Healthcare workers (HCWs) are considered an at-risk population for hepatitis C virus (HCV) transmission. Seroprevalence of HCV in Mexico is 1.4% and is similar in HCWs at 0.5–2%.
AimTo determine the seroprevalence of HCV in HCWs at the Hospital de Especialidades Centro Médico Nacional La Raza (HECMNR) and correlate the positive cases with the viral load.
Materials and methodsAn observational, longitudinal, descriptive study was conducted on HCWs at the HECMNR that voluntarily answered a risk factor questionnaire and gave a capillary blood sample for detecting antibodies to HCV. Anti-HCV antibody detection was performed through the rapid colloidal gold-enhanced immunochromatographic assay for the qualitative identification of antibodies to HCV. When positive, quantitative HCV RNA PCR testing was carried out.
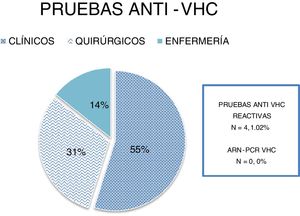
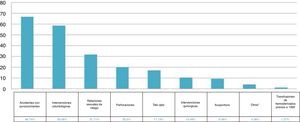
ResultsA total of 391 rapid anti-HCV tests were performed and the mean participant age was 32 years. A total of 214 (54.73%) of the HCWs belonged to clinical services, 120 (30.69%) to surgical services, and 57 (14.57%) to the nursing service; 254 (64.96%) participants were medical residents and 137 (35%) were staff personnel. The most prevalent risk factors were accidental puncture wounds (n = 261, 66.75%) and dental procedures (n = 229, 58.56%). Four samples (1.02%) were positive for anti-HCV antibodies and the HCV RNA PCR test was not positive in any of the samples.
ConclusionsHCWs did not have a greater seroprevalence for HCV, compared with the general population, and no cases of viremia were reported.
Los trabajadores de la salud (TS) se consideran población en riesgo de transmisión del Virus de la Hepatitis C (VHC). La seroprevalencia del VHC en México es del 1.4 % y en los TS es del 0.5 a 2%, siendo similares.
ObjetivoDeterminar la seroprevalencia de VHC en los TS del Hospital de Especialidades Centro Médico Nacional la Raza (HECMNR) y correlacionar los casos reactivos con la carga viral.
Material y métodosEstudio observacional, longitudinal y descriptivo. Se incluyeron a TS adscritos al HECMNR, quienes de manera voluntaria se sometieron a un cuestionario de factores de riesgo y se les tomó muestra de sangre capilar para detectar anti-VHC. La determinación de anti-VHC fue realizada mediante pruebas rápidas inmunocromatográficas de oro coloidal para la identificación cualitativa de anti-VHC. En caso de reactividad, se realizó PCR cuantitativa de ARN-VHC.
ResultadosSe realizaron un total de 391 pruebas rápidas anti-VHC, la edad promedio fue de 32 años, 214 (54.73%) pertenecientes a servicios clínicos, 120 (30.69%) a quirúrgicos y 57 (14.57%) a enfermería; 254 médicos residents (64.96%) y 137 (35%) personal de base. Los factores de riesgo más prevalentes fueron: accidentes con punzocortantes (n = 261, 66.75%) e intervenciones odontológicas (n = 229, 58.56%). Cuatro muestras (1.02%) fueron reactivas a anti-VHC, ninguna de las muestras fue positiva para el ARN del VHC por PCR.
ConclusionesSe observó que los TS no poseen mayor seroprevalencia para VHC en comparación con la población en general, así mismo, no se reportó ningún caso de viremia.
Chronic hepatitis C virus (HCV) infection is a severe public health problem, with 71 million persons reported to be infected. According to recent data from the World Health Organization, HCV infection is responsible for 399,000 deaths annually. It is one of the most prevalent viral infections and is the main cause of chronic hepatopathy in industrialized countries1,2.
