Chronic diarrhea is defined by symptoms lasting longer than 4 weeks. It is a common problem that affects up to 5% of the adult population. Different pathophysiologic mechanisms involve numerous causes, including drug side effects, postoperative anatomic and physiologic alterations, intestinal and colonic wall abnormalities, inflammatory or malabsorption causes, pancreatobiliary diseases, and functional or gut-brain axis disorders associated with dysbiosis or gastrointestinal motility alterations. Due to such a broad differential diagnosis, it is important to categorize chronic diarrhea into five main groups: drug side effect, postoperative, postinfectious, malabsorptive, inflammatory, and functional. The present review is a narrative analysis of the diagnostic approach, emphasizing key aspects of the clinical history, the utility of biomarkers (in breath, stool, urine, and serology) and malabsorption and motility tests, the role of radiologic and endoscopic studies, and the most common histologic findings. A diagnostic algorithm aimed at determining etiology and personalizing therapy is also proposed.
Diarrea crónica es aquella que ocurre por un período mayor de 4 semanas, y es un problema común que afecta hasta 5% de la población adulta. Existen diferentes mecanismos fisiopatológicos que involucran múltiples causas y que incluyen efecto secundario de medicamentos, alteraciones en la anatomía y fisiología posquirúrgicas, trastornos de la pared intestinal y colónica, inflamatorios o malabsortivos, enfermedades pancreatobiliares, y trastornos funcionales y del eje cerebro-intestinal asociados a disbiosis o a alteraciones de la motilidad gastrointestinal. Debido al diagnóstico diferencial tan amplio es importante categorizarla en cinco grupos principales: efecto secundario de medicamentos o cirugías, postinfecciosa, malabsortiva, inflamatoria y funcional. La presente revisión analiza en forma narrativa el abordaje diagnóstico, enfatizando claves en la historia clínica, la utilidad de biomarcadores (aliento, fecales, urinarios, serológicos), así como pruebas de malabsorción, motilidad, y el papel de los estudios radiológicos, endoscópicos, así como los hallazgos histológicos más comunes, y se propone un algoritmo diagnóstico encaminado a determinar la etiología y personalizar la terapia.
Chronic diarrhea is defined as reduced stool consistency or an increase in the number of bowel movements, for a period longer than 4 weeks. It has a prevalence of 3–5% in the adult population and is one of the entities with the broadest differential diagnosis, given that it can be secondary to such heterogeneous causes as drug side effects, structural abnormalities of the small bowel and/or colon, the result of previous gastrointestinal surgery, inflammatory or neoplastic pancreatobiliary disease, or as part of an intestinal functional disorder, such as functional diarrhea, or irritable bowel syndrome (IBS) that is diarrhea predominant (IBS-D) or postinfectious (PI-IBS).1–9
In 2010, the Asociación Mexicana de Gastroenterologia published the clinical guidelines for the management and diagnosis of chronic diarrhea in Mexico,10 but since then, new clinical entities have been described and new diagnostic tests have been developed for the opportune approach to and diagnosis of chronic diarrhea. The aim of the present narrative review was to provide an update of concepts related to the diagnosis of chronic diarrhea.
Materials and methodsThe authors of the present review evaluated and analyzed articles published in the national and international literature on the diagnosis and treatment of chronic diarrhea. A cross-sectional search was carried out in PubMed and IMBIOMED (encompassing the publication time span from January 2011 to December 2020), utilizing the following terms (in English and Spanish): diarrhea, chronic diarrhea, intestinal malabsorption, bacterial overgrowth, celiac disease, enteritis, bile salt malabsorption, inflammatory bowel disease, colitis, diarrhea predominant and postinfectious irritable bowel syndrome, infections, drug side effects, biomarkers, diagnostic tests, radiology, endoscopy, colonoscopy, enteroscopy, and histologic findings. The most relevant articles were identified and information from technical reviews, systematic reviews, meta-analyses, and clinical guidelines on chronic diarrhea were taken into account. Relevant works conducted before the search date were also included. The bibliography was organized, and the review was carried out considering the following sections: pathophysiology, approach and specific clinical entities, diagnostic tests, and algorithms.
Of the 337 articles resulting from the search, information from 106 of the articles was included, given that clinical cases, clinical images, articles on the pediatric population, repeated articles, or articles that could not be accessed because they were written in languages other than English or Spanish, were excluded.
PathophysiologyMultiple pathophysiologic mechanisms keep the balance between secretion, absorption, and luminal content, to prevent diarrhea. The quantity of liquid in stool is determined by its content of solutes, in such a way that the intestinal barrier, permeability mechanisms, and electrolyte transporting pumps enable a balance between extraluminal and intraluminal liquid to be maintained. According to the pathophysiologic mechanism involved, chronic diarrhea can be divided into: osmotic, secretory, inflammatory, malabsorptive, and secondary to dysmotility.1,7 Osmotic diarrhea is typically watery, abundant, and ceases after the patient fasts. In secretory diarrhea, fecal osmolality is entirely due to electrolytes, and in osmotic diarrhea there is a difference between fecal electrolytes and the measured osmolality, called the osmotic gap, caused by poorly absorbed molecules.3 The chronic use of laxatives or antacids, as well as the malabsorption of carbohydrates, such as lactose, fructose, or sorbitol, fall into the category of osmotic diarrhea. Among the secretory causes are microscopic colitis, whose primary mechanism is an inflammatory component,11 non-osmotic laxatives, diabetic enteropathy, and diarrhea associated with hormone-secreting neuroendocrine tumors (vasoactive intestinal peptide, somatostatin and/or gastrin) or vasoactive substances (carcinoid tumors). Malabsorptive diarrhea includes disorders affecting