Surgical resection of gastrointestinal (GI) cancer is the cornerstone of curative treatment but entails considerable morbidity. The surgical Apgar score (SAS) is a practical and objective instrument that provides immediate feedback. The aim of the present study was to evaluate the performance of the SAS for predicting complications at 30 days in patients with primary GI cancer that underwent curative surgery.
Materials and methodsA prospective observational study was conducted that included 50 patients classified into a low SAS (≤ 4) group or a high SAS (≥ 5) group. Complications were defined as any event classified as a Clavien-Dindo grade II to V event. Bivariate and multivariate analyses were performed through the Cox regression and a p<0.05 was considered significant.
ResultsOverall postoperative morbidity was 50.0%, with no mortality. Eighty-six percent of cases were catalogued as having an ASA≥3. Eighty-eight percent had a high SAS, of whom 45.5% presented with a complication, whereas 12.0% had a low SAS and a complication rate of 83.3%. In the multivariate analysis, the BMI (OR: 3.351, 95% CI: 1.218-9.217, P=.019), SAS (OR: 0.266, 95% CI: 0.077-0.922, P=.037), surgery duration (OR: 3.170, 95% CI: 1.092-9.198, P=.034), and ephedrine use (OR: 0.356, 95% CI: 0.144-0.880, P=.025) were significantly associated with the development of adverse outcomes.
ConclusionsSAS was shown to be an independent predictive factor of postoperative morbidity at 30 days in the surgical management of GI cancer and appears to offer a reliable sub-stratification in a high-risk population with an ASA≥3.
La resección quirúrgica del cáncer gastrointestinal (CGI) es el pilar del tratamiento curativo; empero, conlleva una morbilidad considerable. El Apgar quirúrgico (AQ) es un instrumento práctico y objetivo que provee retroalimentación inmediata. El propósito del presente trabajo fue evaluar el rendimiento del AQ para predecir complicaciones a 30 días en pacientes con CGI primario sometidos a cirugía con intento curativo.
Material y métodosSe hizo un estudio observacional prospectivo. Se incluyeron 50 pacientes, quienes se clasificaron en AQ bajo (≤ 4) y alto (≥ 5). Se definió como complicación cualquier evento especificado en los grados II a V del sistema Clavien-Dindo. Se realizaron análisis bivariado y multivariado mediante regresión de Cox, considerando significativa una p<0.05.
ResultadosLa morbilidad postoperatoria global fue del 50.0%, sin mortalidad. El 86.0% se catalogó como ASA≥3. El 88.0% obtuvo un AQ alto; de ellos, el 45.5% presentó alguna complicación, mientras que en el 12.0% con AQ bajo la tasa de complicaciones fue del 83.3%. En el análisis multivariado el índice de masa corporal (RM: 3.351, IC del 95%: 1.218-9.217, p=0.019), el AQ (RM: 0.266, IC del 95%: 0.077-0.922, p=0.037), la duración de la cirugía (RM: 3.170, IC del 95%: 1.092-9.198, p=0.034) y el uso de efedrina (RM: 0.356, IC del 95%: 0.144-0.880, p=0.025) demostraron una asociación significativa con el desarrollo de desenlaces adversos.
ConclusionesEl AQ es un factor predictivo independiente de morbilidad postoperatoria a 30 días en el manejo quirúrgico del CGI y en una población de alto riesgo con ASA≥3 parece ofrecer una subestratificación confiable.