Healthcare workers (HCWs) are constantly exposed to hospitalized patients and their body fluids, which in the context of HCV transmission, belonging to that group of the population is considered a risk factor3. In Mexico, the reported prevalence of HCV is 1.4 %, the 1a genotype is the most common, and blood product transfusions before 1995 are the most reported route of infection4.
Seroprevalence ranges from 0.5 to 1.3% in HCWs, similar to figures that have been reported in Mexico. However, there is little information on that population in our country4.
Therefore, our primary aim was to determine the seroprevalence of HCV in HCWs at the Hospital de Especialidades Centro Médico Nacional la Raza (HECMNR) and correlate the positive cases with the presence of viral load (Table 1).
| Accidental puncture wounds | Dental procedures | Risky sexual behavior | Piercings | Tattoos | Surgical interventions | Acupuncture | Others* | Blood product transfusions prior to 1995 |
|---|---|---|---|---|---|---|---|---|
| 66.75% | 58.56% | 31.71% | 20.2% | 17.13% | 10.48% | 9.46% | 4.08% | 1.27% |
An observational, longitudinal, and descriptive study was conducted on HCWs at the HECMNR from the areas of clinical medicine, surgery, and nursing. The HCWs participated voluntarily, providing statements of informed consent and answering a risk factor questionnaire. Capillary blood samples were collected from the HCWs for the anti-HCV test.
The questionnaire was anonymous and included the demographic data of age, work area, and academic grade. The questions about risk factors included a history of accidental puncture wounds, blood product transfusions or surgical interventions before 1995, intravenous or intranasal drug use, hepatitis B virus infection carrier, human immunodeficiency virus carrier, tattoos or piercings, dental procedures, birth to an HCV-positive mother, risky sexual behavior (men who have sex with men, unsafe sex, intercourse with sex workers, or more than 10 sexual partners), acupuncture or hemodialysis sessions, and previous liver chemistry alterations.
Sample size was calculated with a 95% confidence interval, a 5% margin of error, assuming a proportion of 1.4%, according to that reported in the international literature, and a morning shift HCW population size of 437, resulting in a total of 21 participants. However, it was decided to sample the largest population possible.
The qualitative determination of antibodies to HCV was carried out using the Advanced Quality rapid colloidal gold-enhanced immunochromatographic assay manufactured in China by InTec Products, Inc., with a diagnostic sensitivity of 100% and specificity of 99.4%. If a case was positive, quantitative HCV RNA PCR testing was carried out, utilizing the Abbott RealTime HCV assay, with a lower limit of detection of <15 IU/mL.
Statistical analysisThe results obtained were entered into an Excel database and percentages and frequencies were calculated with the same program. No other statistics program was required for the analysis.
Descriptive statistics were performed with percentages and frequencies.
Ethical considerationsAll participants signed written statements of informed consent in relation to collecting the capillary blood sample, performing the anti-HCV test, and if positive, taking the peripheral blood sample for PCR testing.
The present study met the current bioethics research regulations and was approved by the institutional ethics committee. The authors declare that this article contains no personal information that could identify the participants.
ResultsA total of 391 rapid anti-HCV tests were performed. The mean HCW age was 32 years, 214 (54.73%) of the HCWs belonged to clinical services, 120 (30.69%) to surgical services, and 57 (14.57%) to the nursing service (Fig. 1). A total of 254 participants were medical residents (64.96%) and 137 (35%) were hospital staff. The most prevalent risk factors in descending order were: accidental puncture wounds (n = 261, 66.75%), dental procedures (n = 229, 58.56%), piercings (n = 79, 20.2%), risky sexual behavior (n = 124, 31.71%), tattoos (n = 67, 17.13%), acupuncture (n = 37, 9.46%), surgical interventions (n = 41, 10.48%) and blood product transfusions (n = 5, 1.27%) before 1995, birth to an HCV-positive mother (n = 2, 0.51%), intravenous or intranasal drug use (n = 2, 0.51%), previous liver chemistry alterations (n = 11, 2.81%), and HBV infection carrier (n = 1, 0.25%) (Fig. 2).
