CUCI en dos centros de tercer nivel. Rev Gastroenterol Méx. 2022;87:305–311.