Malignant neoplasias are among the main causes of death worldwide. According to statistics from the GLOBOCAN project, in 2012, they were the cause of 8.2 million deaths, of which 65.0% occurred in countries with fewer economic resources.1 Among the malignant tumors of greater impact are those that affect the gastrointestinal tract. In Mexico, the Instituto Nacional de Estadistica y Geografía (INEGI) reported that they held third place as the cause of cancer death in the age group of 18 to 29 years and first place in the age group of 30 years and older, from 2011 to 2016.2
Surgical resection continues to be the cornerstone of the curative approach for regional and localized disease, but it entails considerable morbidity and mortality. The complication rate varies, depending on the organ involved, and ranges from 2.0-42.0% in colorectal cancer to 17.9-58.0% in esophageal cancer. The mortality rate can be as high as 15.0%.3–7
Postoperative adverse events have a negative impact on short-term and long-term quality of life, resulting in the need for a tool that can objectively determine individual patient risk for suffering some type of deterioration, so that improved care can be offered. At present, there are different methods for such an evaluation but some of them are difficult to calculate, are based on subjective determinations, or have a low predictive value. The American Society of Anesthesiologists (ASA) classification and the Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity (POSSUM) are among the most widely known.8,9
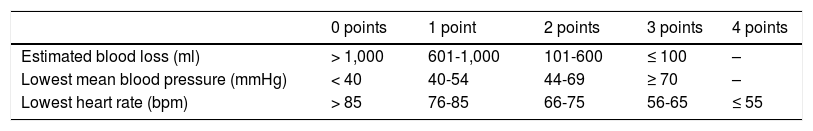
The instrument employed should be simple and fast to apply. It should also be based on objective parameters, make accurate predictions, and be comparable between different groups. The Surgical Apgar Score (SAS) is a practical system consisting of 3 elements obtained during the intra-operative period (estimated blood loss, lowest mean blood pressure, lowest heart rate) that provides immediate feedback and predicts the probability of major complications or death, within the first 30 postoperative days10 (Table 1). It has been validated internationally in general surgeries and vascular operations. However, only one prospective study on an obstetric population, utilizing the score, has been conducted in Mexico.11 Therefore, our primary aim was to evaluate its performance in patients that underwent major gastrointestinal cancer surgery as curative treatment.
Materials and methodsA prospective observational study was conducted on patients diagnosed with primary gastrointestinal cancer (esophagus, stomach, small bowel, colon and rectum, and accessory glands, such as the pancreas and liver). The patients programmed for curative resection, within the time frame of August 2018 and May 2019, at the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” were included in the study and the patients that had undergone any palliative treatment or that presented with any disease in clinical stage IV due to distant metastasis were excluded.
The clinical records of the patients were reviewed, and the sociodemographic variables, personal histories, clinical characteristics, and previous cancer treatments were recorded. The specifications of the intervention and the parameters for calculating the SAS were taken from the anesthesia registration sheet. Respecting the guidelines originally described by Gawande et al.,10 a result of 0 to 4 points was determined as low and a result of 5 to 10 points as high. The preoperative laboratory studies closest to the date of surgery were collected.
Patient follow-up was carried out during hospital stay and outpatient consultation at the Oncologic Surgery Service. Any adverse event that altered the expected recovery of the patient, within the first 30 postoperative days, was defined as a complication. Complication severity was established according to the Clavien-Dindo classification, excluding those of grade I that did not involve any modification in management. Mortality was determined by death, within the first 30 postoperative days, or at any time during hospitalization, regardless of duration.
Statistical analysisIBM SPSS version 21.0 software was utilized for the statistical analysis. A descriptive analysis with measures of central tendency and dispersion was carried out. The differences between the quantitative variables of 2 factors with normal and non-normal distribution were identified using the Student’s t test and the Mann-Whitney U test, respectively. The chi-square test and Fisher’s exact test were employed for the qualitative variables. Likewise, the association of independent factors with the complication rate was estimated using the Cox regression. Statistical significance was set at a p<0.05.
Ethical considerationsThe present protocol was approved by the institutional Research Committee and Ethics in Research Committee. The corresponding informed consent was obtained before accessing any patient’s clinical records and all identifying data were eliminated to ensure participant confidentiality and anonymity. No experiments were performed on animals or humans.
ResultsSeventy-five candidate subjects were initially identified, of which 18 were eliminated due to the absence of malignant neoplasia, according to the definitive histopathologic report, and 7 more due to lack of availability of the anesthesia registration sheet. Fifty patients (26 women and 24 men) were included for the final analysis, with a mean age at the time of surgery of 58.64±15.18 years. According to the nutritional status classification of the World Health Organization, 54.0% of the patients had a body mass index (BMI) above normal, with overweight being the most frequent (36.0%). Chronic comorbidities with metabolic repercussions, such as high blood pressure, diabetes mellitus, and dyslipidemia, were found in 42.0% of the patients, either separately or combined. The most common site of cancer diagnosis was the colon and rectum (46.0%), followed by accessory glands (32.0%). The majority of the patients were in clinical stages I and III, at 37.0 and 33.0%, respectively. A total of 36.0% of the patients received neoadjuvant chemotherapy alone or with radiotherapy.
