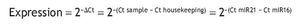Antecedentes: Los microRNA son estructuras moleculares de 20-22 nucleótidos con actividad postranscripional que están implicados en la respuesta inmunitaria, al igual que en las vías de la inflamación de diversas células y tejidos.
Objetivos: Presentamos un estudio prospectivo donde se determina la expresión sérica de microRNA-21 en pacientes con diagnóstico de apendicitis aguda como afección inflamatoria del colon.
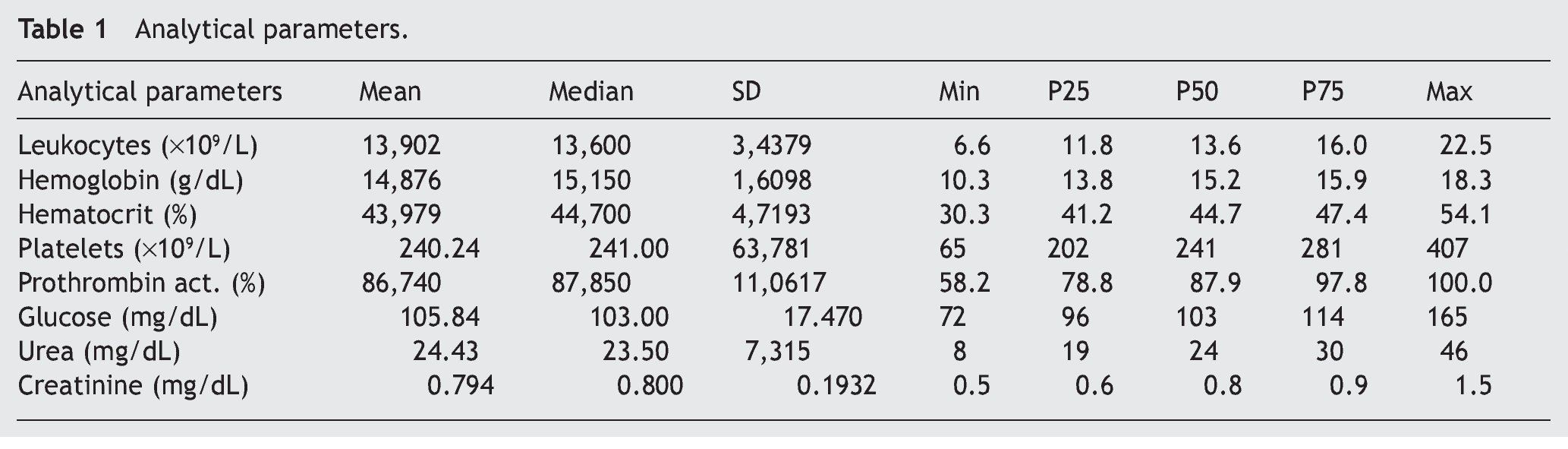
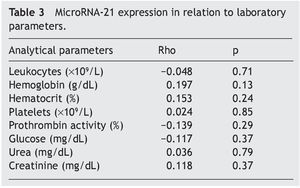
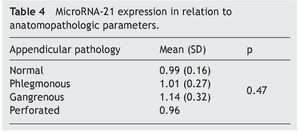
Material y métodos: Estudio de cohorte prospectivo de pacientes con diagnóstico de apendicitis aguda. Se realizó el análisis de microRNA-21 sérico mediante PCR de las muestras sanguíneas de los pacientes obtenidas de forma preoperatoria. Los valores de microRNA-21 se compararon con variables analíticas (leucocitos, hemoglobina, hematocrito, plaquetas, actividad de protrombina, glucosa, urea y creatinina) y anatomopatológicas (apéndice normal, apendicitis aguda flemonosa, gangrenosa y perforada).
