Nonalcoholic fatty liver disease (NAFLD) affects nearly one third of the population worldwide. Mexico is one of the countries whose population has several risk factors for the disease and its prevalence could surpass 50%. If immediate action is not taken to counteract what is now considered a national health problem, the medium-term panorama will be very bleak.
This serious situation prompted the Asociación Mexicana de Gastroenterología and the Asociación Mexicana de Hepatología to produce the Mexican Consensus on Fatty Liver Disease. It is an up-to-date and detailed review of the epidemiology, pathophysiology, clinical forms, diagnosis, and treatment of the disease, whose aim is to provide the Mexican physician with a useful tool for the prevention and management of nonalcoholic fatty liver disease.
La enfermedad por hígado graso no alcohólico (EHGNA) afecta prácticamente a un tercio de la población mundial. México es uno de los países cuya población reúne varios factores de riesgo para esta enfermedad y su prevalencia podría superar el 50%; es por eso que el panorama a mediano plazo es muy pesimista si no se toman acciones inmediatas para contrarrestar lo que ya se considera un problema de salud nacional.
De ahí el interés de la Asociación Mexicana de Gastroenterología y de la Asociación Mexicana de Hepatología para realizar el Consenso mexicano de EHGNA, en el cual se hizo una revisión actualizada y a fondo de temas como epidemiología, fisiopatología, formas clínicas, diagnóstico y tratamiento, con el objetivo de ofrecer al médico mexicano una herramienta útil para la prevención y el manejo de esta enfermedad.
Nonalcoholic fatty liver disease (NAFLD) is a metabolic disorder characterized by the accumulation of fat in the hepatocytes of individuals that do not drink significant amounts of alcohol, take hepatotoxic medications, or have any other known cause of secondary steatosis, and is currently the most common chronic liver disease worldwide.1 Its clinical and pathologic spectrum can progress from simple steatosis to steatohepatitis, cirrhosis, and hepatocellular carcinoma (HCC). NAFLD is considered the hepatic component of metabolic syndrome (MetS) and its prevalence has increased on a par with that of obesity, type 2 diabetes mellitus (DM2), dyslipidemia, and MetS.2 Due to the growing worldwide epidemic of obesity and diabetes, NAFLD is soon expected to be the main cause of HCC and the first indication for liver transplantation.
In 2008, the Asociación Mexicana de Gastroenterología formulated the guidelines for the diagnosis and treatment of nonalcoholic fatty liver disease.3–5 Since then, new concepts involving its correct definition, epidemiology, pathophysiology, diagnosis, and prevention have emerged, and numerous lines of research have been opened in the search for effective therapeutic alternatives. All those advances justify the production of an up-dated document to complement the 2008 diagnosis and treatment guidelines.
In August of 2017, the Asociación Mexicana de Gastroenterología and the Asociación Mexicana de Hepatología agreed to create the Mexican consensus on nonalcoholic fatty liver disease and summoned 6 coordinators and 29 additional participants to make up the consensus group. They carried out an up-to-date review of the theme, evaluated the evidence, and formulated and discussed statements, until reaching agreements.
The aim of the present document is to present a consensus review of the current state of NAFLD, updating the 2008 diagnosis and treatment guidelines, and incorporating the new scientific evidence that has been published worldwide.
MethodThe present consensus was developed utilizing the Delphi method.6 To review the bibliography, the coordinators used the following terms as the search criteria: “nonalcoholic fatty liver disease”, “nonalcoholic steatohepatitis”, “hepatic steatosis”, “steatohepatitis”, and “fatty liver hepatitis” combined with the terms: “epidemiology”, “incidence”, “prevalence”, “pathophysiology”, “inflammation”, “microbiota”, “diagnosis”, “differential diagnosis”, “treatment”, “therapy”, “management”, “review”, “guidelines”, and “meta-analysis”, as well as the equivalent terminology in Spanish. The search was carried out in PubMed and included articles published within the time frame of November 2012 to October 2017 in both English and Spanish. Preference was given to consensuses, guidelines, systematic reviews, and meta-analyses, but the search was not limited to those types of articles. Complementary online and manual searches were also carried out in all the publications up to October 2017 that the coordinators deemed relevant. The complete bibliography was made available to the members of the consensus group for consultation throughout the entire process.
Six Working Groups were formed to cover the main themes in the study of NAFLD:
- 1.
Generalities: definition, nomenclature, epidemiology.
- 2.
Natural history and pathophysiology.
- 3.
Clinical manifestations and involvement of other organs.
- 4.
Diagnosis and evaluation of steatosis and fibrosis.
- 5.
Treatment I: diet and exercise, drugs of limited usefulness, accepted drugs, and surgery.
- 6.
Treatment II: emerging drugs and liver transplantation.
After the review was completed, 90 statements were formulated that were anonymously voted upon electronically from December 22, 2017, to January 7, 2018. The consensus participants placed their votes with the following responses: a) in complete agreement; b) in partial agreement; c) uncertain; d) in partial disagreement; and e) in complete disagreement. When agreement was equal to or greater than 75%, the statement remained unchanged for the next voting round. When disagreement was 75% or greater, the statement was eliminated. The statements in which agreement or disagreement was less than 75% were rewritten by the coordinator of each working group, taking the comments of the participants into account. The second long-distance electronic voting round included 57 statements and was conducted from January 22 to 29, 2018, following the same system. The face-to-face and definitive voting round was carried out in Puerto Vallarta, Jalisco, Mexico, on February 16 and 17, 2018. Fifty-five statements were voted upon by the consensus group and a final total of 54 statements were accepted.
Once the final consensus statements were determined, the coordinators established the quality of evidence that sustained each statement and its strength of recommendation grade, when applicable, employing the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system for all the statements that involved a diagnostic or therapeutic intervention.7 That system was developed in an effort to overcome the limitations of previous ones, optimizing the evaluation of quality of evidence and the grading of strengths of recommendation, and has been used in the most recent consensuses of the Asociación Mexicana de Gastroenterología. In the GRADE system, the quality of evidence is not determined solely by the methodology of the study analyzed, but also on the design employed to respond to a previously posed specific question.7,8 Thus, the quality of evidence is “high” when the publication of further research studies will not change our confidence in the estimate of effect, “moderate” when the publication of further research studies may modify our confidence in the estimate of effect, “low” when the publication of further research studies is very likely to have an important impact on our confidence in the estimate of effect, and “very low” when any estimate of effect is uncertain. In addition, the GRADE system establishes the strength of recommendation as strong or weak and in favor of or against an intervention or statement. The system utilizes classification codes in which a capital letter describes the quality of evidence, followed by a number indicating the strength of a recommendation in favor of or against the intervention or statement.7,8 In the statements referring to definition, epidemiology, natural history, pathophysiology, and involvement of other organs, only the quality of evidence was graded.
Definitions, epidemiology, and risk factorsCoordinator: Dr. Raúl Bernal Reyes
Participants: Dr. Heriberto Rodríguez Hernández, Dr. José Antonio Chávez Barrera, Dr. Mauricio Castillo Barradas, Dr. Javier Lizardi Cervera
1. At present, different terms have been used to denote the same disease, causing confusion among physicians and patients. Therefore, the present consensus proposes the name, nonalcoholic fatty liver disease (NAFLD).
Level of agreement: in complete agreement, 97.05%; in partial agreement, 2.94%.
2. NAFLD is a metabolic disorder characterized by macrovesicular steatosis in more than 5% of hepatocytes in individuals that do not drink significant amounts of alcohol, take hepatotoxic medications, or present with other known causes of secondary steatosis. Its clinical and pathologic spectrum can progress from simple steatosis to steatohepatitis, cirrhosis, and HCC.
Level of agreement: in complete agreement, 94.11%; in partial agreement, 5.88%.
NAFLD is closely related to MetS. One of its characteristics is macrovesicular steatosis, although fat microvesicles can occasionally be observed.9 NAFLD is considered a primary steatosis and therefore other causes of secondary steatosis must be ruled out, especially significant alcohol consumption, understood as drinking more than 30g/day for men and 20g/day for women. Other diseases should also be ruled out, such as hepatitis C, Wilson's disease, and lipodystrophy, as should sequelae from prolonged fasting, bariatric surgery, and parenteral nutrition. Drug toxicity should also be excluded, and the most common agents associated with liver injury are amiodarone, methotrexate, tamoxifen, and steroids.10
NAFLD has clinical and pathologic variants and the most common form is simple steatosis. Nonalcoholic steatohepatitis (NASH), which in addition to steatosis, entails inflammation and liver damage, can present to a lesser degree. Almost one third of those cases can go on to develop cirrhosis11 and a minority of patients can develop the complication of HCC.12
3. Nonalcoholic fatty liver refers to the accumulation of fat vacuoles in more than 5% of the liver parenchyma with no apparent hepatocellular damage in persons that do not drink a significant amount of alcohol or have other causes of secondary steatosis. It can be corroborated through biopsy, biochemical methods, or imaging studies.
Level of agreement: in complete agreement, 88.23%; in partial agreement, 8.82%; uncertain, 2.94%.
Nonalcoholic fatty liver is characterized by the presence of simple steatosis, with no inflammatory changes, fibrosis, or necrosis. It is usually asymptomatic and is considered reversible if the patient can correct the lifestyle factors that promote it, which are extreme sedentary behavior and a diet rich in saturated fats and refined sugar.13 Even though the diagnosis can be made through liver biopsy, that procedure is not justified in the majority of cases due to its inherent risks. There are other noninvasive alternatives, such as ultrasound (US), which is accessible, low-cost, and has high diagnostic accuracy, or serum biomarkers (fatty liver index [FLI] and SteatoTest®). The controlled attenuation parameter (CAP) and hepatic magnetic resonance spectroscopy (1H-MRS) are additional options, but they are less accessible and have a higher cost.14 They will be discussed in more detail further ahead.
4. Nonalcoholic steatohepatitis (NASH) is the name given to the progression of steatosis not associated with alcohol ingestion or other causes of secondary steatosis. Biopsy reveals inflammation and ballooning degeneration of hepatocytes and there can be fibrosis.
Level of agreement: in complete agreement, 91.17%; in partial agreement, 8.82%.
From clinical, biochemical, or imaging study perspectives, it is not possible to distinguish NASH from simple steatosis. Even though there are biochemical markers for inflammation and fibrosis, they are not superior to liver biopsy, which is essential for diagnosis.15
Some cases of NASH can be reversed if good metabolic control is achieved, especially through diet and exercise and occasionally with certain medications. In other cases, the disease can progress to an increase in fibrosis, in which case prognosis is poor, because it can lead to cirrhosis.16
5. Nonobese NAFLD refers to the presence of steatosis in more than 5% of the liver parenchyma, with or without steatohepatitis, in individuals that have a body mass index (BMI) under 25kg/m2and do not drink a significant amount of alcohol or present with any other cause of secondary steatosis.
Level of agreement: in complete agreement, 97.05%; in partial agreement, 2.94%.
Obesity is the clinical phenotype commonly associated with NAFLD and the rest of the comorbidities of MetS. However, nonobese patients can also develop NAFLD and the prevalence range for those patients is 3 to 30%.9
The cutoff point for determining overweight or obesity varies between different populations. In Western countries, nonobese NAFLD is characterized by a BMI under 25kg/mý (9), whereas a BMI <23kg/my is the cutoff point in some Asian countries, and a growing number of cases have been reported.17
The prevalence of MetS is lower in the group of patients with nonobese NAFLD, compared with obese NAFLD patients, but insulin resistance and/or dyslipidemia tend to be common.18
6. NAFLD-related cirrhosis is an advanced disease stage characterized by the development of fibrosis that damages the functional architecture of the liver. It occurs in patients with one or more components of MetS that have not been exposed to damage by alcohol, drugs, viruses, or other recognizable hepatotoxic agents.
Level of agreement: in complete agreement, 91.17%; in partial agreement, 5.88%; uncertain, 2.94%.
NASH represents a greater risk for progression to fibrosis and is the main risk factor associated with progression to cirrhosis and death due to liver-related causes. Approximately 40.75% (95% CI: 34.69-47.13) of the patients with NASH can present with liver fibrosis progression, with an average rate of annual progression from one stage to another of 0.09% (IC 95% CI: 0.06-0.12). In other words, it would take 10 years, on average, to progress from one stage of liver fibrosis to another.19
Studies on patients with cryptogenic cirrhosis have shown that more than 60% have components of MetS, the same as occurs in patients with NAFLD, and that MetS is much more frequent in cryptogenic cirrhosis than in patients with cirrhosis due to other causes, such as viruses or autoimmunity.20,21 ln addition, the prevalence of NASH in patients that underwent a liver transplant due to cryptogenic cirrhosis is very high,22 leading to the supposition that most likely a large percentage of the patients with cryptogenic cirrhosis originally presented with NAFLD that progressed to cirrhosis. Unfortunately, once cirrhosis presents, it is difficult to histologically confirm whether the cause is related or not to NAFLD.