the surface of the small bowel (enteropathies); small intestinal bacterial overgrowth (SIBO); infections, such as giardiasis; or some of the processes involved in the digestion of nutrients (bile acid malabsorption, exocrine pancreatic insufficiency). Inflammatory diarrhea that involves structural damage to the mucosa of the colon is called inflammatory bowel disease (IBD). IBD includes ulcerative colitis (UC), Crohn’s disease, indeterminate colitis, microscopic colitis, Clostridioides difficile (C. difficile) infection, some forms of SIBO that colonize in the colon, ischemic colitis, and certain malignancies (villous adenomas, colorectal cancer [CRC] with extensive involvement, severe tumor activity, or that associated with post-obstructive diarrhea).1–5 Diarrhea associated with food can occur due to enzyme deficiency (e.g., disaccharidase deficiency) or be mediated by immune mechanisms (e.g., cow’s milk protein allergy and celiac disease due to gluten intolerance). In some cases of chronic diarrhea, multiple mechanisms are most likely involved that include autocrine, luminal, paracrine, immune, neural, and endocrine alterations that affect vascular, paracellular, muscular, and epithelial function, finally resulting in alterations in permeability, ion transport, the microbiota, and motility.3,7
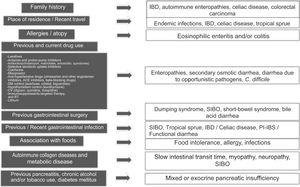
Clinical approach and specific clinical entitiesA detailed clinical history is a key aspect in evaluating chronic diarrhea, given that some past medical events, or their combination, can lead to one or more probable causes (Fig. 1).1–3 First of all, it is very important to understand what the patient defines as his/her normal bowel habit. It can sometimes be characterized as an increased number of bowel movements, but with normal consistency, called pseudodiarrhea, and should not be considered part of the diarrhea spectrum.1,4,7,12 The patient often defines diarrhea as loose stools, increased frequency of stools with decreased consistency, bowel movement urgency, or at times, tenesmus. The physical characteristics of large stool volume, lientery, steatorrhea, creatorrhea, or floating stools indicate malabsorption or a small bowel or pancreatobiliary problem. Small-volume watery diarrhea, with no food remains, is characteristic of disorders of the colon. Temporality with foods can indicate malabsorption, when it is associated with bloating, meteorism, borborygmi, or flatulence, whereas postprandial urgency suggests fast bowel transit time. As a general rule, diarrhea that is structural in origin is both diurnal and nocturnal and diarrhea associated with dysmotility and functional diarrhea, occurs only during the day, especially in the postprandial period. If there is a family history of chronic diarrhea, disorders with a genetic association, such as autoimmune enteropathies, including celiac disease, IBD, or CRC, must be considered. The place of residence or recent travel imply risk for certain endemic infections that could eventually be associated with SIBO. Certain groups of Nordic descent have a higher risk for celiac disease. Tropical sprue is a consideration in subjects born and living in areas at sea level. If there is an important history of atopy (e.g., allergic rhinitis, asthma) or a history of food allergies, gastrointestinal disorders associated with eosinophilic infiltrations, such as enteritis or eosinophilic colitis, are a strong possibility. Reviewing the list of previous and current medications is vitally important, given that some drugs, alone or in combination, can increase the risk for diarrhea. A thorough review must be carried out regarding the previous, recent, or intermittent use of laxatives, antacids, proton pump inhibitors (PPIs), antibiotics (particularly clindamycin, macrolides, amoxicillin, and quinolones), colchicine, antihypertensive drugs (particularly olmesartan and other angiotensin II-receptor blockers, angiotensin-converting enzyme inhibitors, and beta-blockers), drugs used in heart disease (digoxin, quinidine, ticlopidine), drugs taken for controlling overweight, obesity, and diabetes mellitus, lithium use, and oncologic therapy, including immunosuppressants, targeted therapy, and immunotherapy, as well as previous radiotherapy. If chronic diarrhea began as an apparently acute infectious episode that marked a change in bowel habit, SIBO should be considered the first option, and the development of an IBD, enteropathy secondary to a cross-reactive immune system response, functional diarrhea, or PI-IBS should also be considered. When the patient identifies an association between episodes of diarrhea and food consumption, several factors must be taken into account: (a) substances that in sufficient quantities cause diarrhea in the normal intestine (e.g., fructose), (b) foods that cause diarrhea due to a previous condition (dairy products in lactase deficiency), (c) intestinal or hepatobiliary alterations that limit digestion or absorption (cholecystectomy, short intestine, pancreatic insufficiency), (d) idiosyncratic intolerances or allergies (less common in adults: 1–2%), or (d) an infectious component. Some systemic disorders can cause diarrhea as part of their clinical presentation, including thyroiditis and hyperthyroidism, uncontrolled diabetes, rheumatologic diseases, such as systemic sclerosis, and some neuroendocrine tumors that produce vasoactive substances, such as histamine or serotonin (e.g., carcinoids), prosecretory tumors that produce vasoactive intestinal peptide or somatostatin (e.g., VIPomas and somatostatinomas), or substances, such as gastrin, that increase acid secretion and present as difficult-to-control peptic acid disease and diarrhea due to interference in the processes of fat digestion (e.g., gastrinoma). Finally, when there is a history of extensive or recurrent acute pancreatitis, diabetes, or chronic alcoholism or smoking, following an episode of pancreatitis, exocrine pancreatic insufficiency is a strong possibility1–4 (Fig. 1).
Key aspects in the clinical history for determining the cause of chronic diarrhea.
ACE: angiotensin-converting enzyme; AHT: antihypertensive; CV: cardiovascular; DM: diabetes mellitus; IBD: inflammatory bowel disease; CRC: colorectal cancer; RT: radiotherapy; SIBO: small intestinal bacterial overgrowth; PI-IBS: postinfectious irritable bowel syndrome; PPI: proton pump inhibitor.