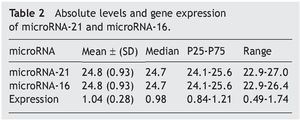
Resultados: Se incluyó, de forma consecutiva, a un total de 60 pacientes con diagnóstico de apendicitis aguda, desde junio del 2009 hasta octubre del 2009, siendo el 66% varones (40 hombres, 20 mujeres), con una edad media ± desviación estándar de 26.2 ± 14.8 años. Los niveles absolutos medios del microRNA-21 fueron de 24.8 ± 0.93, mientras que la expresión genética media del microRNA-21 fue de 1.04 ± 0.28, no observándose correlación con los parámetros analíticos y anatomopatológicos evaluados (p = 0.47).
Conclusiones: Es necesario continuar la búsqueda de cuáles son los microRNA más apropiados para que su determinación en suero conlleve una mayor precisión en el establecimiento del diagnóstico y pronóstico de las enfermedades inflamatorias intestinales.
Background: MicroRNAs are 20-22 nucleotide molecular structures with post-transcriptional activity that are involved in the immune response, as well as in the inflammatory pathways of different cells and tissues.
Aims: We present herein a prospective study in which serum microRNA-21 expression was determined in patients diagnosed with acute appendicitis as a model of bowel inflammation.
Material and methods: A prospective cohort study of patients diagnosed with acute appendicitis was conducted. Serum microRNA-21 was analyzed through the PCR of blood samples taken from the patients prior to surgery. MicroRNA-21 values were compared with the analytic variables (leukocytes, hemoglobin, hematocrit, platelets, prothrombin activity, glucose, urea, and creatinine) and the anatomopathologic variables (normal appendix, phlegmonous, gangrenous, and perforated acute appendicitis).
Results: A total of 60 patients with acute appendicitis diagnosis were consecutively included in the study from June to October 2009. Sixty-six percent of the patients were men (40 men and 20 women), with a mean age of 26.2±14.8 years. The mean absolute level of microRNA-21 was 24.8±0.93, whereas the mean microRNA-21 gene expression was 1.04±0.28. No correlation between the analytic and anatomopathologic parameters evaluated was observed (P=.47).
Conclusions: It is necessary to continue to search for the most appropriate microRNAs, so that their determination in serum can lead to greater precision in establishing the diagnosis and outcome of inflammatory disorders of the bowel.
Introduction
MicroRNAs are 20-22 nucleotide molecular structures with post-transcriptional activity that are involved in gene expression regulation. They participate in different physiologic and pathologic functions such as apoptosis, cell proliferation and differentiation, demonstrating their functionality as tumor-suppressor genes or as proto-oncogenes in carcinogenesis.1
In different studies, microRNAs have also been shown to be involved in the immune response, as well as in the inflammation pathways of different cells and tissues, such as rheumatoid arthritis (microRNA-146a, microRNA-155), psoriasis (microRNA-21, microRNA-125b, microRNA-203), osteoarthritis (microRNA-9), or ulcerative colitis (microRNA-192).2
The aim of the present analysis was to present a prospective study to determine serum microRNA-21 expression in patients diagnosed with acute appendicitis as a model of inflammatory pathology of the colon, during a short period of time for sample collection. This microRNA was chosen because its relation to the inflammatory pathology of the colon3 was demonstrated by the increase of microRNA-21 expression in the mucosa of patients presenting with ulcerative colitis,4 as well as its involvement in other cellular processes.5 In contrast, microRNA-16 has been verified in different human tissues and in serologic samples, and is regarded as a safe endogenous control in the determination of aberrant expressions of other microRNA.6
Despite the fact that there are no other studies that analyze the participation of microRNAs in acute appendicitis, we aim to show the presence of microRNAs in the pathophysiology of inflammatory disease, looking for a correlation with the different clinical stages of the disease. Our hypothesis is that the microRNAs participate in intestinal inflammatory processes, and so we decided to study their role in acute appendicitis and correlate it with the different disease stages; its determination in blood would allow for early and non-invasive diagnosis, and therefore avoiding aggressive therapeutic processes in situations of diagnostic doubt.