In 2004, a Mexican study on the main causes of cirrhosis placed cryptogenic cirrhosis in third place, after alcoholic cirrhosis and disease due to the hepatitis C virus. Thus, the possibility that an important number of Mexican patients originally diagnosed with cirrhosis of cryptogenic origin, were actually cases of cirrhosis secondary to NAFLD.23 There is an increasing trend of cases of cirrhosis secondary to NAFLD worldwide.24
7. Hepatocellular carcinoma due to nonalcoholic fatty liver disease is a complication characterized by the development of malignant neoplasia originating in the hepatocytes in patients with nonalcoholic steatohepatitis, and it is not necessarily preceded by cirrhosis of the liver.
Level of agreement: in complete agreement, 94.11%; in partial agreement, 5.88%.
The development of HCC in patients with NAFLD is associated with older age, obesity, DM2, the PNPLA3 I148M polymorphism, poor dietary habits, and certain drugs.25,26 The annual incidence of HCC in patients with NAFLD is estimated at 0.44 per 1,000 person years, whereas it is estimated at 5.29 per 1,000 person years in patients with NASH.19 An annual 9% increase in the number of HCC cases attributed to NAFLD between 2004 and 2009 was reported in a recent study. Those patients had a lower survival rate, more cardiovascular events, and more probability of death related to liver cancer than the patients with no NAFLD.27 HCC can present in patients with NAFLD in the absence of fibrosis or cirrhosis.28,29
8. The prevalence of nonalcoholic fatty liver disease is variable due to ethnic diversity and the different diagnostic methods utilized. One-third of the total world population is estimated to be affected.
Level of agreement: in complete agreement, 97.05%; in partial agreement, 2.94%.
NAFLD is the most frequent chronic liver disease worldwide, and its prevalence in the general population is estimated at 17 to 46%, with certain differences depending on the variables of diagnostic method utilized, age, sex, and ethnic origin of the groups studied.
The disease has been observed more frequently in the male sex, in persons above 50 years of age, and in the population of Mexican origin.30
There are few reports on the incidence of NAFLD. The figure of 20-86/1,000 person years based on elevated liver enzymes and/or US and of 34/1,000 per year by 1H-RMS have been estimated.14
The authors of a study on a Mexican population of persons with private health insurance that had a medical check-up reported a 14.4% prevalence of hepatic steatosis,31 and in a recent study on volunteers recruited from the Internet, signs of steatosis were found in 62.9% of the participants.32 The diagnostic method utilized in those two studies was US.
9. NAFLD is the manifestation of MetS at the hepatic level and the risk for its development is higher in patients with more components of the syndrome. Obesity is the most common, followed by DM2 and dyslipidemia. Other known risk factors are male sex, advanced age, and the PNPLA3 polymorphism in the Mexican population.
Level of agreement: in complete agreement, 91.17%; in partial agreement, 8.82%.
The prevalence of NAFLD increases with the augmented prevalence of MetS components, especially obesity. In fact, some authors consider NAFLD to be another component of MetS.14
There is a direct relation between BMI, the grade of steatosis, and the severity of the hepatic lesion. It appears that fat distribution at the visceral level is more important than the amount of total body fat for determining liver damage.33
In patients with morbid obesity that have undergone bariatric surgery, the prevalence of NAFLD can exceed 90% and more than 5% of those patients can present with unexpected cirrhosis.14 In Mexico, the prevalence of NAFLD is 82% in obese patients that have undergone bariatric surgery,34 36% in obese women,35 and in 18.5% of diabetic patients, NASH has been confirmed by biopsy.36
High levels of triglycerides and low levels of HDL are quite common in NAFLD, with a prevalence up to 50%. Obesity, DM2, and insulin resistance are the main metabolic risk factors for developing NAFLD.14
Natural History and PathophysiologyCoordinator: Dr. Norberto Chávez Tapia
Participants: Dr. Ylse Gutiérrez Grobe, Dr. Fátima Higuera de la Tijera, Dr. Nahúm Méndez Sánchez, Dr. Francisco Bosques Padilla
10. Patients with NAFLD present with an increase in general mortality and mortality related to cardiovascular diseases and liver diseases. They also have a higher incidence of malignant neoplasias, including HCC (even in the absence of liver cirrhosis), and the risk for DM2.
Level of agreement: in complete agreement, 100%.
In the cohort study that presently has the largest number of patients with NAFLD diagnosed by biopsy and the longest follow-up with a mean of 20 years (range: 0 to 40 years), the presence of NASH did not increase general mortality or liver disease mortality. However, the severity of fibrosis was a determining factor, related to a shorter time for developing decompensated liver disease (22 to 26 years in F0-F1, 9.3 years in F2, 2.3 years in F3, and 0.9 years in F4).37
Patients with NAFLD present with alterations in cardiac remodeling, manifested by an increase in the mass index of the left ventricle, the diameter of the left ventricle at the end of the diastole, and the left atrial volume index. The presence of steatosis, as well as that of fibrosis, in patients with NAFLD, has been correlated with diastolic dysfunction and the deterioration in glucose uptake by the myocardium.38,39 The cardiac morphologic and functional alterations are more important, the more severe the liver fibrosis.40 The presence of advanced fibrosis is the most significant predictor related to an increase in cardiovascular death in those patients.41
In the study by Allen et al.,42 the 10-year general mortality rate was greater in patients diagnosed with NAFLD (19.2%) than in the controls (7.6%; p <0.0001). The relative risk for death associated with the presence of NAFLD was 2.16 (95% CI: 1.41-3.31). In addition, NAFLD was an independent risk factor associated with the development of other metabolic comorbidities and cardiovascular events.
In another study that included 4,406 cases of HCC, its annual incidence was 0.4/1,000. The presence of NAFLD/NASH was the most frequent etiologic factor for the development of HCC, followed by DM2, and then chronic infection due to the hepatitis C virus (59, 36, and 22%, respectively).43
HCC usually occurs in patients with cirrhosis but can develop in its absence. A study on 1,221 patients with HCC with no cirrhosis showed that the most frequently associated factors were female sex and NAFLD. HCC with no underlying cirrhosis was more frequently unifocal, but larger, when compared with HCC developed in patients with cirrhosis. The possibility of resection and survival was comparatively better in the patients with cirrhosis,44 although the risk for recurrence of HCC was also greater.45
The presence of NAFLD in US imaging, as well as altered liver enzymes, are predictive factors for the development of DM2 and MetS, regardless of age and BMI.46,47 The risk for developing DM2 in patients with NAFLD affects both sexes, but women are at greater risk: 4.8 (95% CI: 3-7.8) in men and 14.5 (95% CI: 7-30.1) in women.48 In another prospective study, with a 5-year follow-up, the incidence of DM2 and prediabetes was higher in patients with NAFLD than in patients without NAFLD, registered at 20.6 and 51.6 per 1,000 person years, versus 4.9 and 29.2 per 1,000 person years, respectively. However, in the adjusted multivariate model, the presence of NAFLD was only related as a predictive factor for later DM development (HR 4.5; IC 95% CI: 1.9-10.7, p <0.001).49
11. The presence of NAFLD in pediatric-aged individuals is associated with the development of DM2 and obesity in adulthood.
Level of agreement: in complete agreement, 100%.
There are few epidemiologic studies that have evaluated the natural history of NAFLD in pediatric patients, but it is known that adolescents that present with NAFLD have higher fasting glucose values and deterioration of insulin sensitivity, compared with controls.50 In a study that included 66 children with NAFLD, at diagnosis, 55 of them (83%) had at least one feature of MetS, such as obesity, hypertension, dyslipidemia, or hyperglycemia. Four children developed DM2 4 to 11 years later. Four out of 5 patients that had a baseline liver biopsy and follow-up of a mean 41.4±28.8 months, had fibrosis stage progression. Two children died and 2 underwent liver transplantation due to decompensated cirrhosis. The 2 transplanted patients had post-transplantation recurrence of NAFLD. Transplant-free survival was lower in the children with NAFLD, compared with the general population and paired by age and sex (p <0.00001).51 In another longitudinal study on children with NAFLD, 30% of the patients developed DM2 in their youth and 78% remained obese.52 At two years of follow-up, 10% of the children with NASH developed DM2. Obese children have also been observed to have a higher risk for developing HCC in adulthood.53
12. The quantity of intrahepatic fat in patients with NAFLD can vary, depending on the change in the abdominal perimeter, characteristics of diet, physical activity, and control of metabolic comorbidities.
Level of agreement: in complete agreement, 97.05%; uncertain, 2.94%.
The combination of diet plus exercise is more effective than diet, alone, for reducing intrahepatic triglycerides, according to the findings of a systematic review.54
Both moderate aerobic exercise and intense exercise performed consistently for a minimum period of 6 to12 months are effective strategies for reducing the intrahepatic triglyceride content and the amount of abdominal fat, as well as for improving blood pressure values.55–60 An exercise intervention in patients with overweight and NAFLD was evaluated in a meta-analysis. It included 21 randomized controlled trials, with a total of 1,530 participants. The studies with an exercise intervention that included a total workload in the exercise program> 10,000kcal showed significant improvement in intrahepatic fat content (−3.46% [95% CI:−5.20 to−1.73%], p <0.0001, I2=73%; effect size [SMD]:−1.77 [95% CI:−3.11 to−0.42], p=0.01, I2=77%).61
The quantity and quality of the diet and the type of nutrients ingested have also been shown to influence the development of NAFLD and the amount of intrahepatic fat.62 The presence of NAFLD is related to the consumption of hypercaloric diets and the high content of saturated fatty acids and polyunsaturated fatty acids.63 The hepatic fat fraction and the intrahepatic content of lipids, determined by 1H-MRS, are associated with the total energy or caloric intake and the total fat intake.64 Diets with a high carbohydrate content are also related to greater intrahepatic fat content, because carbohydrates promote lipogenesis through the activation of transcription factors, such as the “carbohydrate-responsive element-binding protein (ChREBP) and the “sterol-regulatory element-binding protein-1c (SREBP-1c).65 Other sugars, such as sucrose and fructose, are also associated with greater intrahepatic fat content.66–68 In contrast, isocaloric diets with a high protein content, rich in methionine and branched-chain amino acids, are associated with a decrease in intrahepatic fat content.69 Low-carbohydrate diets are associated with reduced intrahepatic fat in patients with NAFLD (at up to−11.53% [95% CI:−18.1 to−4.96]; I2=83.2%).70
The intrahepatic triglyceride content is related to different metabolic alterations. In a study that included 352 patients with NAFLD, Bril et al.62 demonstrated that hepatic insulin sensitivity was affected at an early stage when intrahepatic fat was ∼1.5%. Skeletal muscle insulin sensitivity was also impaired early on in patients with NAFLD. When the hepatic triglyceride content reached ∼6±2%, other alterations, such as hypertriglyceridemia and a low HDL cholesterol profile, were also relevant. The worsening of adipose tissue insulin sensitivity was the most significant alteration, with continuous and progressive deterioration that directly correlated with the increase in intrahepatic fat content (r=−0.38; p <0.001). Those findings confirm the close association between insulin resistance in adipose tissue and the theory of lipotoxicity as a factor that regulates the deposit of fat in the liver parenchyma.
In diabetic patients with NAFLD, glycemic control significantly reduces intrahepatic lipid content, regardless of the hypoglycemic agent indicated for diabetes control.63,71–74
13. Fibrosis progression in patients with NAFLD is influenced in a dose-response manner by MetS components.
Level of agreement: in complete agreement, 85.29%; in partial agreement, 11.76%; uncertain, 2.94%.
The association between NAFLD and MetS has been reported in numerous studies. The risk for NAFLD significantly increases in accordance with the number of MetS components. Patients with only one component have a 3.6-fold greater risk for presenting with NAFLD, compared with patients with no components (HR: 3.64; 95% CI: 1.5-8.88%).75 In relation to fibrosis, previous studies have shown that patients with MetS have higher fibrosis scores than patients that do not present with the syndrome (3.3 vs 1.6, p=0.01). There is a significant increase in fibrosis in relation to the number of MetS components (p=0.014).76
14. The prognosis of patients with NAFLD is determined by the grade of fibrosis.
Level of agreement: in complete agreement, 97.05%; in partial agreement, 2.94%.
According to different population studies, long-term prognosis of patients with NAFLD is not the same throughout the disease spectrum, given that patients with a minimum of steatosis and no other histologic liver injury alterations have been reported to present with a more benign disease course.77 Angulo et al.78 followed 619 patients for a mean of 12.6 years and observed that fibrosis, from early disease stages, was the only histologic variable independently associated with mortality and liver transplantation. According to their results, the accumulated risk for mortality or liver transplant was 29.8% in grade 1 fibrosis, 42.3% in grade 2, 50.9% in grade 3, and 77.8% in grade 4. They also observed that fibrosis, regardless of the diagnosis of NASH, was associated with higher mortality and events related to liver disease.
15. In patients with NAFLD, the advance from one stage of fibrosis to another can occur within a time interval of one to two decades.
Level of agreement: in complete agreement, 79.41%; in partial agreement, 14.70%; uncertain, 5.88%.
In the recent meta-analysis by Singh et al.79 focused on estimating the fibrosis progression rate in patients with NAFLD and NASH, they showed that up to 36.1% of the patients had progressive fibrosis. They also observed that the annual fibrosis progression rate in patients with NAFLD that had stage 0 fibrosis at the baseline was 0.07 stages (95% CI: 0.02-0.11). They found that mean progression of a stage occurred in approximately 14.3 years (95% CI: 9.1-50.0). About 20% of the subgroup that developed fibrosis had rapid fibrosis, progressing from stage F0 to advanced fibrosis in a mean of 5.9 years.