Regarding the surgical procedures, 86.0% of the patients were catalogued as high risk with an ASA 3. Previous laboratory test results showed a normal hemoglobin level by sex in 62.0% (mean: 12.65±2.23g/dl), as well as adequate nutritional status by albumin in 68.0% (mean: 3.80±0.65g/dl). Mean surgery duration was 261.04±104.34min. Vasopressor support was administered in 72.0%: ephedrine (52.8%), norepinephrine (16.7%), or both (30.6%). Twenty percent of the patients required a blood transfusion. To calculate the SAS, the following means were obtained: blood loss, 451.40±444.43ml; lowest blood pressure, 64.68±9.60mmHg; and lowest heart rate, 63.30±14.54bpm. In accordance with those figures, only 12.0% of the patients were classified as having a low SAS.
The overall incidence of complications was 50.0%, and the most common were infectious (72.0%), with 38.9% of those patients presenting with abdominal sepsis, followed by surgical wound infection, urinary tract infection, and pneumonia, each at 16.7%. Based on severity, according to the Clavien-Dindo classification, distribution was: grade II 60.0%, grade III 8.0%, and grade IV 32.0%. The mean time interval between surgery and the appearance of the complication was 7.87±7.86 days. No deaths occurred during follow-up.
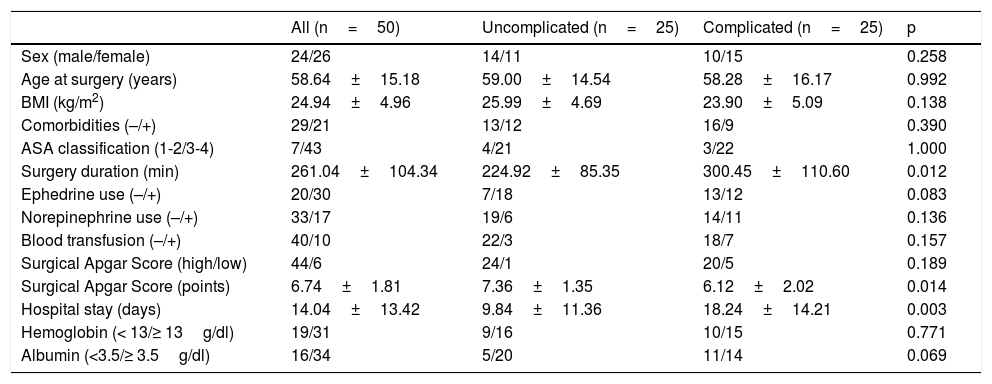
In the comparison of the complicated patients and the uncomplicated patients (Table 2), there were statistically significant differences in relation to surgery duration (300.45 vs. 224.92min, p=0.012), the SAS (6.12 vs. 7.36, p=0.014), and hospital stay (18.24 vs. 9.84 days, p=0.003). On the other hand, when the low vs. high SAS categories were compared, the patients with a low SAS had more norepinephrine use and blood transfusions (83.3%, p=0.014 and 66.7%, p=0.011, respectively), as well as a higher severe complication rate (grade II 0.0% vs. 75.0%, p=0.005; grade III 20.0% vs. 5.0%, p=0.367; grade IV 80.0% vs. 20.0%, p=0.023). Finally, analyzing the performance of the ASA and SAS systems for defining the probability of adverse events, there was a similar complication percentage in the two low-risk groups (42.9% vs. 45.5%, p=1.000), whereas it was higher for the high-risk SAS group, albeit with no statistical significance (51.2% vs. 83.3%, p=0.204).