Over 700 drugs have been implicated as causes of diarrhea, and of all the side effects associated with pharmacotherapy, diarrhea accounts for 7%.13
- 1
Antibiotics: Of all the drug-related causes of diarrhea, antibiotics stand out in first place. By inducing dysbiosis, they can increase the risk for C. difficile infection (CDI). A recent systematic review with a meta-analysis concluded that antibiotics, as a group, have an odds ratio between 8 and 10 for causing CDI, particularly clindamycin (OR 46.95), meaning that 1 of every 10 users develops it. Other parenteral and oral antibiotics also have an elevated association: aztreonam OR 29.95, amoxicillin OR 20.05, carbapenem OR 19.16, cephalosporines OR 15.33, tetracyclines OR 7.54, macrolides OR 5.8, and even quinolones, with an OR of 4.94.14
- 2
Drugs associated with microscopic colitis: Several drug groups increase the risk for lymphocytic microscopic colitis and collagenous microscopic colitis. One study reviewed the association of microscopic colitis with the previous and current use of three types of drugs: nonsteroidal anti-inflammatory drugs (NSAIDs), PPIs, and antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs). Those authors reported an increased risk for microscopic colitis in the 3 groups: NSAIDs OR 1.86, PPIs OR 3.37, and SSRIs OR 2.03. Recent use of NSAIDs or PPIs was also associated with a higher risk (OR 2.09 and 4.00, respectively), as was prolonged use (PPI > 12 months OR 4.69, NSAID > 4 months OR 4.86). Interestingly, the association persisted in the two microscopic colitis subtypes: current use of NSAIDs and PPIs increased the risk for collagenous colitis (OR 2.32 and 5.35, respectively) and recent use of PPIs or SSRIs increased the risk for lymphocytic colitis (PPI OR 2.06, SSRI 2.28).15
- 3
Angiotensin inhibitors (AIs): AI-associated enteropathy is a recently described condition. That group of medications is employed to control high blood pressure and can induce intestinal changes resembling those of celiac disease, with a similar clinical presentation.16 The highest association has been described with olmesartan (94% of cases), but other AIs are occasionally associated, as well (telmisartan 2%, irbesartan 1.6%, valsartan 1.2%, losartan 0.8%, and eprosartan 0.4%).17
- 4
Antineoplastic agents: A new group of drugs used to treat different forms of cancer through immunotherapy are called checkpoint inhibitors. Those agents are monoclonal antibodies that inhibit T cells by blocking cytotoxic T lymphocyte antigen-4 (CTLA-4). Two antibodies, pembrolizumab and nivolumab, regulate T cell activation, blocking programmed cell death protein 1 (PD-1). The result is important antitumor activity that has been associated with tumor regression and prolonged patient survival in different tumors, such as melanoma, non-small-cell lung cancer, mesothelioma, glioblastoma, and kidney cancer. The main secondary effect is excessive immune activation, which can affect different organs, including the digestive tract and the hepatopancreatobiliary apparatus. Severe forms of toxicity in the intestine and colon have been described that are similar to those that occur in IBD. They can manifest as erosions, ulcerations, severe atrophy, and apoptosis, with a clinical variability ranging from mild-to-moderate diarrhea to refractory diarrhea, megacolon, and necrotizing enterocolitis.18–21
- 5
Drugs used for weight control and glycemic control: Various drugs utilized to control diabetes mellitus and/or overweight and obesity can cause diarrhea through different mechanisms: acarbose is an alpha-glucosidase inhibitor that causes carbohydrate malabsorption and is associated with meteorism and diarrhea; orlistat is a lipoprotein lipase inhibitor that reduces fat absorption and causes steatorrhea; metformin is a gluconeogenesis inhibitor that improves insulin sensitivity but can frequently cause dysbiosis and gastrointestinal symptoms, such as meteorism, diarrhea, and abdominal pain.22
Different surgical procedures can temporarily or permanently alter the gastrointestinal anatomy or physiology, causing chronic diarrhea. Truncal vagotomy can result in accelerated gastric emptying (also known as dumping syndrome), particularly when accompanied by antrectomy.23 A small subgroup of patients that undergo fundoplication can experience persistent diarrhea (15%) or postprandial bloating.24 Extensive intestinal resection or resection that includes key absorption areas, such as the terminal ileum or the ileocecal valve, can trigger malabsorptive diarrhea, particularly when the remnant segment is abnormal or short. Ileal resection and cholecystectomy can be associated with bile acid diarrhea (BAD).25 Due to their intrinsic characteristics, bariatric surgery procedures that include diversions (Roux-en-Y gastric bypass, duodenoileal bypass, and biliopancreatic diversion) cause accelerated transit time, malabsorption, and osmotic diarrhea.26 Various procedures can increase the risk for SIBO, through different mechanisms: reduced gastric acidity (vagotomy), stasis (anastomotic stricture, adhesions), blind loop (lateral enteroanastomosis), or ileocecal valve resection (reflux of the content of the colon into the ileum).27
EnteropathiesDiseases that affect the intestinal wall are called enteropathies and can be associated with chronic diarrhea, which clinically presents in that group of patients as malabsorption syndrome, characterized by explosive diarrhea with steatorrhea, creatorrhea, lientery, floating stools, and in some cases, by anemia, abdominal pain, and weight loss. There are different categories, depending on the cause, and can be divided into:
- 1
Autoimmune causes: They include celiac disease, Crohn’s disease, and other autoimmune enteropathies that affect one or several areas of the intestine.
- 2
Drugs: Of the antihypertensives, AIs, especially olmesartan, have been associated with the development of atrophic enteropathy, similar to celiac disease; NSAIDs can cause ulcerous enteritis, particularly in the duodenum and terminal ileum; immunosuppressants, such as azathioprine, methotrexate, and mycophenolate mofetil, as well as the checkpoint inhibitors, such as nivolumab, can cause different grades of enteric mucosal damage.
- 3
Radiotherapy (RT): Up to 20% of the patients exposed to RT can develop intestinal damage; it typically occurs between 1 and 6 years, post-exposure, and is dose-dependent, usually presenting when the dose exceeds 5000 cGy (centi-Gray).
- 4
Infectious causes: They include tropical sprue, SIBO, giardiasis, Whipple’s disease, human immunodeficiency virus infection and associated opportunistic germs, tuberculosis, post-viral enteropathies, and lymphocytic enteritis associated with Helicobacter pylori infection.
- 5
Infiltrative and neoplastic disorders: They include eosinophilic enteritis, collagenous sprue, amyloidosis, T cell or B cell lymphoma associated with enteropathies, lymphoproliferative intestinal lymphoma, and some vasoactive substance-producing neuroendocrine tumors, especially gastrinomas, VIPomas, and intestinal carcinoid tumors.
- 6
Miscellaneous causes: They include conditions as diverse as peptic duodenitis, food allergies, malnutrition, lymphangiectasis, common variable immunodeficiency, or idiopathic sprue, which can also cause malabsorption syndrome.27–32
Diarrhea originating from the colon presents with symptoms that are predominantly postprandial, preceded by cramping or urgency. Stools are liquid but small in volume and can be watery or contain blood and mucus if the cause is inflammatory. From the pathophysiologic perspective, colonopathies can be secondary to an inflammatory process of the wall, as in IBD or infectious diarrhea, or low-grade inflammation that affects the mechanisms of mucus production and fluid absorption, as in microscopic colitis, or BAD. As with enteropathies, colonopathies are divided according to cause, and the classification is similar:
- 1)
Autoimmune causes: UC, Crohn’s disease, microscopic colitis (lymphocytic or collagenous).
- 2)
Drugs: Antibiotics, NSAIDs, immunosuppressants, radiotherapy (acute or chronic, usually when exceeding 6000 cGy), and immunotherapy, particularly checkpoint inhibitors.
- 3)
Chronic infections. C. difficile, Campylobacter jejuni (C. jejuni), Yersinia enterocolitica, viruses (cytomegalovirus, herpes simplex virus), non-opportunistic parasites (Entamoeba histolytica, Cyclospora cayetanensis), and opportunistic parasites (Cryptosporidium, Isospora belli).
- 4)
Infiltrating and neoplastic disorders: Eosinophilic colitis, amyloidosis, neuroendocrine tumors, villous adenomas, and CRC (post-obstructive diarrhea or diarrhea associated with tumor-releasing factors).
- 5)
Previous colon surgery: Diversion colitis, sigmoidectomy, total and subtotal colectomy, and proctocolectomy.