16. A decrease in life-expectancy and quality of life is estimated for the adult and pediatric populations with NAFLD.
Level of agreement: in complete agreement, 85.29%; in partial agreement, 14.70%.
In studies conducted on the general population, the prevalence of NAFLD in children was 7.6% (95% CI: 5.5-10.3%)80 and 2.6 to 3.2% in adolescents.81 In a follow-up study at 20 years, with a total of 409.6 person years of follow-up, conducted on 66 children with NAFLD, 4 patients had fibrosis progression: 2 of them required liver transplantation and 2 of them died. Upon comparing the NAFLD cohort with the general population of the same age and sex, there was a significantly higher number of progression events in the patients with NAFLD, with a standardized mortality rate of 13.6 (95% CI: 3.8-34.8).51
Long-term population studies have shown that the mortality rate and associated morbidity increase in adult patients that progress to NASH. In a cohort of 256 Swedish patients followed for 28 years, the mortality rate was 40% in patients diagnosed with NAFLD.82 On the other hand, at a mean 12.3 years of follow-up, Angulo et al.78 reported that deaths caused by complications of cirrhosis, HCC, or liver transplant occurred in 9.3% of the 619 patients with NAFLD.
17. In NAFLD, the inflammatory and fibrogenic response is regulated by endocrine mechanisms, primarily insulin resistance, but it is also determined by immunologic and endothelial mechanisms, sex hormones, endotoxemia, and genetic variability.
Level of agreement: in complete agreement, 88.23%; in partial agreement, 5.88%; uncertain, 5.88%.
Through the search for genetic targets that explain NAFLD, the type I collagen alpha-1, sex hormone-binding globulin, and amyloid-beta precursor protein genes have been found to participate in the development of the disease.83 In the Mexican population, the PNPLA3 gene is the most widely reported in association with NAFLD.84 Nevertheless, the need for local validations should be considered, as well as determining the impact of genetic variants on the development of NAFLD.
Even though insulin resistance determines the mechanisms that favor the accumulation of fat inside the hepatocyte, local and systemic responses also have an influence,85 in addition to the immunologic response caused by an alteration in the balance of cytokines produced by Th17 cells and regulatory T cells.86 Sex hormones have been shown to influence the inflammatory response and the endotoxin-induced inflammatory response, through the modification of physical activity.87
18. The cardiovascular alterations observed in NAFLD are partially determined by the response of the liver to the presence of fat, to inflammatory activity, and to the fibrogenic response.
Level of agreement: in complete agreement, 85.29%; in partial agreement, 8.82%; uncertain, 5.88%.
It is clear that body weight, physical activity, and dietary characteristics can be risk factors per se for the development of cardiovascular disease. Said risk has classically been related to the increase in adiposity, particularly visceral adiposity, and to humoral mechanisms in the adipose tissue.88 However, there is also a hepatic collaboration in subjects with NAFLD,89 which multiplies the cardiovascular risks already described in those patients, and it is related to disease severity. NAFLD can cause structural alterations in the high-density lipoproteins that negatively affect their functions,90 confirming that alterations at the hepatic level play an independent role in the development of cardiovascular disease.
19. Lipotoxicity participates in the inflammatory and fibrogenic response that is dependent on the hepatocyte and other cells residing in the liver (e.g., Kupffer cells and stellate cells).
Level of agreement: in complete agreement, 97.05%; in partial agreement, 2.94%.
Fatty acids in the cells residing in the liver have an impact at different levels. One of the most important effects is the production of reactive oxygen species that promote liver damage through apoptosis. With the addition of inflammatory cytokine production by the Kupffer cells and hepatocytes, the stellate cells are activated, promoting fibrin formation. Finally, the fatty acids can cause cytokine production that favors fibrogenesis, not only by the stellate cells, but also by the Kupffer cells and hepatocytes.91–94
Clinical manifestations and involvement of other organsCoordinator: Dr. Graciela Castro Narro
Participants: Dr. Rafael Trejo Estrada, Dr. Diego García Compeán, Dr. Carlos Aguilar Salinas, Dr. David Kershenobich Stalnikowits, Dr. José Antonio Velarde-Ruiz Velasco
20. The presence of MetS should be investigated in patients with NAFLD and the presence of NAFLD should be investigated in patients with MetS.
Level of agreement: in complete agreement, 88.23%; in partial agreement, 11.76%.
Evidence supports the common pathogenic mechanisms for NAFLD and MetS that are associated with the development of DM2, cardiovascular disease, and severe forms of liver disease, including cirrhosis and HCC.
The first accepted opinion maintained that NAFLD was simply “the hepatic manifestation of MetS”. Current evidence supports the idea that NAFLD can appear as part of a series of biologic events, culminating in the development of MetS or its clinical characteristics, particularly DM2. That new paradigm is clinically relevant and implies that NAFLD can be a pathogenic determinant of MetS and that treating NAFLD is also an important way to prevent the development of MetS and its associated cardiometabolic complications.95
In a case series of 304 patients with NAFLD,96 the authors found that the prevalence of MetS in patients with NAFLD increased when there was a higher BMI. That increase went from 18% in patients with nonobese NAFLD to 67% in obese patients with NAFLD. In addition, the presence of MetS was associated with a greater risk for NASH and severe fibrosis. A total of 88% of the patients with NASH met the criteria for MetS, compared with 53% of the patients with simple steatosis.
According to data from the NASH Clinical Research Network, MetS confers a 40% increase in the risk for histologically-confirmed NASH, and the highest values in the NAFLD Activity Score (NAS) are associated with higher levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), whereas the diagnosis of NASH is associated with characteristics of MetS.97
In a recent meta-analysis (2016), 117,020 patients from 20 studies were analyzed and the conclusion was that NAFLD, diagnosed through liver enzyme determination or ultrasonography, significantly increased the risk for DM2 and MetS in a mean follow-up period of 5 years, with a relative risk of 1.80 for ALT (last vs first quartile or quintile), 1.98 for gamma glutamyl transferase (GGT), and 3.22 for echography.98 Kwon et al.99 reported a stronger association for MetS and NAFLD in nonobese patients with NAFLD than in obese patients with the disease. Patients with nonobese NAFLD had higher adjusted prevalence rates for certain MetS components (elevated triglycerides in both sexes and high blood pressure, altered fasting glucose, and high-density lipoprotein [HDL] cholesterol in women) than the obese patients with NAFLD.
Finally, not only are MetS characteristics highly prevalent in patients with NAFLD, but also the risk for NAFLD and its severity increase with the number of MetS components.
21. Insulin resistance and abnormal body fat distribution are common in patients with NAFLD.
Level of agreement: in complete agreement, 82.35%; in partial agreement, 14.70%; uncertain, 2.94%.
Obesity is related to a greater risk for metabolic diseases, such as insulin resistance, DM2, dyslipidemia, and NAFLD.100 Hepatic steatosis is a consequence of the alteration of lipid metabolism in the liver. The main contributing factors are hepatic insulin resistance and the greater affluence of free fatty acids in the liver. The pathophysiology of steatohepatitis points to the original two-hit hypothesis, in which a first hit, such as insulin resistance, results in hepatic steatosis, and a second hit, such as oxidative stress, results in the development of steatohepatitis.101,102
The multiple-hit theory has recently been proposed to explain the pathophysiology of NAFLD. Insulin resistance is one of the key factors in the development of steatosis/steatohepatitis, and results in increased de novo hepatic lipogenesis and the inhibition of adipose tissue lipolysis, with the consequent increase in the flow of fatty acids to the liver. Insulin resistance also promotes adipose tissue dysfunction, with the resulting altered production and secretion of adipokines and inflammatory cytokines.103,104
Insulin resistance is a cardinal characteristic of NAFLD and is more prevalent in steatohepatitis than in simple steatosis.105 We know that it plays an important part in the pathogenesis of NAFLD.106
In addition, adipose tissue dysfunction is known to have an essential role in the development of metabolic disorders, such as insulin resistance and NAFLD. In patients with NAFLD, body fat distribution is a more important factor than the total amount of fat. Persons with excess visceral adipose tissue or abdominal obesity are at greater risk of having MetS components than persons whose subcutaneous fat is predominantly in the lower part of the body. Furthermore, lean patients that present with steatohepatitis generally have abdominal obesity or more visceral adipose tissue.107,108 The area of visceral adipose tissue is increased in patients with NAFLD (with and without significant fibrosis) and is independently associated with a greater risk for steatohepatitis.33
In another cohort study that included 2,017 subjects and a median follow-up period of 4.4 years, the area of visceral adipose tissue was related to a higher incidence of NAFLD, with a HR of 1.36 (95% CI: 1.16-1.59). In contrast, larger areas of subcutaneous adipose tissue were longitudinally associated with NAFLD regression. Those data indicate that certain types of body fat are risk factors for NAFLD (visceral adipose tissue), whereas other types can reduce the risk for NAFLD (subcutaneous adipose tissue).109
22. NAFLD is a risk factor for HCC that can also appear in the absence of cirrhosis and the presence of the PNPLA3 gene.
Level of agreement: in complete agreement, 97.05%; in partial agreement, 2.94%.
The development of HCC complicates chronic liver disease. Epidemiologic studies have shown the association of DM2 and obesity with an increased risk for developing HCC.110 The appearance of HCC has also been reported in NAFLD and cryptogenic cirrhosis.27
In the United Kingdom, HCC-related mortality rose 1.8-fold over a 10-year period (from 2.0 to 3.7 per 100,000). In a study that evaluated the demographics of patients referred for cancer in England, a 2 to 3-fold increase was shown in the referral of patients with HCC associated with hepatitis C, alcoholic hepatopathy, or the absence of chronic liver disease, but a 10-fold increase was demonstrated in HCC associated with NAFLD.110
NAFLD is the third cause of HCC in the United States and the second most frequent indication for HCC-related liver transplantation.111 The incidence of HCC associated with NAFLD is estimated to increase at an annual rate of 9%.27
At the time of diagnosis, patients with NAFLD-associated HCC are older and have extrahepatic comorbidities, but with a lower frequency of cirrhosis, when compared with patients that have HCC that is not associated with fatty liver. At Veterans Hospitals, up to 13% of patients with HCC do not have cirrhosis. NAFLD is recognized as an independent associated factor.112
Patients with NAFLD-related HCC probably die more frequently from the HCC, with respect to patients with cirrhosis due to another cause. However, in the study from the United Kingdom, survival of those patients was similar to that of patients with HCC related to other etiologies and was attributed to significantly higher incidental presentation and a lower prevalence of cirrhosis.110
Genetic variation and environmental factors can combine to determine disease progression in NAFLD. The PNPLA3 rs 738409 C>G gene has been associated with a higher risk for progressive steatohepatitis and fibrosis, but also with an increased risk for HCC. In a recent study, the PNPLA3 rs 738409 gene was determined by allelic discrimination in 100 European Caucasians with NAFLD-associated HCC and 275 controls with histologic characteristics of NAFLD and no HCC. The genotypic frequencies were significantly different between the cases with HCC and the controls. In a multivariate analysis adjusted by age, sex, diabetes, BMI, and the presence of cirrhosis, being a carrier of the minor allele (G) of rs 738409 conferred an added risk for HCC. Those results suggest that genotyping could provide a risk stratification that would enable HCC surveillance in patients with NAFLD, albeit such a strategy is not yet considered cost-effective.113
23. The identification of cases of NAFLD with fibrosis is recommended, especially in high-risk patients (age> 40 years, DM2, MetS, AST/ALT> 1).
Level of agreement: in complete agreement, 97.05%; in partial agreement, 2.94%.
Fibrosis is the most important histologic characteristic of NAFLD associated with the risk for long-term mortality. The different stages, F2 to F4, are independent predictors of liver disease-related mortality.114
Elevated BMI and waist circumference are a reflection of visceral adiposity. They are related to NAFLD and predict more severe disease, especially in persons of advanced age. DM2 is related to the progression of NAFLD, the development of steatohepatitis, the presence of advanced fibrosis, and HCC.115
Fatty liver accompanied by necro-inflammatory changes is defined by the NAS. In a recent study, NAFLD-specific death was determined and the NAS and fibrosis stage were evaluated as general and disease-specific prognostic mortality markers. In that study, data from 229 patients with NAFLD demonstrated by biopsy were evaluated and compared with a reference population. The mean follow-up period was 26 years. Mortality was higher in the patients with NAFLD, with an increased risk for cardiovascular disease, HCC, infectious disease, and cirrhosis. There was no increase in the general mortality rate in patients with F0-F2 stages of fibrosis, but mortality was higher in the patients with F3 and F4 stages, regardless of the NAS.114
24. Patients with NASH and fibrosis have an elevated risk for cirrhosis and liver-related mortality.
Level of agreement: in complete agreement, 94.11%; in partial agreement, 5.88%.