Comparison of clinical and pathologic characteristics.
| All (n=50) | Uncomplicated (n=25) | Complicated (n=25) | p | |
|---|---|---|---|---|
| Sex (male/female) | 24/26 | 14/11 | 10/15 | 0.258 |
| Age at surgery (years) | 58.64±15.18 | 59.00±14.54 | 58.28±16.17 | 0.992 |
| BMI (kg/m2) | 24.94±4.96 | 25.99±4.69 | 23.90±5.09 | 0.138 |
| Comorbidities (–/+) | 29/21 | 13/12 | 16/9 | 0.390 |
| ASA classification (1-2/3-4) | 7/43 | 4/21 | 3/22 | 1.000 |
| Surgery duration (min) | 261.04±104.34 | 224.92±85.35 | 300.45±110.60 | 0.012 |
| Ephedrine use (–/+) | 20/30 | 7/18 | 13/12 | 0.083 |
| Norepinephrine use (–/+) | 33/17 | 19/6 | 14/11 | 0.136 |
| Blood transfusion (–/+) | 40/10 | 22/3 | 18/7 | 0.157 |
| Surgical Apgar Score (high/low) | 44/6 | 24/1 | 20/5 | 0.189 |
| Surgical Apgar Score (points) | 6.74±1.81 | 7.36±1.35 | 6.12±2.02 | 0.014 |
| Hospital stay (days) | 14.04±13.42 | 9.84±11.36 | 18.24±14.21 | 0.003 |
| Hemoglobin (< 13/≥ 13g/dl) | 19/31 | 9/16 | 10/15 | 0.771 |
| Albumin (<3.5/≥ 3.5g/dl) | 16/34 | 5/20 | 11/14 | 0.069 |
Data are expressed in number of patients or mean±standard deviation.
BMI: body mass index.
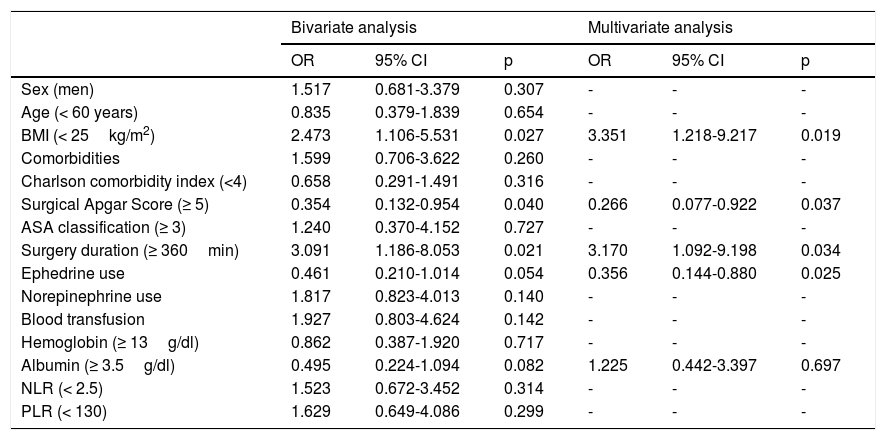
The study variables correlated with the appearance of postoperative complications (Table 3). The parameters with a p<0.1 in the bivariate analysis were included in the multivariate model, establishing a BMI<25kg/m2 (OR: 3.351, 95% CI: 1.218-9.217, p=0.019), a high SAS (OR: 0.266, 95% CI: 0.077-0.922, p=0.037), surgery duration≥360min (OR: 3.170, 95% CI: 1.092-9.198, p=0.034), and ephedrine use (OR: 0.356, 95% CI: 0.144-0.880, p=0.025) as independent prognostic factors for the development of adverse outcomes.
Association between prognostic factors and postoperative complications.
| Bivariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Sex (men) | 1.517 | 0.681-3.379 | 0.307 | - | - | - |
| Age (< 60 years) | 0.835 | 0.379-1.839 | 0.654 | - | - | - |
| BMI (< 25kg/m2) | 2.473 | 1.106-5.531 | 0.027 | 3.351 | 1.218-9.217 | 0.019 |
| Comorbidities | 1.599 | 0.706-3.622 | 0.260 | - | - | - |
| Charlson comorbidity index (<4) | 0.658 | 0.291-1.491 | 0.316 | - | - | - |
| Surgical Apgar Score (≥ 5) | 0.354 | 0.132-0.954 | 0.040 | 0.266 | 0.077-0.922 | 0.037 |
| ASA classification (≥ 3) | 1.240 | 0.370-4.152 | 0.727 | - | - | - |
| Surgery duration (≥ 360min) | 3.091 | 1.186-8.053 | 0.021 | 3.170 | 1.092-9.198 | 0.034 |
| Ephedrine use | 0.461 | 0.210-1.014 | 0.054 | 0.356 | 0.144-0.880 | 0.025 |
| Norepinephrine use | 1.817 | 0.823-4.013 | 0.140 | - | - | - |
| Blood transfusion | 1.927 | 0.803-4.624 | 0.142 | - | - | - |
| Hemoglobin (≥ 13g/dl) | 0.862 | 0.387-1.920 | 0.717 | - | - | - |
| Albumin (≥ 3.5g/dl) | 0.495 | 0.224-1.094 | 0.082 | 1.225 | 0.442-3.397 | 0.697 |
| NLR (< 2.5) | 1.523 | 0.672-3.452 | 0.314 | - | - | - |
| PLR (< 130) | 1.629 | 0.649-4.086 | 0.299 | - | - | - |
BMI: body mass index; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio.