- 6)
Motility and functional disorders: Functional diarrhea, IBS-D, and PI-IBS.3–12,30,33–36
Given the broad differential diagnosis and pathophysiology of chronic diarrhea, there are numerous diagnostic tests directed at the site of origin and pathophysiologic mechanism(s). There are also numerous biomarkers measurable in breath, stool, urine, and serology testing, as well as malabsorption tests, motility tests, and radiologic, endoscopic, and histologic findings, all of which guide us, and in the majority of cases, lead to the diagnosis of the cause of chronic diarrhea (Table 1).
Utility of diagnostic tests in chronic diarrhea.
| Type of test or biomarker | Examples | Diagnostic utility | Sensitivity (S) |
|---|---|---|---|
| Specificity (Sp) | |||
| Breath | Glucose breath test | SIBO | S 20−93, Sp 30−86 |
| Lactulose breath test | SIBO | S 31−68, Sp 44−100 | |
| Stool | Calprotectin | IBD screening | S 81−92, Sp 82−87 |
| Lactoferrin | IBD screening | S 79−88, Sp 79−93 | |
| Urine | Lactulose/mannitol ratio | Intestinal permeability | S 61, Sp 90.3 |
| Serology | High-sensitivity C-reactive protein | Tissue inflammation | S 90, Sp 78 |
| Tissue transglutaminase-IgA antibodies | Celiac disease screening | S 95, Sp 97 | |
| HLA DQ2, DQ8 | Genetic risk for celiac disease | S 98, Sp 75 | |
| Anti-CdtB and anti-vinculin antibodies | Postinfectious IBS | S 52, Sp 93 | |
| Perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCAs) | UC vs Crohn’s disease in indeterminate colitis | S 44, Sp 86 | |
| Anti-Saccharomyces cerevisiae antibodies (ASCAs) | Crohn’s disease vs UC in indeterminate colitis | S 16, Sp 97 | |
| Malabsorption | Serum carotenoids | Malabsorption syndrome | S 78, Sp 82 |
| Fecal fat | Malabsorption syndrome | S 76−94, Sp 95−99 | |
| Fecal elastase-1 | Exocrine pancreatic insufficiency | S 97, Sp 84 | |
| SeHCAT | Diarrhea due to bile acids | S 100, Sp 94 | |
| Motility | Wireless motility capsule | Slow or fast transit time | N/A |
| Radiologic | Intestinal transit | Intestinal filling defects | S 20, Sp N/A |
| CTE, MRE | Thickening or stricture | S 23, Sp N/A | |
| Endoscopic | Colonoscopy/ileoscopy | Ulcerations in the colon/ ileum | S 15−31 |
| Endoscopy | Nodular or cobblestone pattern in the duodenum | S 95, Sp 98 | |
| Video capsule endoscopy | Small bowel ulcerations | S 93, Sp 100 | |
| Enteroscopy | Small bowel ulcerations | S 87.5, Sp 90.9 | |
| Histologic | Biopsy ± immunohistochemistry | Definitive diagnosis or microscopic description | N/A |
CTE: computed tomography enterography; IBD: inflammatory bowel disease; MRE: magnetic resonance enterography; N/A: not applicable; SIBO: small intestinal bacterial overgrowth; S: sensitivity, Sp: specificity, UC: ulcerative colitis.
Breath tests consist of determining hydrogen or methane, in parts per million (ppm), in the air that is exhaled for a certain length of time after the administration of a substrate, such as glucose, lactulose, lactose, fructose, or sorbitol. The theoretic basis is that hydrogen production in humans is due to the metabolism of carbohydrates by bacteria, in such a way that the carbohydrates are not absorbed. The bacteria of the colon metabolize the carbohydrates, releasing hydrogen, which diffuses through the intestinal wall and then into the bloodstream, and is excreted by the lungs, enabling its detection in breath, measured in ppm. The tests conducted with glucose and/or lactulose establish whether there is intestinal bacterial fermentation of the substrate, which would suggest SIBO. In the case of other carbohydrates, a positive test signifies intolerance to the carbohydrate ingested. There are different protocols and durations for each test: generally, a test is considered positive if, with respect to the baseline expiration, there is an increase of 10–20 ppm (> 10 ppm of methane, > 20 ppm of hydrogen) 90 min after substrate ingestion for the glucose and/or lactulose test and up to 5 h for the lactose test. Sensitivity (S) and specificity (Sp) depend on the type of substrate and the criterion utilized. For SIBO, at 90 min, the glucose breath test has 20−93% S and 30−86% Sp and the lactulose test has 31−68% S and 44−100% Sp. The main utility of breath tests, in relation to chronic diarrhea, is as screening for SIBO, for predicting the response to rifaximin, or for ruling out intolerance to specific carbohydrates.37–39
Fecal biomarkersDuring an episode of acute or subacute diarrhea, the presence of leukocytes in stool indicates inflammatory diarrhea. The fecal leukocyte test is less useful for chronic diarrhea. The ova and parasite exam and stool culture have low S and Sp and are not recommended.10 The analyses aimed at determining the physical, chemical, and osmotic composition of stool, such as weight at 24 h, determination of the osmotic gap, and the presence of food or fiber could guide the clinician toward the mechanism involved, but they are not clinically useful in chronic diarrhea.4–7 A test has recently emerged that determines the presence of bacterial, viral, or parasitic genetic material in stool, through polymerase chain reaction (PCR), called the FilmArray GI Panel® (BioFire Diagnostics, Salt Lake City, UT, USA). That testing system has greater utility in subacute or persistent acute diarrhea, given that in chronic diarrhea it has 54.8% S and a high false positive rate, especially for different strains of enteropathogenic or enterotoxigenic Escherichia coli. However, in selected cases, the presence of pathogenic germs that cause chronic diarrhea can be determined, such as Giardia, or C. difficile. The test is more useful in cases of the latter, when there is discrepancy between the determination of toxins A/B and fecal glutamate dehydrogenase.40 Three fecal markers are derived from leukocytes: calprotectin, lactoferrin, and polymorphonuclear elastase. The first two are available in some laboratories and are useful as IBD screening in chronic diarrhea. S and Sp depend on the cutoff value utilized and the tests can be reported qualitatively or quantitatively. A cutoff point of 50 mcg/g for fecal calprotectin has 81−92% S and 82−87% Sp, and if the cutoff point is raised to 1000 mcg/g, the odds ratio for IBD diagnosis is 27.8. For lactoferrin, the cutoff value varies from 4.0 to 7.25 mcg/g, and so S varies from 79-88% and Sp from 79-93%, depending on the cutoff value.41–44 A positive result indicates inflammation of the wall of the intestine or colon and the need to perform colonoscopy and ileoscopy. Other markers detected in stool are fecal chymotrypsin and fecal elastase, which are discussed in the malabsorption test section.