In general, NAFLD is a relatively benign disease, but some individuals develop hepatic and cardiovascular complications. NAFLD can affect from 17 to 46% of the general population and NASH can affect from 3 to 5%. However, up to 30% of the patients with NAFLD can have NASH at the time of NAFLD detection.116 Patients with NASH have an increased risk for progression to liver cirrhosis, compared with patients without NASH, because many have some grade of fibrosis. In cases of NASH with isolated inflammation, progression to cirrhosis is approximately 5 to 18%, whereas the presence of fibrosis increases that progression to 38%.79,117 Recent evidence from prospective cohort studies on patients with NAFLD suggests that fibrosis is a more reliable predictor of chronic liver disease than inflammation, alone. A current study showed that a NAS> 4 (the cutoff point used for defining NASH in clinical studies) did not correlate with liver disease-related mortality.118 In contrast, another prospective study on 209 patients with NAFLD that were followed for 12 years, showed that advanced fibrosis was the only histologic lesion independently associated with liver-related mortality. In that study, as in the previous one described, the patients with a NAS> 4 with no significant fibrosis showed no increase in liver-related mortality, compared with the reference population.119
The hepatic complications of NAFLD include progressive liver disease, cirrhosis, and HCC. The prevalence of liver disease-related mortality (hepatic encephalopathy, ascites, esophageal variceal bleeding, and hepatorenal syndrome) varies. A study on the general population, conducted in the United States within the time frame of 1988 to 1994, with a cohort of 4,083 patients with NAFLD followed up to 2006, with a mean follow-up period of 14.5 years, showed that 779 of the patients had died. The most frequent cause of death was cardiovascular disease (37%), followed by HCC (21%) and liver-related disease (2.4%).41 In another study with a follow-up period of 8 to 18.5 years, liver-related mortality was higher: from 11 to 18%, compared with 2 and 3% in the patients that did not present with NASH or fibrosis, respectively.120,121 Those data indicate that looking for fibrosis in patients with NAFLD is essential for estimating prognosis and deciding on preventive and corrective treatment.
25. In the case of cryptogenic cirrhosis, the investigation of MetS, overweight, and obesity is recommended because they can correspond to NAFLD.
Level of agreement: in complete agreement, 97.05%; in partial agreement, 2.94%.
Cryptogenic cirrhosis appears to have a causal relation to NAFLD. In a study conducted in Mexico, it was the cause of cirrhosis in 10.4% of the patients.23 A similar prevalence was reported in a study carried out in Japan.122 The prevalence of cryptogenic cirrhosis can vary up to 30% in relation to the presence of risk factors.
Histopathologically, the association of NAFLD with cryptogenic cirrhosis has not been easy to demonstrate, given that clear signs of NASH are not found in biopsies, particularly in cases of hepatic steatosis, resulting in cryptogenic cirrhosis being considered a “burned-out” steatohepatitis. Nevertheless, in a histologic analysis of patients with cirrhosis described as cryptogenic that had previous biopsies identified as NASH, residual changes consistent with steatohepatitis (ballooning degeneration, Mallory-Denk bodies, and megamitochondria) were shown, which reflected a pathophysiologic relation between the two pathologic entities.123
A Japanese study122 conducted on 404 patients with cryptogenic cirrhosis reported more obesity and DM2, compared with controls (53 vs 20% and 40 vs 18%, respectively). A study in a Mexican population with 134 patients with cryptogenic cirrhosis showed a significant prevalence of MetS (29.1 vs 6%), obesity (16.4 vs 8.2%), and DM2 (40 vs 22.4%), compared with the control subjects.124
Based on the above, MetS, obesity, dyslipidemia, and diabetes mellitus should be looked for in all patients with cirrhosis of undefined etiology.
26. NAFLD can be associated with other endocrine diseases, such as polycystic ovarian syndrome, hypothyroidism, osteopenia, osteoporosis, growth hormone deficiency, or hypercortisolism.
Level of agreement: in complete agreement, 94.11%; in partial agreement, 5.88%.
NAFLD is associated with several extrahepatic multisystemic diseases.125 That is due to the fact that NAFLD shares pathophysiologic mechanisms with diseases related to MetS, such as cardiovascular diseases associated with atherosclerosis, DM2, polycystic ovarian syndrome, chronic kidney disease, certain tumors, and osteoporosis.126 The relation to bone disease was identified in epidemiologic studies that showed a lower bone mineral density in persons with NAFLD, even when confounding variables were adjusted. The most solid evidence has been in the pediatric population. There is no predominance in any region of the skeleton. The mechanisms involved in the association are vitamin D deficiency and chronic inflammation. However, other associations have alternate explanations. Some endocrine diseases can be the cause of NAFLD, as occurs with hypothyroidism, hypogonadism, growth hormone deficiency, and hypercortisolism.127 Those conditions can be the cause of a decrease in the systemic action of insulin or an increase in lipogenesis. Estrogen and androgen deficiencies are associated with hepatic steatosis. There is insufficient evidence about the effect of hormone replacement on the progression of NAFLD and there is no consensus on whether the evaluation of the abovementioned diseases should be included in screening for NAFLD. Nevertheless, the healthcare professional should be aware of the existence of those associations.
27. Obstructive sleep apnea is associated with obesity and is a risk factor for NAFLD.
Level of agreement: in complete agreement, 85.29%; in partial agreement, 11.76%; uncertain, 2.94%.
The association between NAFLD and obstructive sleep apnea has been documented in case-control studies. Several studies have reported greater fibrosis severity in the cases with apnea, but methodological deficiencies of the reports limit the clinical implications of their findings. The association has even been described in patients with normal adiposity. Both conditions share the reduced action of insulin in peripheral tissues and a state of chronic inflammation.128 In addition, repeated hypoxia facilitates the activation of the hypoxia-inducible factor 1 alpha subunit (HIF1-alpha), which aggravates the chronic inflammation and mitochondrial dysfunction.129 The treatment of apnea through continuous positive airway pressure (CPAP) does not modify the metabolic alterations and there is insufficient quality evidence to make a clinical recommendation.
28. NASH is associated with an increased prevalence of chronic kidney disease (CKD).
Level of agreement: in complete agreement, 94.11%; in partial agreement, 5.88%.
Evidence associates NAFLD and CKD, suggesting that NAFLD has a role in the development and progression of CKD. The participation of the renin-angiotensin-aldosterone system (RAAS) merits attention. Experimental studies have shown the presence of key RAAS elements in the normal liver and their increase in NAFLD. In particular, excessive RAAS activation occurs not only in progressive kidney damage, but also in liver fibrogenesis, relating both liver and kidney damage. The severity of liver damage is associated with greater deterioration in kidney function. That association appears to be independent of insulin resistance, obesity, diabetes, and hypertension.130
NAFLD appears to be associated with a greater risk for the prevalence (OR: 2.12, 95% CI: 1.69-2.66) and incidence (HR: 1.79, 95% CI: 1.65-1.95) of CKD. Patients with NASH have an even higher risk for the prevalence (OR: 2.53, CI: 1.58-4.05) and incidence (HR: 2.12, CI: 1.42-3.17) of CKD, compared with patients that have simple steatosis. The same risk can be doubled in NASH with advanced fibrosis, compared with NASH with mild/absent fibrosis, and the severity of liver damage is positively associated with stages of CKD. In all the analyses, those associations were not affected by diabetes status, abdominal/whole-body obesity, insulin resistance, or other cardiometabolic risk factors.131
Screening and diagnosisCoordinator: Dr. René Malé Velázquez
Participants: Dr. Miguel Stoopen Rometti, Dr. Mario Arturo Ballesteros Amozurrutia, Dr. Paris Ramos Martínez, Dr. Ignacio Aiza Haddad, Dr. Jorge Luis Poo Ramírez, Dr. Misael Uribe Esquivel, Dr. Laura Ladrón de Guevara
29. Screening is recommended for the detection of NAFLD in patients with obesity and/or MetS through the determination of liver enzymes and/or imaging methods.
Quality of evidence and strength of recommendation: GRADE A2, strong in favor of the statement.
Level of agreement: in complete agreement, 76.47%; in partial agreement, 8.82%; uncertain, 2.94%, in partial disagreement, 5.88%; in total disagreement, 5.88%.
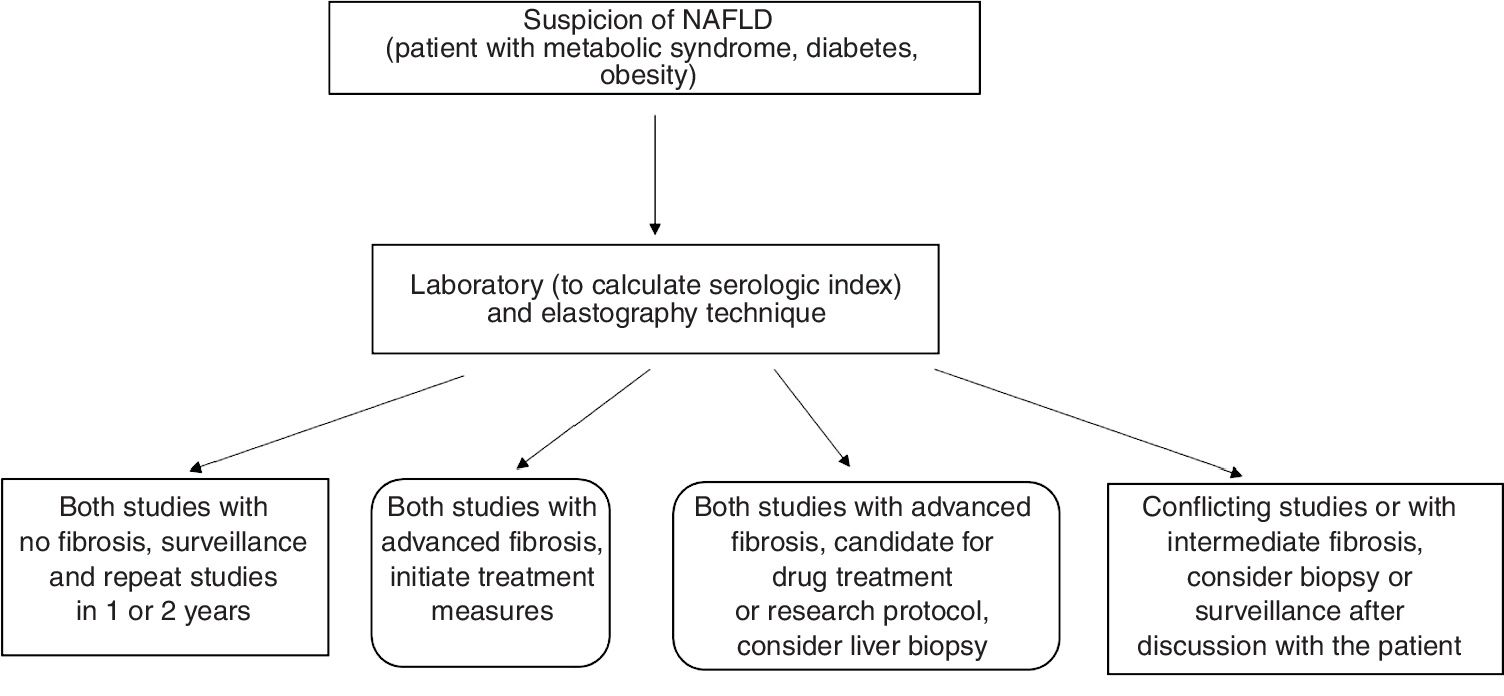
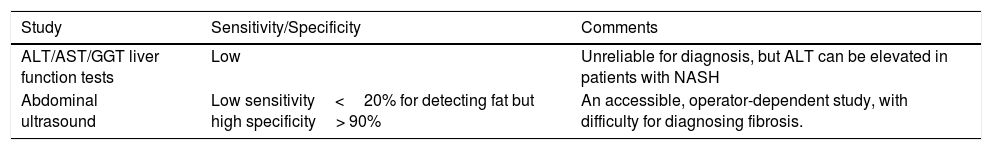
In patients with risk factors for the development of NAFLD (obesity, DM2, and/or MetS), screening through abdominal US and/or liver function tests (AST, ALT, GGT, and alkaline phosphatase) is recommended as a valuable strategy for the early diagnosis of hepatic steatosis. Once the diagnosis of NAFLD is made, fibrosis grade evaluation is recommended through biologic indexes and/or US or magnetic resonance (MR) elastography studies.14,15,132 Liver enzyme level determination (ALT, AST, GGT) is limited in the diagnosis of NAFLD (Table 1) because those levels can be normal even in patients with NAFLD and advanced fibrosis. However, NASH tends to be associated with elevated ALT levels.133,134 Screening for diagnosing NAFLD is a subject of debate, given that there are no studies that validate its usefulness or that assess the cost-benefit of early NAFLD diagnosis in the at-risk population.135,136 On the one hand, screening presents the risk for saturating the health systems with patients with NAFLD, and on the other, it is important to opportunely diagnose patients with advanced NASH and/or fibrosis, especially in at-risk populations.14,15,132 The present consensus group believes that screening for NAFLD is necessary in the Mexican at-risk population (patients with MetS, obesity, and/or DM2).