Four participants (1.02%) had a positive qualitative anti-HCV antibody test and their mean age was 40 years. One belonged to the surgical service, three belonged to the clinical service, three were staff physicians, and one was a medical resident. The risk factors identified in that group were: dental procedures, risky sexual behavior, accidental puncture wound, and surgical interventions. None of the samples were positive for HCV RNA PCR testing.
Discussion and conclusionsIn Mexico, the main HCV transmission route is a blood product transfusion before 1995 (in relation to the definitive version of the NOM-003-SSA2-1993 that determines HCV antibody detection in blood banks, issued on July 18, 1994)5. Likewise, other reported factors are intravenous drug use6,7 and surgical interventions8,9.
In 2010, González et al.4 studied the prevalence of antibodies to HCV in HCWs at the Instituto de Seguridad Social in the State of Mexico and its municipalities. They analyzed blood samples from 374 HCWs, with a mean age of 35.5 years. The main risk factors were a history of surgery (n = 220, 58.8%), a history of a workplace accident (n = 58, 15.5%), and transfusions prior to 1992 (n = 25, 6.7%). A total of 1.3% of the HCWs had a positive anti-HCV test and none of the samples presented with viremia. Martínez et al.5 reported RT-PCR-confirmed seroprevalence of 1.02%, in a high-risk population that included HCWs. Cano-Contreras et al.10 described seroprevalence and viremia of 1.2% in HCWs. In 2011, the Fundación Mexicana para la Salud Hepática reported a prevalence of HCV in an open Mexican population of approximately 1.4%11. Prevalence in northern Mexico (2.0%) was significantly different from that in the southern (1.5%) and central (1.1%) regions of the country12. Based on the 2012 ENSANUT survey, Gutiérrez et al.13 determined that the seroprevalence of HCV in the general population of individuals between 15 and 49 years of age was 0.27%.
In 2016, the Polaris Observatory HCV Collaborators conducted a systematic review that included 100 countries, and they reported a prevalence of HCV of 0.4% in an open population, which is the equivalent of 532,000 cases in Mexico2.
Regarding the analysis of prevalence in blood banks, in a 12-year study published in the medical journal of the Hospital General de México, Rojo-Medina reported a prevalence in the general population of 0.57% (10,217 donors)14. In a 17-year study on an open population in Guanajuato, Sangrador et al. reported a prevalence of HCV of 0.87% in a sample of 340,215 blood donors15.
The majority of reference texts on risk factors for being an HCV infection carrier include HCWs in that category, given that they are exposed to contact with HCV-infected blood through the mucosa or accidental puncture with HCV-contaminated needles. However, in the present study, we found no greater seroprevalence in HCWs, compared with the general population, which concurs with previously published data on the Mexican population.
Our results could be related to the implementation of the universal measures of protection from and management of infectious biologic material.
The participants that were reactive to anti-HCV antibodies in the present study were negative for viral RNA quantification, with a low probability of false positives, given the high sensitivity and specificity of the test, concluding that those participants had seroconversion.
Our analysis has three weaknesses: the first was the mean age of 32 years of the participants, given that individuals receiving blood product transfusions before 1995 are in the fifth and sixth decades of life; the second was that 64.96% of the participants were medical residents with less workplace exposure time than the staff physicians, and the third was that 54.73% of the participants belonged to clinical services, whereas surgical services have greater exposure to infectious biologic material.
From our study results we conclude that the HCWs surveyed had the same seroprevalence for HCV as the general population and we reported no cases of viremia in our analysis.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Ramírez-Zamudio LC, Castillo-Barradas M. Seroprevalencia del virus de hepatitis C en personal de salud del IMSS. Rev Gastroenterol Méx. 2021;86:335–339.