Urine biomarkersThe measurement of the lactulose/mannitol ratio in urine is an intestinal permeability (IP) test, through which, similarly to lactoferrin and calprotectin, inflammatory intestinal causes can be distinguished from noninflammatory ones. The test consists of ingesting a 50 ml solution with 10 g of lactulose and 5 g of mannitol, after a nighttime fast. Urine is collected for a period of 6 h and refrigerated at −20 °C. Sugar levels are measured by liquid chromatography and the IP is expressed as the ratio of the percentage of lactulose and mannitol (L/M) excreted in urine. A result equal to or greater than 0.030 is considered abnormal. A recent study compared the performance of IP measured by that method with fecal lactoferrin, and although it was inferior (S 87.8% vs 61%, Sp 93.6% vs 90.3%), IP enabled improved identification of the structural causes that originated in the small bowel. Therefore, in the future, it could be used as a complementary test to fecal biomarkers for inflammation.45 Two other biomarkers that are measurable in urine, the d-xylose test and pancreolauryl test, are discussed in the malabsorption test section.
Serologic biomarkersSeveral biomarkers measurable in serum enable the detection of tissue inflammation or the presence of antibodies, with varying S and Sp, for the detection of different illnesses associated with chronic diarrhea.
- a)
Erythrocyte sedimentation rate (ESR): It is one of the most widely used tests, but the values considered normal vary according to age (above 30 years of age, normal value is one-third of the age or less). Studies comparing ESR values in populations with IBD and functional disorders have found numerous overlaps between groups, thus an elevated value neither distinguishes IBS nor predicts IBD.7,10,44
- b)
C-reactive protein: C-reactive protein and high-sensitivity C-reactive protein are markers with elevated S for detecting tissue inflammation, but low Sp, and so do not distinguish the inflammation site. C-reactive protein has a cutoff point of 5–6 mg/l, 73% S, and 78% Sp, for predicting IBD; and high-sensitivity C-reactive protein, at a cutoff value < 6 mg/l, has >90% S, for detecting tissue inflammation.1,2,7,42–44
- c)
Antibodies for detecting celiac disease: Various tissue biomarkers have been tested for identifying celiac disease as the cause of chronic diarrhea. Anti-gliadin antibodies were the first to be used but they have low S, given that they can be positive in 12% of the healthy population and up to 17% in patients with IBS. Anti-endomysial antibodies have been reported to have high S and Sp, but the determination methods vary among laboratories, and so S and Sp can vary, as well.46,47 The determination of IgG antibodies against deamidated gliadin peptides (DGPs) is a more recent tool, whose positivity depends on active gluten ingestion. Eighty percent of cases become negative after a gluten-free diet implemented for more than 6 months. However, said test is not available at the majority of clinical laboratories.48 Anti-tissue transglutaminase (anti-TTG)-IgA antibody measurement is the best noninvasive test in persons above 2 years of age, with 95% S and 95–97% Sp, and the higher the titer value, the greater the probability of a true positive. Anti-TTG-IgA production requires the secretion of normal IgA levels. However, 2–3% of the healthy population does not produce IgA, which can turn the test into a false negative. The test can also become negative, after a gluten-free diet for weeks or months, as with the DGPs.46–47,49 Finally, the most important genetic risk factor for celiac disease is the presence of the HLA DQ2 and DQ8 haplotypes, which are positive in 98% of the subjects with celiac disease, but also in 25-30% of the healthy population. They can be positive in other non-related diseases, as well, such as hepatopathies (45%), functional digestive disorders (53%), and other gastrointestinal illnesses (46%). A recent review concluded that they have 95% S and a negative predictive value (NPV) of 98%.50 National and international guidelines recommend their use in special situations, such as conflicting results between histologic findings and serology, or in patients on a gluten-free diet for whom there is high suspicion.46,49 Other autoimmune enteropathies and colonopathies frequently present with positive autoantibodies. For example, autoimmune enteritis is associated with the presence of positive anti-erythrocyte or anti-caliciform cell antibodies in 85–90% of cases.51
- d)
Antibodies for the diagnosis of inflammatory bowel disease: Patients with inflammatory diseases of the colon can develop autoantibodies, even years before the onset of symptoms (UC: antineutrophil cytoplasmic antibodies that target proteinase 3 [C-ANCA]; Crohn’s disease: anti-Saccharomyces cerevisiae antibodies [ASCA] and anti-flagellin X). The utility of those antibodies is limited to differentiating between UC and Crohn’s disease when endoscopic and/or histologic findings are insufficient, or in cases of indeterminate colitis. P-ANCAs have 44% S and 86% Sp for UC and ASCAs have 16% S and 97% Sp for Crohn’s disease.52 Finally, even though patients with microscopic colitis can more frequently present with positive antinuclear antibodies, cases have been described with positive anti-gliadin antibodies or ASCAs, none of which are specific or of diagnostic utility.53
- e)
Anti-CdtB and anti-vinculin antibodies: The concept that infectious gastroenteritis can affect gastrointestinal motility has been shown in murine models, in which a bacterial toxin of C. jejuni, called cytolethal distending toxin B (CdtB), causes symptoms similar to those of IBS. Anti-CdtB antibodies were also shown to react against vinculin, a cytoskeletal protein required for neuronal migration in the cells of Cajal and myenteric ganglia, and thus may alter gastrointestinal motility. The test has been compared in patients with IBS, healthy controls, and patients with IBD, showing a higher optical density of anti-CdtB in IBS, as well as a higher concentration of anti-vinculin antibodies. The first-generation test had low S (<40%) and Sp close to 90%.54 After epitope correction, the second-generation test showed 90–93% Sp and 52% S. It is mainly useful when IBS or postinfectious functional diarrhea is suspected.55 In special cases of suspicion of diarrhea associated with neuroendocrine tumors, serum levels of vasoactive intestinal peptide, gastrin, chromogranin, or calcitonin can be measured, or 5-hydroxy-indolacetic acid levels can be measured. However, given the low pre-test probability and the false positive rate, those tests should not be part of the routine evaluation of chronic diarrhea.3
Various tests enable alterations in the different mechanisms of digestion and/or intestinal absorption to be detected:56
- 1
Serum beta-carotenes: Beta-carotenes need the absorption mechanisms of the intestinal wall to be intact, thus a random decrease in their serum level can be indicative of malabsorption or malnutrition. In general, a value >130 mcg/dl (range 112–385) is considered normal, a value between 100 and 130 is not diagnostic, and a value <100 mcg/dl is suggestive of malabsorption syndrome, with 78% S and 82% Sp. Values are highly suggestive of malabsorption, when <60 mcg in women and <50 mcg in men.10,56–57 Carotene loading can differentiate a patient with malnutrition from a patient with malabsorption, when its administration normalizes the level in the patient with the former condition.