Usefulness of liver enzymes and ultrasound for diagnosing NAFLD.
| Study | Sensitivity/Specificity | Comments |
|---|---|---|
| ALT/AST/GGT liver function tests | Low | Unreliable for diagnosis, but ALT can be elevated in patients with NASH |
| Abdominal ultrasound | Low sensitivity<20% for detecting fat but high specificity> 90% | An accessible, operator-dependent study, with difficulty for diagnosing fibrosis. |
30. Single or combined serum biomarkers are not sufficiently accurate for distinguishing NAFLD from NASH, nor do they accurately detect early stages of fibrosis.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 94.11%; in partial agreement, 2.94%; uncertain, 2.94%.
The diagnosis of NASH has been attempted through noninvasive serologic tests (cytokeratin 18, metabolomics tests, terminal peptide of procollagen III, NASH test, etc.), but they are not sufficiently reliable or reproducible. Therefore, liver biopsy continues to be the main tool for the accurate diagnosis of NASH.15,137,138
31. Liver biopsy is recommended for accurately diagnosing nonalcoholic steatohepatitis, for precisely determining the grade of fibrosis, and for ruling out other pathologies. Its performance should be a case-by-case decision, especially in patients that are candidates for pharmacologic treatment.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 91.17%; in partial agreement, 5.88%; uncertain, 2.94%.
NAFLD affects 17 to 46% of the general population, thus it is not possible to perform a liver biopsy in all the patients suspected of having NASH or liver fibrosis. That is true, not only due to the lack of economic and human resources, but also to the limitations of the procedure itself, such as the risk for complications, the variability in fibrosis grade in different regions of the liver in the same patient, and the variability in the interpretation of the biopsy results between the different observers.139–143
Liver fibrosis is the most significant histologic finding in patients with NAFLD because it is associated with increased liver disease-related mortality and the need for liver transplantation.78,144 Liver biopsy, despite its limitations, continues to be the gold standard, but its performance should be considered case-by-case when there is diagnostic doubt or when an accurate diagnosis of NASH or liver fibrosis is needed (the use of drugs for treatment or study protocols).145
32. Liver ultrasound is the first-line radiologic method for the detection of NAFLD, not only because of its widespread availability, but also because it provides complementary information on other possible hepatobiliary pathologies.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 97.05%; uncertain, 2.94%.
Liver US is a basic screening tool for NAFLD. Among its advantages are its noninvasiveness, the absence of radiation, its widespread availability, and its low cost.146,147 When the liver has no steatosis, the texture of the parenchyma is homogeneous, with an optical density similar to that of the renal cortex or the parenchyma of the spleen. In contrast, when there is steatosis, the echogenicity (brilliance) of the liver increases and is greater than that of the kidney, and the clarity of the gallbladder wall, intrahepatic vessels, and diaphragm decrease. US sensitivity for detecting steatosis varies from 60 to 94% and specificity from 84 to 95%.148–153
In a meta-analysis of 49 studies, US sensitivity and specificity were evaluated for detecting moderate to severe fatty liver (> 30%), compared with biopsy.154 Sensitivity was 84.8% (95% CI: 79.5-88.9) and specificity was 93.6% (95% CI: 87.2-97.0). That same study pointed out that abdominal ultrasound sensitivity and specificity for the diagnosis of fatty liver were similar to those of other imaging techniques (computed axial tomography and MR imaging). Even though US was highly sensitive for diagnosing moderate to severe steatosis, in cases of steatosis> 5% and <30%, sensitivity decreased to 53.5-66.6% and specificity fluctuated between 77 and 93.1%.146
US without elastography can suggest the presence of advanced fibrosis (cirrhosis) by determining the irregularity and nodularity of the surface of the liver. In fact, it has been described as a complementary study to elastography techniques.155,156
33. Screening for cardiovascular diseases is recommended in patients with NAFLD, according to individual risk factors.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 91.17%; in partial agreement, 5.88%; uncertain, 2.94%.
Patients with NAFLD, with or without fibrosis, are at risk not only for developing inflammation and liver fibrosis, but also for presenting with greater mortality due to cardiovascular disease.157
NAFLD contributes to accelerated atherogenesis, suggesting a bidirectional relationship between NAFLD and cardiovascular diseases.158 NAFLD is an independent risk factor for cardiovascular events.159 Patients should be instructed with respect to lifestyle modifications for MetS control, including the control of high blood pressure and dyslipidemia, according to international guidelines for the prevention of cardiovascular disease.160–163
34. Techniques based on MR have high diagnostic accuracy (similar to that of biopsy) in relation to hepatic steatosis, but their availability in Mexico is limited.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 94.11%; in partial agreement, 5.88%.
Magnetic resonance (MR) and US-based techniques can be used for determining NAFLD. Techniques based on MR, whether through spectroscopy164 or the measurement of the proton density fat fraction (PDFF), are the most accurate for the diagnosis of NAFLD.165,166 MR spectroscopy has preferably been used in research studies because of its technical difficulty and its limited availability.
A recent meta-analysis of 28 publications shows that MR imaging-PDFF has greater accuracy and reproducibility for measuring fat content in the liver and for patient follow-up.167 MR imaging adequately classifies hepatic steatosis grade, with results similar to those of liver biopsy, and has been used for follow-up in the response to pharmacologic therapy, but its availability in Mexico is limited.
35. Liver stiffness* and fat* can be simultaneously determined by ultrasonographic techniques through controlled attenuation parameter measurement (transitory elastography).
Quality of evidence and strength of recommendation: GRADE *A1, strong in favor of the statement, **B2, weak in favor of the statement.
Level of agreement: in complete agreement, 88.23%; in partial agreement, 11.76%.
Controlled attenuation parameter (CAP) measurement, connected to the imaging technique equipment of transient elastography (FibroScan®), is a good diagnostic method for quantifying hepatic steatosis. When integrated into liver stiffness measurement, CAP can simultaneously classify the grades of fibrosis and steatosis in a single procedure. Compared with MR imaging-PDFF, CAP quantifies liver fat less accurately.168 Evaluated in 5,323 patients by De Ledinghen et al.,169 CAP detected hepatic steatosis, especially in patients with a BMI above 25kg/m2 or with MetS, in alcoholics, and in patients with liver stiffness above 6kPa. Liver biopsy was performed on 440 of the patients. In the patients with hepatic steatosis>10%, CAP had an AUROC of 0.79 (95% CI: 0.74-0.84, p=0.001), in steatosis> 33%, it was 0.84 (95% CI: 0.80-0.88, p=0.001), and in steatosis> 66%, it was 0.84 (95% CI: 0.84-0.88, p=0.001).
36. The serologic indexes for hepatic steatosis determination are an acceptable alternative when imaging studies (resonance or controlled attenuation parameter) are not available.
Quality of evidence and strength of recommendation: GRADE B2, weak in favor of the statement.
Level of agreement: in complete agreement, 82.35%; in partial agreement, 14.70%; in total disagreement, 2.94%.
The serologic or biologic indexes, such as the fatty liver index (FLI), SteatoTest®, and steatosis score, have been used as alternatives in NAFLD detection and screening, especially when other techniques, such as MR imaging-PDFF or CAP, are not available. Those indexes have been validated in the general population and evaluate the presence of steatosis, but not its severity.
Described in 2006, the FLI is calculated through a formula that incorporates BMI, hip circumference, triglycerides, and GGT levels. It has been used in epidemiologic studies for NAFLD screening and has been validated in different populations.170,171
The SteatoTest® is a patented formula with 12 serologic variables. The extra cost for obtaining its results is a disadvantage.172
The steatosis score was first described in a Finnish population and was later validated in other groups. The index incorporates simple variables, such as the presence of MetS, DM2, fasting serum insulin, and the AST/ALT ratio.173,174
37. The combined and/or simultaneous and/or sequential use of serologic tests and elastography studies is recommended for establishing the grade of fibrosis, to reduce the use of liver biopsy.
Quality of evidence and strength of recommendation: GRADE B2, weak in favor of the statement.
Level of agreement: in complete agreement, 91.17%; in partial agreement, 5.88%; uncertain, 2.94%.
US or MR elastography are modalities that are sensitive to tissue stiffness. Imajo et al.168 compared MR elastography (MRE) vs transient elastography (TE) in 142 patients identified through biopsy and found that the AUROC for establishing liver fibrosis> F2 was 0.82 for TE and 0.91 for MRE. Park et al.175 confirmed that MRE was more accurate than TE for detecting fibrosis in patients with NAFLD.
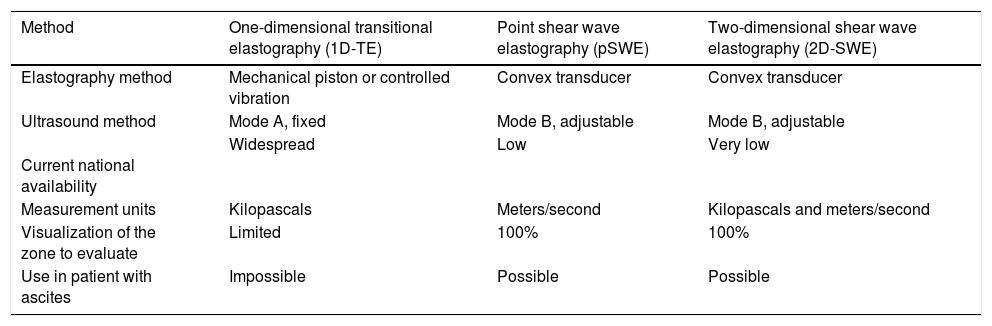
Fibrosis is the main component of “liver stiffness”, but in US-based elastography, other factors, such as inflammation and hepatic congestion, cholestasis, and steatosis grade, over-evaluate the grade of liver stiffness.176 The US elastography methods are based on the determination of tissue elasticity, through which the grade of fibrosis can be estimated. Table 2 describes the characteristics of the different US elastography methods and Table 3 describes the specific details of their sensitivity and specificity.176–180
Physical methods for measuring the elasticity of the liver through ultrasound, according to Sigrist et al.179
| Method | One-dimensional transitional elastography (1D-TE) | Point shear wave elastography (pSWE) | Two-dimensional shear wave elastography (2D-SWE) |
|---|---|---|---|
| Elastography method | Mechanical piston or controlled vibration | Convex transducer | Convex transducer |
| Ultrasound method | Mode A, fixed | Mode B, adjustable | Mode B, adjustable |
Current national availability | Widespread | Low | Very low |
| Measurement units | Kilopascals | Meters/second | Kilopascals and meters/second |
| Visualization of the zone to evaluate | Limited | 100% | 100% |
| Use in patient with ascites | Impossible | Possible | Possible |
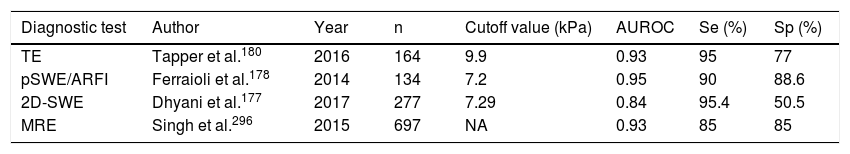
Sensitivity (Se) and specificity (Sp) of the ultrasound-based elastography methods.
| Diagnostic test | Author | Year | n | Cutoff value (kPa) | AUROC | Se (%) | Sp (%) |
|---|---|---|---|---|---|---|---|
| TE | Tapper et al.180 | 2016 | 164 | 9.9 | 0.93 | 95 | 77 |
| pSWE/ARFI | Ferraioli et al.178 | 2014 | 134 | 7.2 | 0.95 | 90 | 88.6 |
| 2D-SWE | Dhyani et al.177 | 2017 | 277 | 7.29 | 0.84 | 95.4 | 50.5 |
| MRE | Singh et al.296 | 2015 | 697 | NA | 0.93 | 85 | 85 |
MRE: magnetic resonance elastography; NA: Not applicable because the values are in m/sec, instead of kilopascals; pSWE/ARFI: point shear wave elastography (pSWE) using acoustic radiation force impulse (ARFI); TE: transient elastography; 2D-SWE: 2D-shear wave elastography.
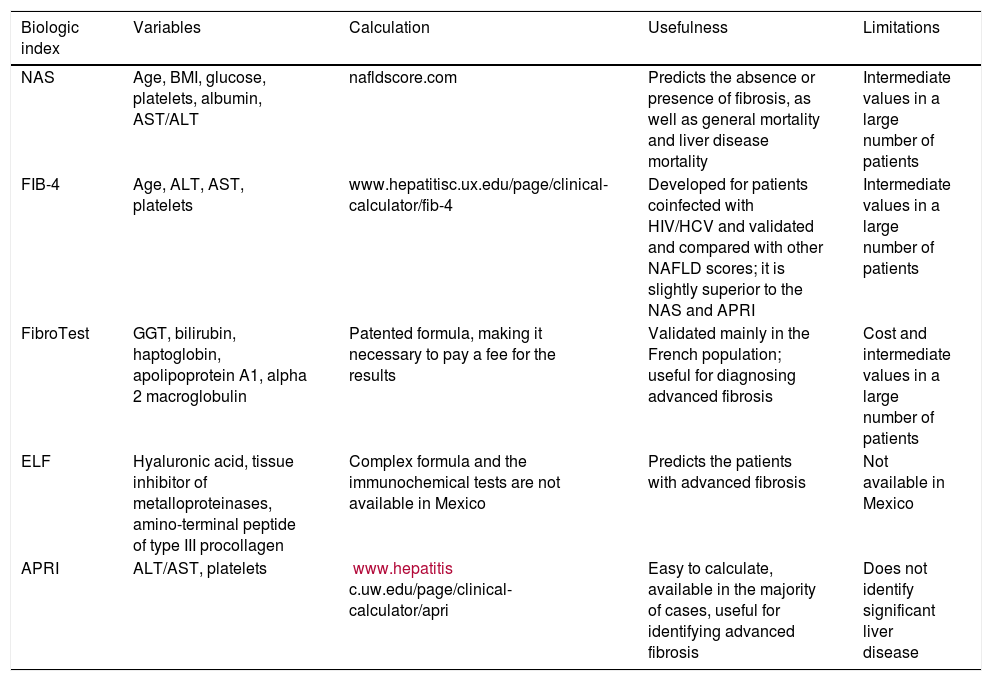
The biologic indexes for establishing liver fibrosis in NASH are determined in serum and have an acceptable diagnostic reliability. Those indexes are useful for establishing the presence of advanced fibrosis or the absence of fibrosis. Intermediate fibrosis grades are difficult to determine through noninvasive techniques, particularly through the serologic indexes.15,181–183 The most widely studied serologic indexes for determining fibrosis in patients with NAFLD are (Table 4):
- 1.