Methods
Sample obtention
A prospective cohort study was conducted on patients admitted to the hospital for surgery, with the diagnosis of acute appendicitis made by diagnostic ultrasound as well as clinical and analytic suspicion and physical examination. Informed consent was requested prior to integrating the patients' corresponding medical histories into the study project. Preoperatively, a specific 5 ml peripheral blood sample was collected in a BD Vacutainer® tube. After centrifuging the blood to obtain 2 aliquots of 2 ml of serum, the Clinical Analysis Service immediately stored the samples in a freezer at -80 ºC. The surgical specimen obtained from appendectomy was fixed in formaldehyde 3.7-4.0%, buffered to pH=7, and stabilized with methanol DC and then sent to the Pathologic Anatomy Service.
Sample processing
RNA was isolated from the serum samples and then underwent the quantitative technique of real time polymerase chain reaction (qRT-PCR), following the manufacturer's processing instructions. MicroRNA-21 and microRNA-16 were processed using the "High Pure miRNA Isolation" Kit-Roche Diagnostics SL, Spain (July 2009 version). To obtain the microRNA-21 and microRNA-16 complementary DNA (retrotranscription), the cDNA Synthesis Kit, Strand First Transcriptor-Roche Diagnostics SL, Spain (version April 2007 version) was used. As inverse retrotranscription specific primers the Taqman® microRNA Assay miR-21 and miR-16 and TIB Molbiol Syntheselabor GmbH, Berlin (Germany) kits were used. For the microRNA quantification, amplification was carried out through PCR in a LightCycler® 2.0 Roche Diagnostics SL, Spain, using the amplification probes included in the Taqman® microRNA Assay kits.
MicroRNA expression analysis and statistical analysis
The absolute Ct values according to the amplification curves of each microRNA, as well as the gene expression analysis that determines the microRNA-21 expression with respect to the control microRNA (microRNA-16), were estimated using the methodology proposed by Livak and Schmittgen (Methods 2001).
The evaluated parameters were divided into 3 categories: clinical parameters, analytic parameters (total leukocytes, hemoglobin, hematocrit, platelets, prothrombin activity, glucose, urea, creatinine, serum microRNA-21, and serum microRNA-16) and anatomopathologic parameters (grade of appendicular pathology).
The statistical analysis of the study was done using the PASW 18.0 (SPSS Inc, Chicago) statistical program. A 5% alpha risk was chosen (p<0,05) in all the contrasts. The contrast between quantitative and qualitative variables (the gene expression levels in relation to the anatomopathologic parameters) was carried out through non-parametric tests for comparing 2 groups (the Mann-Whitney U test) and more than 2 groups (the Kruskal-Wallis H test). In the correlations between the quantitative variables (gene expression level and analytic parameter correlations) the non-parametric Spearman correlation (Rho) was used.
Results
A total of 60 consecutive patients diagnosed with cute appendicitis from June 2009 to October 2009 were enrolled in the study. Sixty-six percent were men (40 men and 20 women) and they had a mean age of 26.2 ± 14.8 years. Expressed as means and standard deviation, the analytic parameter values were, among others: leukocytes (×109/L) 13.90 ± 3.44; hemoglobin (g/dL) 14.87 ± 1.61; platelets (×109/L) 240 ± 63; (Table 1).
The most frequent anatomopathologic diagnosis was acute phlegmonous appendicitis, in 48 cases (77.4%), followed by acute gangrenous appendicitis in 11 cases (17.7%), normal ileocecal appendix in 2 cases (3.2%), and acute perforated appendicitis, in one case (1.6%).