- 2
Fecal fat: Qualitative or quantitative fecal fat determination in chronic diarrhea is also suggestive of malabsorption syndrome. The qualitative determination is carried out through Sudan III staining, with glacial acetic acid (abnormal >100 blood cells or blood cells with a diameter >4 μg per high power field), with 76–94% S and 95–99% Sp. Quantitatively, levels >7 g/24 h (depending on the percentage of fat in the diet) are considered abnormal and stool weight above 1000 g/24 h indicates severe causes of malabsorption, such as pancreatic insufficiency, biliary disorders, or a neuroendocrine cause. Fecal fat can be complementary to beta-carotene, when levels of the latter are in the nondiagnostic range.4–5,56,58–59
- 3
d-xylose test: d-xylose is an actively absorbed and passively diffused pentose that evaluates intestinal absorption capacity. It has been used for many years to establish whether malabsorption is secondary to an intestinal wall defect, among other causes. The test consists of administering 25 g of d-xylose orally +500 ml of water, followed by collecting urine over a 5 h period (abnormal <4 g or 16% excretion) or by determining the serum level 1-2 h post-ingestion (abnormal < 20 mg/dl). If the d-xylose test is abnormal, the following step is to rule out an intestinal wall problem, albeit there can be false positives in cases of dehydration, kidney failure, ascites, or if the volume of urine is below 150 ml during the 5 h of the test.10,56,60
- 4
Tests for pancreatic insufficiency: Three tests can determine whether chronic diarrhea is secondary to exocrine pancreatic insufficiency: the pancreolauryl, fecal elastase-1, and secretin or cholecystokinin (CCK) stimulation tests. In the pancreolauryl test, fluorescein dilaurate is administered, together with a portion of carbohydrate and fat, and after the collection of urine over an 8 h period, the percentage of fluorescein excreted in urine is determined. A result above 30% is considered normal, between 20 and 30% is nondiagnostic, and below 30% is abnormal. A cutoff value of 4.5 mcg/mL can also be used, which has 82% S and 91% Sp.56,61 Fecal elastase-1 determination is a more recent test that discerns whether chronic diarrhea is secondary to exocrine pancreatic insufficiency. With a cutoff value <200 mcg/g, S varies from 77 to 96% and Sp is 88%, whereas with a lower cutoff value (100 mcg/g), S is 97%, Sp is 84%, the positive predictive value (PPV) is 66%, and the NPV is 100%.62–63 The secretin or CCK stimulation tests are performed by endoscopically collecting secretions from the ampulla of Vater, after endovenous stimulation, but given their invasive nature and the fact that the alternative tests of pancreolauryl and fecal elastase-1 have reasonable S and Sp, they are less frequently employed.10
- 5
Bile acid malabsorption: Bile acids are metabolically active substances that when they exceed a level in the colon >3 mmol/l, they can modify secretion physiology. Colonic bacteria have the ability to turn the primary prosecretory bile acids into secondary secretor acids, which reduce sodium absorption and increase chloride secretion, causing diarrhea.33 An increasingly recognizable cause of chronic diarrhea is BAD. Up to 22.5% of patients with functional diarrhea or IBS have been described as presenting with BAD.64 Several tests can detect bile acid malabsorption, such as the measurement of serum levels of 7-alpha-hydroxy-4-cholesten-3-one or fibroblast growth factor 19, the measurement of fecal bile acids for 48 h, or the scintigraphic method employing the selenium-labelled homotaurocholic acid test (SeHCAT). A whole-body retention value < 15% on day 7 has 100% S and 94% Sp, but the availability of the test is low. More practically, the positive clinical response to bile acid sequestrants, such as cholestyramine or colestipol, can also be used as a therapeutic test for BAD.1,3–4,7,10,32,64
The wireless motility capsule is an ingestible device with pH and pressure sensors that enables transit time to be measured by intestinal segments, based on changes in pH, pressure, and temperature, enabling normal values to be established for each gastrointestinal segment.65 Its utility in chronic diarrhea is limited to measuring small bowel transit time (SBTT), given that slow transit may predispose to SIBO,66 and rapid transit to premature emptying of the intestinal content into the colon, enabling the passage of undigested substances that are osmotically active. The median SBTT measured by the motility capsule is 4.6 h, and SBTT <2.5 h is considered rapid, between 2.5–6 h, normal, and longer than 6 h, slow.65
Radiologic studiesThere are different imaging studies that can indirectly indicate the presence of structural anatomic abnormalities, such as strictures, fistulas, or intestinal diverticula, to identify calcifications in the pancreatic area, delineate the grade and extent of IBD, and detect potentially secretory tumors, particularly neuroendocrine tumors, or motility alterations associated with rapid transit time. Simple studies, such as plain abdominal films, can be useful by demonstrating calcifications in the area of the pancreas, suggesting chronic pancreatitis. An intestinal transit study with oral contrast can measure ileocecal valve filling time, as well as identify the presence of filling defects or flocculation zones suggestive of malabsorption, in one or several areas of the small bowel. However, it has a low diagnostic yield in chronic diarrhea (20%). Abdominal computed tomography (CT) and magnetic resonance imaging (MRI), in addition to identifying tumors, especially when performed together with a positron emission tomography (PET) scan, have protocol variations that enable better visualization of the intestine (CT enterography [CTE] and magnetic resonance enterography [MRE]) that help identify focal or segmental thickening that suggest the presence of Crohn’s disease or other intestinal or colonic inflammatory causes. The diagnostic yield of CTE is 23% when chronic diarrhea is the only symptom, but increases when there is pain, as well as diarrhea.67–68
Endoscopic studiesEndoscopic studies may help to diagnosis of the cause of chronic diarrhea in two ways: by confirming macroscopic lesions that are diagnostic of the disease and by enabling biopsies to be performed for the final histologic diagnosis.