The NAFLD fibrosis score (NFS).184
- 2.
The fibrosis-4 calculator (FIB-4).183
- 3.
Enhanced liver fibrosis (ELF).181
- 4.
FibroTest®.182
- 5.
The AST to platelet ratio index (APRI).185
The most widely studied serologic indexes to determine fibrosis in patients with NAFLD.
| Biologic index | Variables | Calculation | Usefulness | Limitations |
|---|---|---|---|---|
| NAS | Age, BMI, glucose, platelets, albumin, AST/ALT | nafldscore.com | Predicts the absence or presence of fibrosis, as well as general mortality and liver disease mortality | Intermediate values in a large number of patients |
| FIB-4 | Age, ALT, AST, platelets | www.hepatitisc.ux.edu/page/clinical-calculator/fib-4 | Developed for patients coinfected with HIV/HCV and validated and compared with other NAFLD scores; it is slightly superior to the NAS and APRI | Intermediate values in a large number of patients |
| FibroTest | GGT, bilirubin, haptoglobin, apolipoprotein A1, alpha 2 macroglobulin | Patented formula, making it necessary to pay a fee for the results | Validated mainly in the French population; useful for diagnosing advanced fibrosis | Cost and intermediate values in a large number of patients |
| ELF | Hyaluronic acid, tissue inhibitor of metalloproteinases, amino-terminal peptide of type III procollagen | Complex formula and the immunochemical tests are not available in Mexico | Predicts the patients with advanced fibrosis | Not available in Mexico |
| APRI | ALT/AST, platelets | www.hepatitis c.uw.edu/page/clinical-calculator/apri | Easy to calculate, available in the majority of cases, useful for identifying advanced fibrosis | Does not identify significant liver disease |
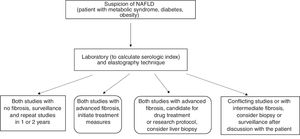
Different authors have suggested the combined performance of serologic tests and elastography studies when first evaluating patients with NAFLD, to establish the absence and/or presence of fibrosis. Concordance between the two studies would eliminate the need for liver biopsy, but it could be considered if there were a discrepancy between the two studies.139.186–189 In patients with no fibrosis or in cases of diagnostic doubt, surveillance with noninvasive tests is suggested for the evaluation of disease progression187,189 (fig. 1).
38. Liver biopsy in patients with NASH should be classified according to the severity of inflammation (mild, moderate, or severe) and the results of the previously validated scoring systems (the NAS and the SAF score).
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 100%.
Liver biopsy is the gold standard for NASH diagnosis. It enables the evaluation of a group of histologic alterations: steatosis, steatohepatitis, fibrosis, and cirrhosis, with and without steatohepatitis. If biopsy is necessary, it should be evaluated according to previously validated scoring systems.
Liver biopsy distinguishes patients with steatohepatitis from those that “only” have steatosis (which includes a spectrum called steatosis with inflammation).190 NASH is histopathologically defined by the presence of steatosis, inflammation (lobular and portal), and damage to hepatocytes (ballooning degeneration). The initial stages of ballooning injury and fibrosis begin in zone 3 (near the central vein/site with less oxygenation). The ballooning degeneration tends to disappear in advanced stages.190
The nonalcoholic fatty liver disease activity score (NAS) is the most widely used histologic scale for NASH. It was designed to evaluate treatment response.97 The steatosis-activity-fibrosis (SAF) scale includes steatosis, ballooning degeneration, lobular inflammation, and fibrosis.191
39. If a patient presents with fibrosis or cirrhosis due to NASH, clinical follow-up with liver ultrasound every 6 months should be carried out for the opportune detection of HCC.
Quality of evidence and strength of recommendation: GRADE B1, weak in favor of the statement.
Level of agreement: in complete agreement, 85.29%; in partial agreement, 14.70%.
HCC is the fifth most prevalent form of cancer and the second leading cause of cancer-related mortality.192 The increase in new cases of HCC is due to the high prevalence of NAFLD and MetS. Patients with fibrosis or cirrhosis due to NAFLD have a higher risk for developing HCC. One-third of the patients with cirrhosis will develop HCC, and follow-up studies have found that approximately 1-8% of the patients with cirrhosis develop HCC per year, which is why liver ultrasound every 6 months is recommended.193 That would enable early stage detection and its consequently opportune treatment of resection, liver transplant, or ablation, resulting in greater survival. Thus, the implementation of said follow-up for early HCC detection in at-risk populations would contribute to a decrease in deaths associated with HCC.
Treatment ICoordinator: Dr. Saraí González Huezo
Participants: Dr. Francisco Sánchez Ávila, LN Sophia Martínez Vázquez, Dr. Jorge Alejandro López Cossio, Dr. Ernesto Márquez Guillén, Dr. Laura Cisneros Garza
40. Weight reduction through diet and exercise is the most effective strategy in NAFLD. Weight loss of at least 7% decreases histologic activity and weight loss of more that 10% reduces fibrosis.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 88.23%; in partial agreement, 11.76%.
Different meta-analyses and randomized clinical trials state that body weight reduction of at least 5% of the initial weight produces changes in the biochemical markers of the disease and of MetS, specifically in relation to insulin sensitivity, transaminases, and lipids.14,194,195 Weight loss> 7% produces histologic changes, particularly in steatosis grade, ballooning, and inflammation, with resulting changes in the NAS.196–198 A 10% reduction in body weight produces remission of steatohepatitis in up to 90% of patients and decreased fibrosis in 45%.199
Different types of diets have been proposed and studied for the treatment of NASH and the common denominator has been found to be caloric reduction. Some studies and clinical practice guidelines recommend a 25% reduction in a person's customary energy intake.200,201 Macronutrient distribution is also important. A carbohydrate-based diet (50-60%) can be beneficial if the patient has signs of insulin resistance or diabetes, and a low-fat diet (20-25%) if the patient presents with dyslipidemia.197,202,203 Avoiding foods high in fructose and those high in trans fats is also recommended.
41. A personalized diet designed by a nutrition professional to aid in weight loss in the treatment of NAFLD is recommended.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 94.11%; in partial agreement, 5.88%.
The amount of energy a person should ingest should be individualized by a nutrition profesional.200 A complete evaluation of nutritional status, preferably of body composition, should be carried out.201,204
The nutrition professional should consider energy expenditure, whether through body composition or through prediction formulas (Table 5), such as those used for treating obesity,205 in which at least 16-17 kcals should be provided per kg of adjusted weight (Table 6). The following dietary nutrient distribution is recommended: 20% proteins, 30% fats, and 50% carbohydrates; more monounsaturated and polyunsaturated fats than saturated fats (10:13:7, respectively); less than 10% of simple carbohydrates, preferably derived from fruit;201 25-35g/day of dietary fiber; and 2.5 to 3 liters of plain water daily.206 Sweetened drinks, soft drinks, and products with a high fructose content should be reduced.14,204 Products with a high content of saturated fats, such as fried foods, breaded foods, and highly processed foods that contain trans fats or saturated fats as ingredients or preservatives, should be eliminated.14,201
Prediction formulas for calculating energy expenditure validated for persons with overweight and obesity.297
| Name of the formula | Formula |
|---|---|
| Mifflin St. Jeor | Male: 10 (weight)+6.25 (heighta) – 5 (age)+5 |
| Female: 10 (weight)+6.25 (heighta) – 5 (age) – 161 | |
| Valencia | Male: |
| 10- 30 year: (13.37 x weight)+747 | |
| 30- 60 years: (13.08 x weight)+693 | |
| >60 years: (14.2 x weight)+429 | |
| Female: | |
| 10- 30 years: (11.02 x weight)+679 | |
| 30- 60 years: (10.92 x weight)+677 | |
| >60 years: (10.98 x weight)+520 | |
| Rapid estimate (Carrasco) | Male: 17 kcals x kg weight |
| Female: 16.2 kcals x kg weight | |
| Livingston | Male: 293 x weight 0.4330 – (age x 5.92) |
| Female: 248 x weight 0.4356 – (age x 5.09) |
42. Exercise reduces body fat and thereby insulin resistance and thus is recommended as an essential part of the treatment of NAFLD.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 97.05%; in partial agreement, 2.94%.
Exercise on its own, independent of diet, favors the decrease in liver fat. Different studies have shown that it promotes the reduction of hepatic triglycerides and visceral adipose tissue.14,201,204 The current recommendation is 150 to 200min of aerobic or anaerobic exercise per week, which is less than that indicated for the treatment of obesity. Said quantity is sufficient for reducing steatosis, but no modifications have been seen in relation to fibrosis. Exercise should be carried out together with other lifestyle interventions. That same amount of exercise, especially the aerobic type, reduces insulin resistance.14,59,201
43.Bariatric surgery improves histopathology in patients with obese morbidity and NAFLD and therefore can be considered in those patients.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 94.11%; in partial agreement, 5.88%.
Bariatric surgery as a therapeutic option in the treatment of NASH was previously thought to be premature, given that prior studies had very small samples and were not prospectively designed with a paired biopsy program, thus hindering its evaluation as a feasible treatment. 207–209
As a result, a prospective study was conducted on 109 patients with morbid obesity and biopsy-documented NASH. Over a 19-year period (1994-2013), patients underwent bariatric surgery and paired biopsies.210 The patients had morbid obesity (BMI> 40kg/m2) or severe obesity (BMI of 35-40kg/m2) and associated comorbidities (high blood pressure or diabetes for a minimum of 5 years that were refractory to medical treatment). They were placed in a nutritional program and then underwent surgical treatment within the time frame of 1994 to 2001. The procedures included biliointestinal bypass or sleeve gastrectomy. Roux-en-Y gastric bypass was begun in 2001. The variables evaluated were: BMI, weight, blood pressure, ALT, GGT, triglycerides, cholesterol, glucose, and insulin. In addition, a liver biopsy was performed at the beginning of the surgery and repeated one year after surgery.
Also evaluated were: steatosis grade; the severity of necroinflammatory activity with the Brunt score211 (mild: grade 1, moderate: grade 2, and severe: grade 3); the NAS,196 which includes steatosis (0-3), lobular inflammation (0-3), and ballooning degeneration (0-2), with a range of 0-8; and fibrosis grade according to the Kleiner scale97 and METAVIR score.212 The biopsy comparison showed that bariatric surgery induced significant improvement in all the NASH components (Table 7).
Changes in baseline clinical, biochemical, and histologic characteristics one year after bariatric surgery.
| Characteristics | Before surgery | 1 year post-surgery | p value |
|---|---|---|---|
| BMI, mean±SD | 49.2±8.2 | 37.4±6.9 | <0.0001 |
| Biochemical characteristics | |||
| HDL cholesterol, mmol/l, mean±SD | 1.08±0.27 | 1.29±0.35 | <0.0001 |
| LDL cholesterol, mmol/l, mean±SD | 2.78±0.98 | 2.67±0.90 | 0.4 |
| Triglycerides mmol/l median (IQR) | 1.63 (1.31-2.54) | 1.24 (0.92-1.73) | <0.0001 |
| Total cholesterol, mmol/l | 4. 81±1.17 | 4.64±1.06 | 0.12 |
| ALT, IU/l, mean±SD | 52±26 | 25±19 | <0.0001 |
| GGT IU/l, median (IQR) | 51 (34-87) | 23 (14-33) | <0.0001 |
| Fasting glucose, mg/dl, median (IQR) | 130 (100-181) | 94 (87-113) | <0.0001 |
| Insulin resistance index, mean±SD | 3.58±0.50 | 2.94±0.47 | <0.0001 |
| Histologic characteristics | |||
| Ballooning, n (%) | <0.0001 | ||
| 0 | 1 (1.2) | 66 (80.5) | |
| 1 | 51 (62.2) | 10 (12.2) | |
| 2 | 30 (30.6) | 6 (7.3) | |
| inflammation, n (%) | <0.0001 | ||
| 0 | 1 (1.2) | 50 (61.0) | |
| 1 | 60 (73.2) | 28 (34.1) | |
| 2 | 20 (24.4) | 3 (3.7) | |
| 3 | 1 (1.2) | 1 (1.2) | |
| Fibrosis (Kleiner), n (%) | <0.0001 | ||
| 0 | 8 (9.9) | 26 (32.1) | |
| 1a | 10 (12.3) | 7 (8.6) | |
| 1b | 6 (7.4) | 4 (4.9) | |
| 1c | 9 (11.1) | 13 (16.1) | |
| 2 | 26 (32.1) | 15 (18.5) | |
| 3 | 19 (23.5) | 14 (17.3) | |
| 4 | 3 (3.7) | 2 (2.5) | |
| Fibrosis (Metavir), n (%) | 0.003 | ||
| 0 | 22 (27.5) | 35 (43.8) | |
| 1 | 32 (40) | 26 (32.5) | |
| 2 | 17 (21.3) | 11 (13.7) | |
| 3 | 6 (7.5) | 6 (7.5) | |
| 4 | 3 (3.7) | 2 (2.5) | |
ALT: alanine aminotransferase; BMI: body mass index; GGT: gamma-glutamyl transpeptidase; HDL: high-density lipoprotein; IQR: interquartile range; LDL: low-density lipoprotein; SD: standard deviation.