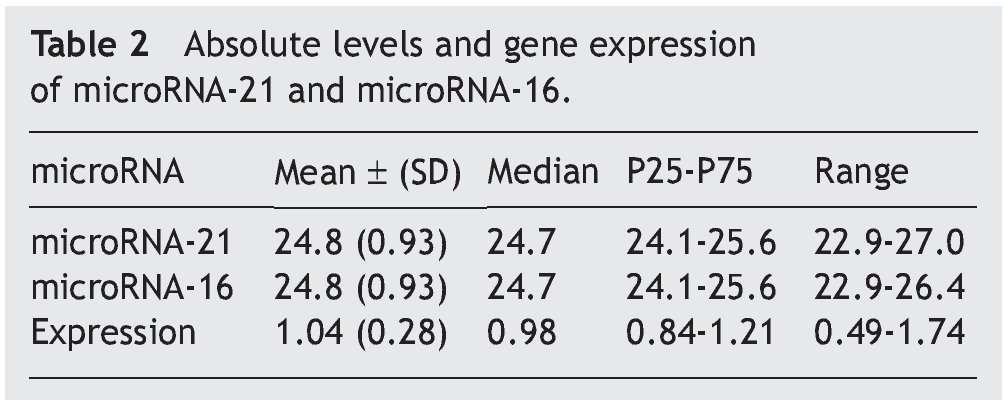
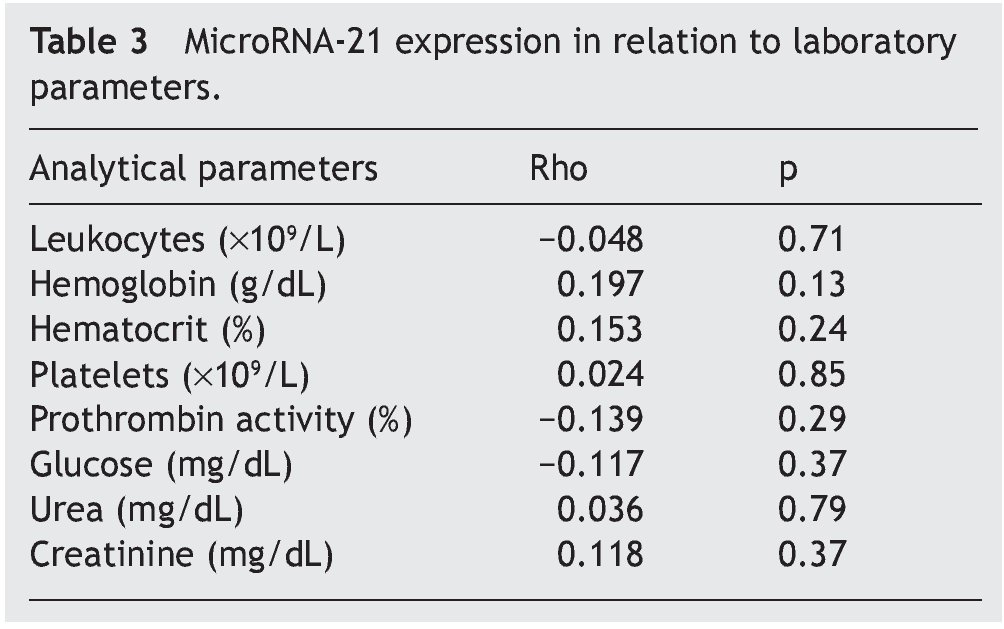
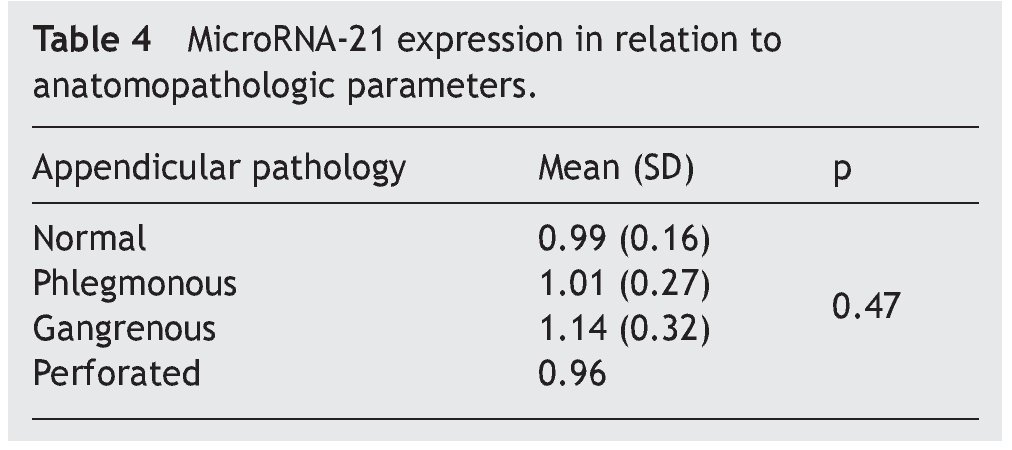
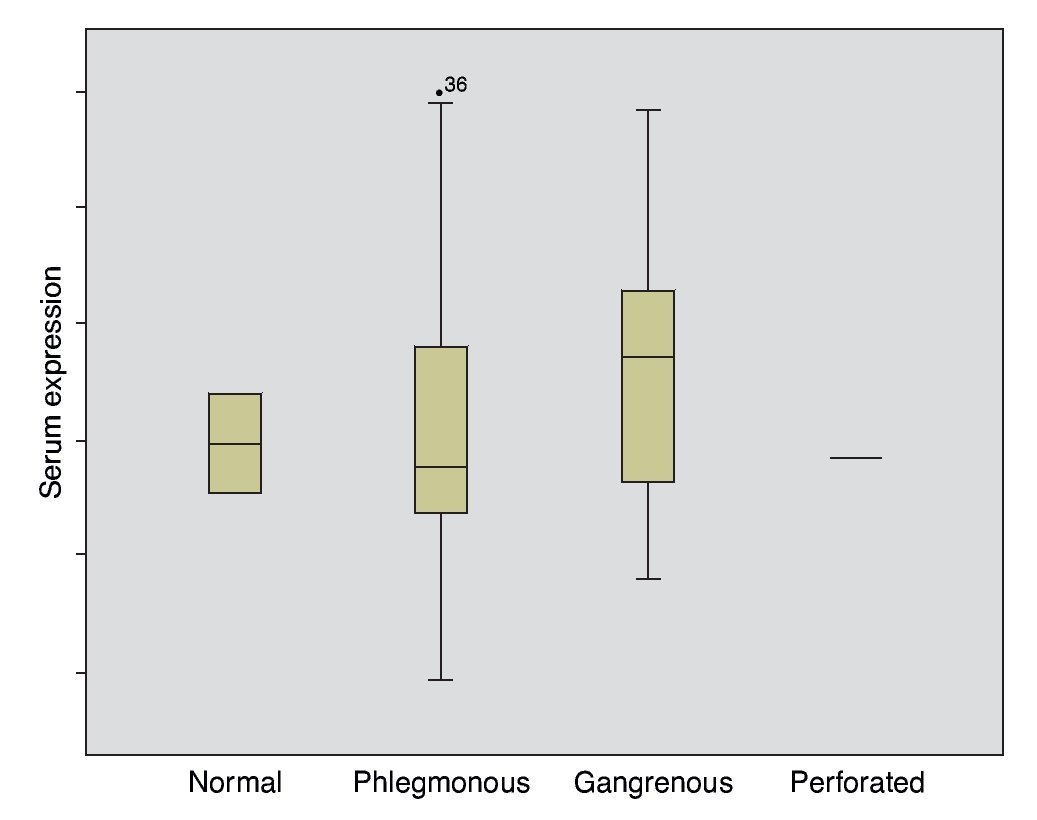
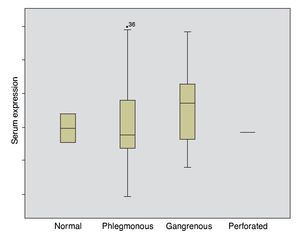
The mean absolute expression of both microRNA-21 and MicroRNA-16 was 24.8 ± 0.93. The mean microRNA-21 gene expression was 1.04 ± 0.28 (Table 2, Fig. 1), indicating microRNA-21 overexpression, in regard to the microRNA-16 control (housekeeping). There was no type of correlation in microRNA-21 expression in relation to the laboratory test results, nor were there any differences in expression with respect to the anatomopathologic types (Tables 3 and 4).
Figure 1. Gene expression according to appendicular disease grade.