- 1
Colonoscopy: The role of colonoscopy and ileoscopy in chronic diarrhea is to identify macroscopic findings that imply mucosal damage, suggestive of IBD, and to take biopsies in the cases with normal-appearing intestine or colon to determine whether there is enteritis or microscopic colitis or other inflammatory conditions. In chronic diarrhea, colonoscopy has an overall diagnostic yield of 15–31% (2–15% in cases of watery chronic diarrhea), but is superior to sigmoidoscopy because it enables biopsies to be taken of each segment of the colon, as well as in the terminal ileum, increasing the possibility of diagnosing microscopic colitis and/or enteritis.69–72 The authors of two Mexican studies concluded that colonoscopy with biopsy made specific histologic diagnosis possible between 28%71 and 36%72 of the cases of chronic diarrhea. The diagnostic yield of biopsies of the ileum varied according to the indication (an average of 10% in chronic diarrhea) and was higher in cases with abnormalities detected through imaging studies, or by viewing erosions, ulcerations, or even macroscopic alterations, utilizing high-definition endoscopes with virtual chromoendoscopy.73–74
- 2
Esophagogastroduodenoscopy: Certain diseases have a predilection for a particular area of the intestine. For example, celiac disease is more easily detectable in the duodenum, where a nodular, reticular, or cobblestone pattern is viewed with white light endoscopes, or a macroscopic reduction of villi is seen with magnification endoscopes. In addition, upper endoscopy enables duodenal biopsies to be taken, and in selected cases, aspiration is performed to obtain cultures and antibiograms. Even though endoscopic findings in the duodenum, such as nodularity or a reticular aspect, can be nonspecific, a report evaluated the diagnostic yield of typical endoscopic duodenal findings and stated that the presence of any of them had 95% S and 98.4% Sp, compared with the gold standard (histopathologic findings). Of the typical findings, reduced thickness of the circular folds had the least diagnostic accuracy, whereas a mosaic or reticular pattern, as well as fissures, were associated with 98–99% Sp and odds ratios above 12.75
- 3
Enteroscopy and video capsule endoscopy: Studies evaluating the middle intestine, such as video capsule endoscopy (VCE) or single or double-balloon enteroscopy, have a diagnostic yield of 42–77% in chronic diarrhea, depending on the identification of erosions, aphthous ulcers, serpiginous ulcers, erythema, edema, or luminal stricture.76–77 In a recent analysis, VCE was reported to change the diagnosis in 1 out of every 3 cases,77 whereas it was concluded, in another work, that although diagnostic yield was 44%, it was considerably reduced in chronic diarrhea, if inflammation markers, such as calprotectin, were negative.78 Specifically in celiac disease, VCE showed 87.5% S and 90.9% Sp in a multicenter study, using histology as the gold standard, comparing the suggestive findings, such as a mosaic or nodular appearance, fissures, or flat mucosa.79 Enteroscopy, in any of its modalities (balloon, double-balloon, spiral, anterograde, retrograde), enables segment-guided biopsies to be taken, thus confirming the histologic diagnosis in 86% of enteropathies and 78% of small bowel Crohn’s disease.80 Its diagnostic yield in chronic diarrhea is lower (55%) when there are no macroscopic lesions suggestive of IBD.81–82
Histopathologic analysis can define the type of inflammation associated with damage of the intestine or colon, which in many cases can be specific to a given entity. It can also guide the clinician to the pathophysiologic mechanism involved or to the differential diagnosis, when the findings are not definitive. A pathologist with expertise in gastrointestinal disorders can distinguish between normal and abnormal findings at each site:
- 1
The presence of atrophy: Height of the villi and their relation to the depth of the crypts, flattening, or grade and distribution of the atrophy (Marsh and Marsh-Oberhuber classifications)
- 2
Type of inflammatory infiltrate: Presence of lymphoid aggregates, with or without hyperplasia, pattern, and number of inflammatory cells per high power field (eosinophils, lymphocytes, plasma cells, neutrophils), as well as their distribution (intraepithelial, lamina propria), presence of erosions, ulcers, abscesses, cryptitis, or crypt microabscesses, fissures, and granulomatous inflammation.
- 3
Specific stains: Certain stains can detect more specific findings (hematoxylin and eosin: contrast of intracellular components; periodic acid-Schiff [PAS]: differentiation of glycogen from other elements, macrophages, parasites; Masson stain: collagen; immunohistochemistry: lymphocyte subtypes; Congo red: amyloidosis; CRP: Tropheryma whipplei or Giardia lamblia).83
- 4
Distribution pattern of intestinal damage: In malabsorption pathology, as well as in inflammatory pathology of the colon, it should be stated that the majority of the diseases (with the exception of a few, such as UC) develop a pattern in patches, throughout the duodenum, jejunum, and ileum, as well as in the colon. Therefore, it is important to take biopsies from the intestine and colon – including each segment of the colon – and in specific cases, emphasize a particular area, such as the duodenal bulb in celiac disease. Taking at least 2 biopsy samples of the bulb (at positions 9 and 12), and at least 4 in the postbulbar duodenum, has been reported to increase the possibility of diagnosing celiac disease by at least 5%, with 96% S.84 In a study that evaluated the diagnostic yield of duodenal biopsies in 28,210 patients, suspicion of sprue, anemia, diarrhea, and weight loss were predictors for biopsy and abnormal findings, and in the patients with chronic diarrhea, 8.6% had a significant finding related to celiac disease. In the patients with suspicion of sprue, 8.9% had intraepithelial lymphocytosis, 11.2% had villous atrophy, and 12% had conclusive data of sprue.85
- 5
Diagnostic criteria, differential diagnoses, and nonspecific findings of enteropathies associated with chronic diarrhea: Of the duodenal disorders associated with chronic diarrhea, some, such as celiac disease, have well defined histologic criteria. It is typical to observe villous atrophy and intraepithelial lymphocytosis, with an intraepithelial lymphocyte (IEL) count >40 per high power field, criteria defined by the Marsh and Oberhuber classifications or the simplified Corazza classification.86–88 However, the differential diagnosis for villous atrophy is broad and different conditions can present atrophy in different grades, such as tropical sprue, SIBO, autoimmune enteritis, drug-related enteropathy, Whipple’s disease, collagenous sprue, eosinophilic enteritis, Crohn’s disease, intestinal lymphoma, intestinal TB, infectious enteritis due to Giardia, enteropathy associated with HIV, and severe malnutrition, whereas the differential diagnosis for intraepithelial lymphocytosis (normal value < 25/HPF), in addition to celiac disease, encompasses diseases, such as tropical sprue, SIBO, autoimmune enteropathy, drug-related enteropathy, and even some functional disorders.89–93 In certain cases, and despite histologic findings – which can show borderline findings – it can be difficult to make the final diagnosis. Therefore, it is important to take the general context of the patient into account, such as place of residence, family history of similar diseases, and previous recent gastrointestinal infections, as well as additional studies, such as serologic or fecal biomarkers, particularly for differentiating certain forms of celiac disease, tropical sprue, SIBO, or postinfectious functional diarrhea.27,92–94
- 6