Modified from Lassailly et al.210.
NASH disappeared in 85% of the patients (95% CI: 75.8%–92.2%). The mean BMI was significantly reduced, from 49.3±8.2 to 37.4±7 (p <0001), as were the ALT levels (from 52.1±25.7 IU/l to 25.1±20 IU/l, p <0.0001). The mean GGT levels decreased from 51 IU/l to 23 IU/l (p <0.0001), and the mean fasting glucose (130mg/dl, range: 100-181) decreased to 94mg/dl (range: 87-113), with statistical significance (p <0.0001). Finally, insulin resistance also decreased from 3.58±0.50 to 2.94±0.47 (p <0.0001). Patients with mild NASH had the highest improvement percentage, compared with those that had moderate-to-severe NASH (94.2 vs 70%, p=0.007). The NAS improved from a median baseline score of 5 (4-5) to 1 (1-2) (p <0.0001). Steatosis decreased from 60 to 10%, ballooning decreased 84.2% (n=69 patients, 95% CI: 74.4-91.3%), and lobular inflammation decreased 67.1% (n=55 patients, 95% CI: 55.8-71%). Fibrosis grade improvement, evaluated by both the Kleiner scale and the METAVIR score (p <0.0001 and p <0.003, respectively), was observed. According to the METAVIR score, fibrosis decreased 33.8% (95% CI: 23.6-45.2) in all the cases and in 46.6% (95% CI: 33.3-60.1) of the cases with a baseline METAVIR score for fibrosis> 1. Those results were in agreement with the Kleiner score, which showed 46.3% improvement in the total population (95% CI: 35.8-55.8) and 51.4% (95% CI: 39.3-63.4) of the patients with baseline fibrosis> 1. In the sleeve gastrectomy and gastric bypass comparison, persistent NASH was more frequent in the group that underwent gastric sleeve surgery (30.4 vs 7.6%, p=0.015).
The authors of that study concluded that bariatric surgery induced the disappearance of NASH in close to 85% of the patients and improved the clinical, biochemical, and histologic conditions. It should be considered an option in a select group of patients with morbid obesity and NASH that do not respond to lifestyle modifications.
44. There is currently no safe and effective pharmacologic therapy for the management of NASH. The available options are useful in specific contexts.
Quality of evidence and strength of recommendation: GRADE C1, strong in favor of the statement.
Level of agreement: in complete agreement, 82.35%; in partial agreement, 11.76%; uncertain, 2.94%, in partial disagreement, 2.94%.
NASH is a heterogeneous disease with a complex pathophysiology. Said complexity has made it difficult to find a single agent that is both effective and safe for the majority of patients.213 Moreover, it is difficult to enroll subjects (many of whom are asymptomatic) in clinical trials, given that it involves serial liver biopsy and the consequent demonstration of histologic improvement.214,215 In addition, the research protocol designs for each drug differ in the populations evaluated, complicating their generalized applicability. Promising results obtained in initial studies were not confirmed, when later compared with placebo. Even though two therapeutic options (vitamin E and pioglitazone) are recommended in international management guidelines,14,135 histologic benefits have been achieved in only about 50% of patients with medium-term and long-term treatment.216–220 Neither option has been approved by the main regulatory agencies215 and there is concern related to their long-term safety.135 Therefore, the use of the available pharmacologic options should be limited to patients at risk for progression of advanced liver disease and only in very specific scenarios.
45. Pharmacologic treatment is currently reserved for patients with NASH with evidence of fibrosis (F ≥ 2) or in patients that have a higher risk for progression (DM2, MetS, persistently increasing ALT).
Quality of evidence and strength of recommendation: GRADE B1, strong in favor of the statement.
Level of agreement: in complete agreement, 85.29%; in partial agreement, 11.76%; in partial disagreement, 2.94%.
Advances in the pharmacologic management of NASH have been slow. Numerous therapeutic options directed at the different pathophysiologic mechanisms of the disease have been evaluated in an effort to prevent complications, such as cirrhosis and HCC. However, evidence on effective pharmacologic therapy is scarce. At present, no drug is considered standard therapy in NASH. Only certain drugs are useful in specific contexts, such as antidiabetic and lipid-lowering agents in cases of coexistence of those MetS components with NASH.
Fibrosis is the main predictor of morbidity and mortality in NASH. Patients with no inflammation or fibrosis have a good prognosis, in relation to patients with inflammation and a fibrosis grade F ≥ 2. Those patients are at greater risk for progression and can benefit the most from potentially effective drug strategies.
Pharmacologic treatment should be focused on liver disease and the associated metabolic alterations, such as DM2, obesity, and dyslipidemia. Those comorbidities render a greater risk for disease progression. Ideally, the drug should reduce liver inflammation and hepatocellular damage, as well as correct insulin resistance and have antifibrotic effects.214,221,222
46.The prolonged use of pioglitazone in NASH has demonstrated a decrease in inflammatory activity and possibly of fibrosis. The side effects related to that drug should be considered.
Quality of evidence and strength of recommendation: GRADE B2, weak in favor of the statement.
Level of agreement: in complete agreement, 88.23%; in partial agreement, 8.82%; uncertain, 2.94%.
Pioglitazone is used in the treatment of DM2. It belongs to the group of thiazolidinediones and has an insulin-sensitizing effect, as well as improving lipid metabolism.
A proof-of-concept study included 55 patients with NASH and prediabetes or DM2. They were randomly assigned to receive pioglitazone (45mg/day) or placebo for 6 months. There was statistically significant improvement in the pioglitazone group in relation to insulin resistance and a decrease in transaminases. Histologically, there was an association with a decrease in steatosis, ballooning degeneration, and inflammation, as well as reduced necroinflammation (85 vs 38%, p=0.001). There was only a tendency toward fibrosis improvement (p=0.08).218
Another study included 101 patients with NASH and prediabetes or DM2, all on a hypocaloric diet. They were randomized to receive pioglitazone (45mg/day) or placebo for 18 months, followed by an open phase for another 18 months with pioglitazone. The primary outcome measure was the reduction of at least 2 points on the NAS in two histologic categories, with no worsening of fibrosis. Of the patients that received pioglitazone, 58% achieved the primary outcome measure (difference of 41%, 95% CI: 23-59%) and 51% had NASH resolution, as well as improved fibrosis (p=0.039). The adverse effect rate did not differ between groups, although there was greater weight gain with pioglitazone (2.5kg vs placebo).219
Pioglitazone has also been studied in patients with NASH and no diabetes. In a study on 74 nondiabetic patients with NASH, randomly assigned to pioglitazone (30mg/day) or placebo for 12 months, therapy with pioglitazone significantly reduced fibrosis and hepatocellular injury.217 In the PIVENS study that included 247 patients with NASH and no diabetes that were randomized to receive pioglitazone (30mg/day) or vitamin E (800 IU/day) or placebo for 24 months, the primary outcome measure was histologic improvement, defined as a decrease of 2 or more points in the NAS, with improvement by at least 1 point in ballooning, as well as in lobular inflammation or steatosis, and no increase in fibrosis. Nineteen percent of the placebo group reached the primary outcome measure, compared with 34% of the pioglitazone group (p=0.04) and 43% of the vitamin E group (p=0.001 vs placebo). Even though pioglitazone did not reach the primary outcome measure, a significantly higher percentage of NASH resolution was achieved in the patients that received pioglitazone vs those that received placebo (47 vs 21%, p <0.001).220
Pioglitazone is well-tolerated, and the most frequent side effect is weight gain. Its supposed association with bladder cancer is a subject of debate. Results in some populations suggest that pioglitazone increases said risk,223 whereas that risk was not confirmed in a cohort of 193,099 persons with follow-up.224 On the other hand, thiazolidinediones can promote bone loss and are associated with osteoporotic fractures in postmenopausal women.225
47. Vitamin E can be used in patients with NASH that do not have diabetes or cirrhosis. The related side effects should be considered.
Quality of evidence and strength of recommendation: GRADE B2, weak in favor of the statement.
Level of agreement: in complete agreement, 82.35%; in partial agreement, 14.70%; uncertain, 2.94%.
Vitamin E reduces oxidative stress, which is a recognized pathophysiologic mechanism in NASH.226 Several studies have shown improvement in steatosis and inflammation, but others have produced contradictory conclusions, perhaps due to the fact that different doses and presentations have been used. In addition, the diverse methodologies and inclusion criteria used make it difficult to compare results between studies.
The abovementioned PIVENS study220 is the largest that has been conducted on nondiabetic patients with biopsy-confirmed NASH. It showed improvement in steatosis, inflammation, and ballooning at a dose of 800 IU/day vs placebo (43 and 19%) for 96 weeks. No improvement in fibrosis was observed.
The TONIC study216 utilized a dose of 800 IU/day vs placebo in children, with no effect on the aminotransferase level, steatosis, or inflammation, but there was improvement in ballooning.
A meta-analysis relating increased mortality from any cause to a dose of vitamin E> 400 IU220 has sparked concern about its prolonged use. However, that result has not been confirmed by other studies.227 Another randomized controlled trial demonstrated an increase in the risk for prostate cancer in healthy subjects (1.6 per 1,000 person years)228 and hemorrhagic stroke.229 In short, vitamin E use is associated with reduced aminotransferase levels in subjects with NASH, and the effects of its long-term use in the prevention of cirrhosis and survival have yet to be evaluated.
48. Ursodeoxycholic acid, metformin, and omega-3 fatty acids are not recommended for the treatment of NASH.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 85.29%; in partial agreement, 5.88%; uncertain, 5.88%, in partial disagreement, 2.94%.
Ursodeoxycholic acid has been assessed as treatment for NAFLD in different studies.230–234 A pilot study on 40 patients demonstrated a potential benefit, but it has not been confirmed in later prospective, randomized, and controlled studies.
Metformin235–237 reduces blood glucose levels, decreases hepatic gluconeogenesis, and increases glucose uptake in muscle and fatty acid oxidation in adipose tissue. Nevertheless, studies on metformin in patients with NASH have not produced favorable results. A meta-analysis with three randomized placebo-controlled trials showed no improvement in aminotransferases or liver histology in a period of 6-12 months, regardless of the presence of diabetes mellitus.
Omega-3 fatty acids have been studied in animal models and humans.238–244 The initial evidence suggested that they reduced liver fat, but two controlled clinical trials did not demonstrate sufficient evidence for documenting benefit with its use in NAFLD or NASH.
Treatment 2Coordinator: Dr. Ignacio García Juárez
Participants: Dr. Rosalba Moreno Alcantar, Dr. Judith Flores Calderón, Dr. Gonzálo Torres Villalobos, Dr. Aldo Torre Delgadillo
49. Some emerging drugs, such as obeticholic acid, cenicriviroc, elafibranor, liraglutide, and selonsertib, are currently being studied and have shown favorable results in inflammation and fibrosis. They may be a treatment option for selected patients in the future.
Quality of evidence and strength of recommendation: GRADE B1, strong in favor of the statement.
Level of agreement: in complete agreement, 82.35%; in partial agreement, 14.70%; uncertain, 2.94%.