Correlations between microRNA-16 and the different parameters were not analyzed, given that it was regarded as the endogenous control and so its main use was to determine the relative expression of microRNA-21.
Discussion
A large number of studies have been conducted whose aim has been to identify noninvasive screening methods focused on the early detection of different diseases, such as hematologic tests or DNA analysis in feces.7
MicroRNA involvement in the stability of RNA regulation in gene expression has led to these "non-encoding" RNA molecules being found in different organic fluids (plasma, serum, urine, tears, and saliva) and a high free microRNA concentration has been determined in the peripheral blood.5 Certain modifications in the concentrations of microRNAs in inflammatory diseases have also been observed, such as the increase of microRNA-146a and microRNA-223 in septic states due to the systemic inflammatory response syndrome.5,8
In inflammatory diseases, a different microRNA expression in peripheral blood and its participation in inflammatory and autoimmune diseases have been seen.9 The first determination of microRNAs in feces showed a different expression in colorectal cancer in relation to ulcerative colitis and these results coincided with the histopathologic findings in both situations.10 Different microRNA expression was also demonstrated in patients presenting with ulcerative colitis, infectious colitis, and irritable bowel syndrome.11 Despite the bibliographic references that establish microRNA overexpression in the inflammatory process, the results of the present study did not show them to be useful in the diagnosis of acute appendicitis.
In the present study, microRNA-16 was used as the control or housekeeping microRNA; microRNA-16 has been found in different human tissues and serologic samples and is regarded as a safe endogenous control in microRNA determination. Two different studies coincided in the establishing microRNA-16 as a normalizing microRNA. Different studies have used other endogenous controls such as RNU6B, 5S rRNA, microRNA-26a, microRNA-425, microRNA-454, or let-7a.6,12
Despite the fact that in the present study the determination of microRNA-21 in serum has shown low diagnostic performance for acute appendicitis, there are possible implications that could explain these results: microRNAs can be inflammation-dependent mediators, given that modification of their concentrations has been found in inflammatory diseases and in septic states.5,8 These are functional results that concur with those obtained by Bihrer et al., in the sense that microRNA-21 expression is clearly related to necro-inflammatory activity.3
According to what has been presented, the participation of microRNA-21 in the inflammatory processes, just as it has been described in the medical literature,5,8,13 as well as the vascular modifications that are secondary to the systemic inflammatory response syndrome, could indicate the limitations of the present study, given that the choice of the pathology per se causes bias in the comparison of microRNA-21 expressions. The decision to study patients with acute appendicitis was based on easy serum and surgical sample obtention of an inflammatory colic pathology that had not been reported on in the medical literature. A repetition of the study establishing a group of healthy controls would probably result in a greater discriminant capacity of microRNA-21 in relation to intestinal inflammatory diseases.
Conclusions
In the present study, the microRNA-21 determinations in blood did not establish diagnostic usefulness in acute appendicitis. These results cannot be considered conclusive (the absence of evidence is not necessarily evidence of absence), given that the results were obtained without a control group comparison.
It is necessary to continue to look for the most appropriate microRNAs whose determination in serum will lead to greater accuracy in establishing diagnosis and outcome in intestinal inflammatory diseases. It is essential to continue to advance the knowledge not only of the functions the circulating microRNAs perform but also to understand their true origin. Likewise, it is also necessary to continue to conduct preoperative studies in relation to the variations in microRNA expression in order to establish the prognostic usefulness of their postoperative serum determination.
Financial disclosure
This study received financial support from La Fundación para la Investigación Sanitaria en Castilla La Mancha (FISCAM) and from La Fundación Mutua Madrileña Investigación Médica.
Conflict of interests
The authors declare that there is no conflict of interest.
Received 15 September 2012;
accepted 27 February 2013
* Corresponding author at:
C/ Julio Palacios 29, Esc. B. 7.o B. 28029. Madrid.
Tel.: +34 660 333 554.
E-mail addresses: pablomensan@hotmail.com, pablo.menendez.sanchez@gmail.com (P. Menéndez).