Diagnostic histologic criteria of colonopathies associated with chronic diarrhea: Among the colonopathies associated with chronic diarrhea, the diseases encompassed in IBD present specific histologic findings, such as microabscesses and crypt distortion in UC95 or granulomatous inflammation, fissures, and transmural involvement in Crohn’s disease.96–97 Microscopic colitis, as its name indicates, is associated with histologic inflammatory findings, in the absence of endoscopic damage. The characteristic finding of lymphocytic colitis is intraepithelial lymphocytosis, defined as >20 IELs per 100 epithelial cells and an acute or chronic mixed infiltrate in the lamina propria. Similar findings can be seen in collagenous colitis, with a lower level of intraepithelial lymphocytosis, but with a subepithelial collagenous band >7 μm (normal <5 μm).98 Eosinophilic colitis requires the presence of tissue eosinophilia, which can vary, depending on the area. On average, it requires at least 65 eosinophils per high power field, but upon observing tissue eosinophilia in other entities, including IBD, an expert panel has recommended a count >50 eosinophils per high power field in the right colon, >35 in the transverse colon, and >25 in the left colon, with or without peripheral eosinophilia, and with or without small intestinal involvement, especially in the terminal ileum, to meet the criteria for eosinophilic colitis.99–100
- 7
Histologic findings suggestive of drug-related damage: Other entities that can cause damage to the colon and chronic diarrhea are infections, particularly CDI, and drugs. As previously stated, certain drugs can increase the risk for microscopic colitis (PPIs, NSAIDs, SSRIs, antibiotics), whereas others can cause ulcerations (NSAIDs, chemotherapy, immunotherapy, targeted therapy and immunotherapy for cancer, and RT). Different histologic patterns in drug-related damage have been described, that include active focal colitis, pseudomembranous colitis, hemorrhagic colitis, ischemic colitis, and erosive colitis.101–102 The histologic diagnosis of damage due to immunotherapy and checkpoint inhibitors is similar to that observed in IBD, with mixed infiltrates characterized by lymphocyte and neutrophil aggregates, cryptitis, and in severe forms, apoptosis.19–20
Functional diarrhea is defined as chronic diarrhea that is not caused by organic or structural disease.6,8,39 Its diagnosis is based on the Rome IV clinical criteria, characterized by diarrheic stools that occur more than 25% of the time, for at least 3 previous months, 6 months before diagnosis, and is distinguished from IBS-D by the absence of pain as the predominant symptom.12
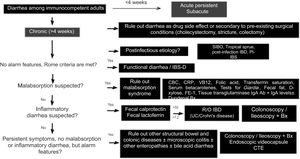
Diagnostic algorithmThere are several published guidelines on the approach to chronic diarrhea, with general recommendations, and different diagnostic algorithms have been described, with the prevalence bias of certain entities presenting in the publishing country.1,7,10 The American guidelines on functional diarrhea recommend basic screening with fecal calprotectin, celiac serology, and the search for Giardia and BAD in the majority of patients, as conditional recommendations, varying in quality of evidence.6,39 A Latin American review was recently published that emphasized the pathophysiology of chronic diarrhea, along with an algorithm that included the fecal fat test and anion gap as major subclassification criteria.103 Given the broad differential diagnosis, to formulate an uncomplicated algorithm, it is important to consider family history, systemic disease, manner of diarrhea onset, bowel movement periodicity or pattern, stool frequency and appearance, previous abdominal surgery, prior drug use, alarm symptoms (rectal bleeding, melena, weight loss, nocturnal symptoms, family history of IBD, polyps, or colon cancer), red flags in the abdominal physical examination (masses, adenomegaly), and associated extraintestinal symptoms to determine whether it is probable that the diarrhea is malabsorptive, inflammatory, or functional and to reduce the need for tests, or direct them towards the probable subtype. We propose herein a simple diagnostic algorithm for establishing the etiology of chronic diarrhea (Fig. 2) that initially excludes causes that are secondary to drug use or to anatomic or functional alterations resulting from gastrointestinal surgery, such as cholecystectomy or colectomy. Next, if there is a history of a potentially infectious episode, with a resulting change in bowel habit, then SIBO, secondary IBD, functional diarrhea, or PI-IBS should be considered. Based on the clinical and macroscopic characteristics of the diarrhea, as well as the presence or absence of alarm symptoms, the algorithm divides chronic diarrhea into 3 groups: functional, malabsorptive, or inflammatory. If there are symptoms suggestive of malabsorption, we recommend ruling out malabsorption syndrome through basic malabsorption studies (CBC, high-sensitivity C-reactive protein, serum carotenoids, celiac serology, serum levels of vitamin B12 and folic acid, and iron kinetics). If there are no data suggestive of malabsorption syndrome, inflammatory diarrhea should be investigated through the determination of fecal calprotectin and/or fecal lactoferrin. Based on the results, the subsequent conduct is established. For example, if celiac serology is weakly positive, the next step is to perform upper endoscopy with duodenal biopsies, and if either calprotectin or lactoferrin is positive, then colonoscopy and ileostomy should be performed to rule out IBD. In cases in which there is no evidence of malabsorption or inflammation of the colon in the biochemical parameters, but there are alarm symptoms, such as weight loss or nocturnal symptoms, then colonoscopy and ileoscopy, with biopsies taken at both sites, should be performed to rule out microscopic or eosinophilic enteropathy and/or colitis. When there are previous surgical or medical events suggestive of BAD, a trial with a bile salt sequestrant test should be given. If patients have no alarm signs or symptoms and meet the Rome criteria, or if the entire diagnostic evaluation is negative, the diagnosis of functional diarrhea can be made. If there is a history of infection and no evidence of IBD or SIBO, the diagnosis of PI-IBS or postinfectious functional diarrhea can be made, based on the main symptoms.1–11 The diagnosis of a causal entity of chronic diarrhea does not rule out the possibility of a second overlapping or simultaneous problem. Cases of post-inflammatory IBS following IBD and cases of overlap between celiac disease and IBS have been reported.104–106
Diagnostic algorithm for chronic diarrhea.
Bx: biopsies; CBC: complete blood count; CTE: computed tomography enterography; FE-1: fecal elastase-1; hsCRP: high-sensitivity C-reactive protein; IBD: inflammatory bowel disease; PI-IBS: postinfectious irritable bowel syndrome; R/O: rule out; SIBO: small intestinal bacterial overgrowth; UC: ulcerative colitis.
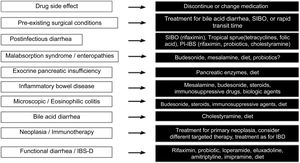
Finally, Fig. 3 describes the therapeutic options, according to the cause of the diarrhea.
ConclusionsDue to the complexity of the mechanisms and the multiple causes of chronic diarrhea, the present review analyzed the diagnostic approach, emphasizing key aspects in the clinical events that can guide the clinician in determining the cause, or otherwise, categorize it in a subgroup, as well as evaluating the role of the different biomarkers and diagnostic tests in each subtype of diarrhea. A clinical approach was detailed, which, rationally supported by diagnostic tests, will be useful in daily practice for determining the etiology of chronic diarrhea, a problem both common and complex, and thus provide a specific treatment.
FundingThere was no source of funding.
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Gómez-Escudero O, Remes-Troche JM. Abordaje de la diarrea crónica en el adulto: Revisión de la literatura. Revista de Gastroenterología de México. 2021;86:387–402.