The pathogenesis of NAFLD is complex and has multiple metabolic pathways, which is why it has not yet been possible to establish a standard treatment for the disease.245 At present, the majority of interventions for NAFLD have been focused on controlling the associated metabolic comorbidities.246 One of the main problems is the long amount of time it takes for clinical outcomes to be seen. Therefore, short-term histologic changes have been accepted as an alternative for evaluating therapeutic response.247 Those changes include steatohepatitis resolution, reduced disease activity and/or improved fibrosis stage.14,245,247 Given the above, pharmacologic treatments are recommended to be limited to patients with the progressive liver diseases of steatohepatitis and/or fibrosis.246
Obeticholic acid (OCA) belongs to the group of farnesoid X receptor (FXR) agonists, and is a semisynthetic derivative of chenodeoxycholic acid (a potent FXR activator).14,245,247,248
In the FLINT phase 2 study, OCA at a dose of 25mg/day was compared with placebo for 72 weeks in patients with NASH and no cirrhosis.249 The primary outcome measure was histologic improvement, with no worsening of fibrosis, from baseline measurement to the end of treatment.249 A planned interim analysis showed that OCA was superior to placebo (p=0.0024), and so biopsies were not performed at the end of treatment. The criterion for early ending of treatment was met by 64 patients.247 Histologic improvement was achieved in 45% (n=50/110) of the patients in the OCA group vs 21% (n=23/109) of the patients that received placebo (RR: 1.9, 95% CI: 1.3-2.8, p=0.0002). Pruritus was the most significant adverse effect, presenting in 23% (n=33/141) of the patients with OCA vs 6% (n=9/142) in the patients with placebo.249 Another effect to consider is an increase in LDL and a decrease in HDL, findings that have not yet been associated with clinical significance. Currently 2 studies, REGENERATE (NCT02548351) and REVERSE (NCT03439254) (phase 3) are looking for improved fibrosis in at least one stage.250,251
Elafibranor (GFT-505) is a dual peroxisome proliferator-activated receptor alpha/delta agonist (PPARαδ) that improves insulin sensitivity, glucose homeostasis, and lipid metabolism and reduces inflammation.252 In the GOLDEN-505 study, a total of 276 patients with NASH and no cirrhosis were assigned to 80mg or 120mg of elafibranor or placebo for 52 weeks. Improvement in NASH was looked for, with no worsening of fibrosis. The primary outcome measure was achieved in 23% of the patients in the group receiving 80mg/day, in 21% in the group receiving 120mg/day, and in 17% in the placebo group.247,252 In a later analysis, reversal of NASH was shown to be a significant outcome measure in patients with a baseline NAS of 4 or more in the group receiving 120mg.247,252 In that same group, there was a significant reduction in fibrosis stage and improvement in liver enzymes, lipids, glucose homeostasis, and systemic inflammation markers. A transitory increase in serum creatinine (4.31±1.19μmol/l, p <0.001) was the only adverse effect.252
Cenicriviroc (CVR) is a dual antagonist of C–C chemokine receptor types 2 and 5. In animal models and phase 2 studies on humans, interesting anti-inflammatory and antifibrotic activity has been shown.247,253,254 The CENTAUR study included 289 patients with NASH and no cirrhosis and a NAS> 4. They were given CVR 150mg, a crossover from placebo to CVR, or just placebo. The first year, inflammation improvement with no fibrosis worsening was evaluated and the second year, complete resolution of NASH with no fibrosis worsening was assessed.253 The final results showed improvement in fibrosis but did not demonstrate changes in steatosis or ballooning degeneration.247
Antidiabetics, such as liraglutide (LG), are drugs that act on the glucagon-like peptide-1 receptor agonist (GLP-1RAs).247 That drug has been studied in diabetic and nondiabetic patients and compared with interventions, such as exercise. Initial results showed a satisfactory effect on patients with NASH, with good ranges of safety and tolerance.254–256 In the Lira-NAFLD study,255 80 patients with poorly-controlled diabetes were treated with a dose of 1.2mg/day for 6 months. In the analysis of the 68 patients that completed the study, there was a decrease in glycated hemoglobin (from 9.8 to 7.3%), a decrease in body weight (from 99.5kg to 95.9kg), BMI, visceral fat, and serologic markers. There was a reduction from 17.3%±10.9 to 11.9%±9.3 (p <0.0001) in liver fat.
Upcoming results are expected from the CGH-LiNASH (NCT02654665), comparing LG vs bariatric surgery.257
Selonsertib (SEL) is a kinase-1 inhibitor that signals apoptosis (ASK-1), which is key to the activation of stress-associated inflammation.247,258 In murine models, ASK-1 inhibition improved metabolic parameters and fibrosis associated with NASH.258 The use of SEL, with and without simtuzumab (the humanized monoclonal antibody that acts against the molecule lysyl oxidase-like molecule 2 [LOXL2]) was evaluated in humans. A dose of 18mg of SEL for 24 weeks produced a reduction of 1 or more stages of fibrosis in 43% of the patients (n=13/30) and in 30% of the patients that received the 6mg dose (n=8/27).258 Improvement was evaluated through biopsy, magnetic resonance, and serologic markers for apoptosis.
50. Silymarin, silybin-phosphatidylcholine, pirfenidone, ademetionine, and probiotics, among others, are medications that have shown encouraging results in preliminary studies, but at present there is no solid evidence of their clinical usefulness. Controlled clinical trials demonstrating histologic improvement are needed to support their recommendation.
Quality of evidence and strength of recommendation: GRADE B2, weak in favor of the statement.
Level of agreement: in complete agreement, 79.41%; in partial agreement, 14.70%; uncertain, 5.88%.
Silymarin (SIL) is a flavonoid extract from the seeds of the milk thistle that belongs to the family Asteraceae/Compositae. It consists of four isomers (silybin, isosilybin, silydianin, and silychristin) and antioxidant, anti-inflammatory, and antifibrotic effects are attributed to it.259 It has been assessed as monotherapy and associated with other supplements. 259–260
SIL was evaluated in a multicenter study at a dose of 700mg for 48 weeks of treatment and compared with a placebo (n=99 patients). There was no improvement in the NAS in the placebo group, but biopsy revealed improvement in the grade of fibrosis in the SIL group (p=0.023).261 Studies with a larger number of patients are required. In a meta-analysis that included 8 controlled clinical trials (n=587), SIL produced a change in transaminase values, but no changes in fibrosis or mortality were reported.262
Oxidative stress induced by free fatty acids and insulin resistance are essential for the production and perpetuation of damage in NAFLD.263 Thus, antioxidant therapies are a reasonable preventive and therapeutic option.263–265 The antioxidant effect of the silybin complex, phosphatidylcholine, and alpha-lipoic acid, given their better bioavailability, improved the ratio of reduced glutathione to oxidized glutathione, favored mitochondrial function, and prevented free radical formation and the activation of apoptotic and fibrogenic mechanisms.264,266,267 Those results were shown in in vitro studies,263 in experimental studies,263–265 and in clinical trials,264,265,268,269 suggesting that the complex can improve the histology, fibrosis markers (TGF-b and MMP-2), and metabolic profile,266,267,269 especially in patients with DM2 and carbohydrate intolerance. We believe more clinical trials that confirm those results are required.270,271
Pirfenidone (PFD) has been used in the treatment of pulmonary fibrosis,272 but its mechanism of action is not clear. There are some reports on its use in patients that have hepatitis C virus (HCV), with a decrease in steatosis in 61% of the patients and decreased levels of IL-6, TGF- β1, and TNF-α. Again, more studies confirming those findings are required.273
S-adenosyl methionine is a methyl donor and is synthesized from methionine and ATP in a reaction catalyzed by methionine adenosyltransferase (MAT).274 Elevated doses in murine models have not produced the expected benefits.274,275
Probiotics are live nonpathogenic microorganisms that have been used in certain diseases. They can improve the gut microbiota involved in the pathogenesis of insulin resistance and NAFLD.276,277 Among the mechanisms proposed are TNF-α inhibition and adiponectin enhancement, possibly resulting in the regulation of blood glucose and lipid metabolism, modulating the microbiota, intestinal permeability, and inflammatory response.278,279 In a meta-analysis of 7 studies with treatments lasting 2 to 7 months, there was a decrease in BMI, ALT, AST, HOMA-IR, and in the grade of steatosis, as identified through US. However, there was great heterogeneity between the studies.278 Probiotics have advantages that must be recognized: low cost, widespread availability, and the absence of serious adverse effects.278
51. Cirrhosis of the liver associated with NAFLD is one of the main indications for liver transplantation worldwide.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 94.11%; in partial agreement, 2.94%; uncertain, 2.94%.
During the 1980s and 1990s, NAFLD was considered an infrequent cause of orthotopic liver transplantation (OLT).280 Nevertheless, in recent years it has become the most common cause of HCC and the second most common for OLT.14,247,281 From 2000 to 2014, the number of patients on the waiting list due to NAFLD increased 410%.282 By the year 2030, cirrhosis is estimated to increase by 168% and the incidence of HCC by 137%.280,283
In 2013, NASH became the second most frequent cause of OLT in the United States (surpassing alcohol). Of 63,061 adults with OLT, 18.25% (n=8,266) of the cases were associated with fatty liver.284 Annual incidence has been estimated at 14%.285 With the new treatment for HCV and the high cure rates, it is considered that OLTs due to that etiology will be decreasing,280,283 making NAFLD the main indication for OLT within a few short years.
Patients with NAFLD-related OLT tend to be older, have a higher BMI, and have more comorbidities, such as DM2 and high blood pressure,280 increasing the wait-list mortality rate and making a more careful selection of cases necessary.286
52. Risk factors for disease recurrence orde novoNAFLD in liver transplant recipients are similar to those in effect before transplantation.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 91.17%; in partial agreement, 5.88%; uncertain, 2.94%.
The existing pre-transplantation risk factors for the recurrence of NAFLD include obesity (in up to 30% of the candidates for OLT), age, high blood pressure (prevalence of 10-15%), DM2, kidney disease, and peripheral arteriopathy. Upon analyzing the factors associated with disease recurrence in the patients after transplantation, they were found to be exactly the same as the pre-transplantation risk factors283 (fig. 2).
Other factors described in the recurrence of NAFLD are the presence of the PNPLA3 c44G allele (in the donor [genotype G], OR: 1.62)287 and immunosuppressants, such as the calcineurin inhibitors that lead to major metabolic complications.283,288 Steroids are associated with greater insulin resistance, obesity, hyperlipidemia, and high blood pressure and the mammalian target of rapamycin (mTOR) inhibitors increase insulin resistance and hypertriglyceridemia.283
Cases of de novo NASH with progression from NAFLD to NASH are rare. They are produced in 5 to 8% of the patients and are more frequently associated with MetS.284
53. The recurrence of NAFLD after liver transplantation increases the risk for developing fibrosis and cirrhosis at 5 years.
Quality of evidence and strength of recommendation: GRADE B1, strong in favor of the statement.
Level of agreement: in complete agreement, 97.05%; in partial agreement, 2.94%.
Outcomes after transplantation due to NAFLD are generally good at 1, 3, and 5 years, with survival rates of 88, 82, and 77%, respectively, and are similar to those for other etiologies.283 Recurrence of NAFLD at 5 years is not associated with mortality or graft loss.289 Steatosis above grade 2 (34-66% established by biopsy) is seen in up to 60% of recipients the second year after transplant. Of those cases, 20 to 50% progress to inflammation, a higher percentage than that in patients with other non-NAFLD indications for transplant.290 NASH with progressive fibrosis and the formation of bridging septal fibrosis or cirrhosis (METAVIR≥2) occurs in 5% of recipients at post-transplantation year 5.291,292 A greater incidence of advanced fibrosis (>27%) was reported in a recent study, albeit the sample size was small, with bias selection.290 Given the increase in prevalence, re-transplantation associated with NASH-related cirrhosis is a possibility, but experience is limited.289 One-year and 5-year survival for re-OLT due to NASH is 65 and 52%, respectively.
54. Cardiovascular diseases are the main cause of death in patients with transplantation due to NAFLD.
Quality of evidence and strength of recommendation: GRADE A1, strong in favor of the statement.
Level of agreement: in complete agreement, 100%.
In general, cardiovascular complications are the cause of morbidity and mortality in liver transplantation, not graft complications.
Metabolic complications, such as MetS (50%), high blood pressure (60–70%), and hyperlipidemia (50-70%), can occur after transplant.280,283,288 An increase in BMI from 24.8kg/m2 to 28.1kg/m2 within a period of two years has been reported in different case series.283 Therefore, patients with NASH are at greater risk for developing post-transplant DM2. Immunosuppression contributes to the development of those metabolic alterations. In addition, the age of the recipient is a risk factor for atherosclerosis.283 Said metabolic alterations are an important factor for the development of cardiovascular complications. Numerous studies show greater morbidity and mortality 5 years after transplantation, ranging from 13.5 to 32%, depending on the case series analyzed.293–295
Financial disclosureLogistic support for the face-to-face vote and financial support for the present Consensus were provided by the Laboratorio MEDIX.
Conflict of interestDr. René Male Velázquez: speaker and advisory board member of Abbvie, MSD, and speaker for Gileard, consultant for the Bayer, Bristol, Falk, and Roche laboratories.
Dr. Ramón I. Carmona Sánchez: advisory board member of Asofarma, speaker for Mayoly-Spindler, Asofarma, and Chinoin.
Dr. María Saraí González Huezo: speaker for Bayer, Janssen, Abbvie, Roche, Falk, Asofarma, Ferring, and Gileard.
Dr. Juan Francisco Sánchez Ávila: has participated in research for Abbvie Farmacéuticos, Gileard Sciences, and Galmed Pharmaceuticals and is a speaker for Bayer.
Dr. Laura Cisneros Garza: is a consultant for Bayer, Abbvie, Roche, Bristol, and Falk.
The rest of the participants declare they have no conflict of interest.
Please cite this article as: Bernal-Reyes R, Castro-Narro G, Malé-Velázquez R, Carmona-Sánchez R, González-Huezo MS, García-Juárez I, et al. Consenso mexicano de la enfermedad por hígado graso no alcohólico. Revista de Gastroenterología de México. 2019;84:69–99.