Unlike other systems, the SAS has the advantage of including the progression of the patient throughout the procedure, which not only depends on his/her previous physical status, but also on the performance of the surgical team. Even though that point has been criticized, precisely because of vital sign alterations that can be caused by anesthesia, both tachycardia and hypotension, regardless of the cause, have been shown to be associated with worse outcome, reflecting a low level of tissue oxygenation.12 Nevertheless, there is a clear disadvantage with respect to the variable of blood loss, given that the amount of bleeding can differ, depending on the surgery that is being performed.
Internationally, different studies have been conducted, with results that favor SAS use, including its application in gastrointestinal oncologic disease. Aoyama et al.13 confirmed its utility as an independent morbidity and mortality factor in patients with pancreatic cancer that underwent different interventions: distal pancreatectomy and pancreatoduodenectomy (p=0.046). In addition, Eto et al.14 compared the SAS with the Estimation of Physiologic and Surgical Stress (E-PASS), revealing that the former correlated better with the complication rate (p<0.0001 vs. p=0.06).
Other authors have made changes to the instrument. On the one hand, Tomimaru et al.15 found a cutoff point between 6 and 7 to have greater sensitivity (75.00%) and specificity (60.00%). Janowak et al.16 employed adjusted blood loss values for esophagectomies, finding a strong association between a low SAS and greater later morbidity: the probabilities were 2.5-times higher (p=0.005). Likewise, Miki et al.17 demonstrated that their modified scale in gastrectomies was an independent predictor in the multivariate analysis, whereas the original score was not (p=0.048 vs. p=0.995).
To the best of our knowledge, the present study is the first in Mexico to encompass such different surgeries. Applying the cutoff points initially established by Gawande et al.,10 there was a direct relation between a score<5 and an adverse postoperative result. In those cases, strict follow-up is important for providing opportune treatment.
Given that the largest number of patients analyzed were defined as high-risk, the fact that the SAS offers a more detailed categorization of the subgroup is relevant: the percentage of complication predictions was higher with the SAS than with the ASA, albeit with no statistical significance, most likely as a consequence of the small sample size. It is true that the SAS cannot replace more detailed methods that have widely demonstrated efficacy, but it does provide a risk evaluation that is simple and fast.
Other variables also influence prognosis. For example, the intraoperative administration of ephedrine was recognized as a protective element, which can be explained by its link to combined anesthesia, being the drug of choice for induced hypotension. That anesthetic technique is frequently used in major abdominal procedures because it has the advantages of reducing the neuroendocrine response to stress and conserving immunologic activity, which consequently results in lower morbidity and mortality.18,19
Certain limitations must be kept in mind when interpreting our study results. The present analysis was conducted at a single institution with a small sample size, reducing its power. In addition, due to the absence of deaths, the role of the SAS as a mortality predictor could not be evaluated. New prospective multicenter studies are needed to validate the utility of the SAS in optimizing postoperative care and reducing complication rates.
In conclusion, the SAS is an independent predictive factor for postoperative morbidity in major gastrointestinal oncologic surgery, and when used in a predominantly high-risk population with an ASA≥3, it appears to offer a reliable sub-stratification. Routine implementation of the SAS would enable healthcare personnel to design strategies for continuous improvement and quality control in the service.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
Conflict of interestThe authors declare there is no conflict of interest.
None.
Please cite this article as: Padilla-Leal KE, Flores-Guerrero JE, Medina-Franco H. Apgar quirúrgico como predictor de complicaciones en cirugía oncológica gastrointestinal. Revista de Gastroenterología de México. 2021;86:259–264.