Gastrointestinal stromal tumors (GISTs) are the most frequent mesenchymal neoplastic tumors of the gastrointestinal tract and their expression and management is dependent on their aggressivity. Our aim was to characterize the GISTs diagnosed at our center, analyzing the epidemiologic, anatomopathologic, imaging study, and management variables.
Material and methodsA retrospective, observational cohort study was conducted on adult patients diagnosed with GIST through imaging studies and biopsy, within the time frame of 2017 and 2022 at our center, characterizing the epidemiologic, imaging study, anatomopathologic, and therapeutic data.
ResultsThirty-three patients with a mean age of 59 years were included. The majority of cases were incidental findings (54.5%), and of the symptomatic cases, the most frequent presentation was gastrointestinal bleeding (24%). The most common location was the stomach (19/33 cases), and the most frequent presentation was exophytic (48.5%). In the imaging studies, impregnation was mainly heterogeneous and progressive and 54% had an intrinsic characteristic, with necrosis/cystic areas the most frequent feature. There were no cases of local invasion or distant metastasis. Histologically, the spindle cell type was the most frequent (78.8%) and the majority had fewer than 5 mitoses per high power field. Thirty cases were treated at our center and surgery was the most frequent form of resolution (29/30). No deaths caused by GIST were registered in our case series.
ConclusionsThe epidemiologic and imaging study characteristics in our case series were similar to those reported in the literature, but our results were different, with respect to early diagnosis and the low aggressivity of the cases.
Los tumores del estroma gastrointestinal (GIST) son las neoplasias mesenquimáticas más frecuentes del tubo digestivo y su expresión y manejo dependen de su agresividad. Nuestro objetivo es caracterizar los GIST diagnosticados en nuestro centro, a partir de variables epidemiológicas, anatomopatológicas, imagenológicas y de manejo.
Material y métodosEstudio observacional de cohorte retrospectiva en pacientes adultos con diagnóstico de GIST mediante imágenes y biopsia entre los años 2017 y 2022 en nuestro centro, caracterizando datos epidemiológicos, imagenológicos, de anatomía patológica y terapéuticos.
ResultadosSe incluyeron 33 pacientes, promedio de edad 59 años. La mayoría fue un hallazgo incidental (54,5%) y de los sintomáticos, lo más frecuente fue hemorragia digestiva (24%). La ubicación más frecuente fue el estómago (19/33 casos) y su forma de presentación exofítica fue la más habitual (48,5%). Imagenológicamente, su impregnación fue mayormente heterogénea y progresiva y 54% presentaron alguna característica intrínseca, siendo la necrosis/áreas quísticas lo más frecuente, sin casos de invasión local ni diseminación a distancia. Histológicamente la variante fusada fue la más frecuente (78,8%) y la mayoría presentaron menos de 5 mitosis por campo mayor. 30 casos fueron tratados en nuestro recinto, siendo la cirugía la forma de resolución más frecuente (29/30). No se registraron muertos a causa de GIST en nuestra serie.
ConclusionesNuestra serie tiene características epidemiológicas e imagenológicas similares a la literatura, diferenciándose por un diagnóstico precoz y baja agresividad de los casos.
Gastrointestinal stromal tumors (GISTs) are the most frequent mesenchymal tumors of the gastrointestinal tract. They account for 80% of this type of tumor and can develop from the esophagus to the rectum, as well as outside of those locations.1 Their prevalence is unknown but their incidence is estimated at 3,300 to 6,000 new cases per year in the United States1 and at 8.5 to 10 cases per one million inhabitants in France.2 In Chile, there is no specific register for determining epidemiologic variables.3 GISTs originate from mutations in the KIT protein (c-kit) in interstitial cells of Cajal, which are found in the myenteric plexus of the entire gastrointestinal system and function as pacemakers in peristalsis.4
Pathologically, GIST diagnosis is based on morphology and immunohistochemistry.5 Advances in GIST characterization through histopathology and immunohistochemistry enable them to be differentiated from other intestinal tumors.6 GISTs are characterized by the overexpression of the tyrosine kinase growth factor receptor (CD117), the importance of which lies in the fact that, unlike other types of mesenchymal tumors, the presence of this protein provides alternative therapeutics for the tyrosine kinase receptor inhibitors, representing a complementary option to surgery, chemotherapy, or radiotherapy.1,7
GISTs tend to more frequently affect persons from 50 to 80 years of age, but a greater incidence has not been determined by geographic zones, sex, or ethnicities,1 albeit there is a slightly higher association with male sex in the gastric or small bowel location.3 The stomach is the most frequent location of GISTs (70%), followed by the small bowel (20-30%). The rest of the gastrointestinal tract is affected to a lesser degree, and exceptionally, tumors at an extraintestinal location. The type of presentation depends on the site and size of the tumor, and diagnosis at the asymptomatic stage is not unusual.8,9
Radiologically, GISTs are expressed as masses that are dependent on the intestinal wall; they can be endophytic or exophytic and they are usually well-delineated. Their contrast medium-enhancement can be variable, and the presence of bleeding, necrosis, cystic areas, and calcifications are findings that are more frequent in larger tumors. Tumor characteristics are expressed, depending on the imaging study utilized, and the most adequate methods for their diagnosis are computed tomography (CT) and magnetic resonance imaging (MRI).8,10,11
Surgical resection is the treatment of choice. Management with immunotherapy or chemo-radiotherapy is evaluated case by case, in accordance with national and international guidelines.3
Numerous international case reports and retrospective cohort studies have helped characterize this pathology and the majority of them report anatomopathologic and clinical characteristics. In Chile, there are few case reports, and they do not include imaging findings or their correlation with clinical and pathologic data.12–17
The aim of our study was to characterize the GISTs diagnosed at the Clínica Alemana de Santiago, by analyzing the epidemiologic, anatomopathologic, imaging study, and management variables.
Materials and methodsA retrospective, observational cohort study was conducted at the Clínica Alemana de Santiago, in Chile. The STROBE checklist was utilized as a reference for developing this article. Information was collected from the clinical records and imaging studies of patients diagnosed with GIST, within the time frame of 2017 and 2022. Patients at our institution who had an anatomopathologic diagnosis of GIST and imaging study support were included, resulting in a total of 33 patients, 32 CT scans, and 9 MRIs.
The epidemiologic, morbidity, and disease presentation variables of the patients were collected from the electronic clinical records. The number of comorbidities per person was recorded, the mean of the sample was calculated, and multimorbidity was defined as the presentation of two or more comorbidities. The anatomopathologic reports were evaluated, registering tumor size, histologic subtype, the presence of necrosis, the mitotic index, progressive disease risk, and immunohistochemical results. Images were analyzed in our PACS system, by a radiologist specializing in abdominal images with 15 years of experience and a radiologist who is a fellow in abdominal images (5 years of experience) to specify the tumor characteristics and measure the tumor attenuation in Hounsfield Units (HU) at the different CT phases, and the mean tumor apparent diffusion coefficient (ADC) in the MRI studies. In an MRI sequence, the ADC measures the magnitude of diffusion of water molecules in tissue, commonly utilized for detecting cerebral infarctions or diagnosing cancer.
The type of management of the patients was registered, whether surgical, pharmacologic, or mixed, and the type of surgery performed was identified.
The calculation for aggressive behavior risk was carried out utilizing the categories proposed by the National Institutes of Health (NIH) in 2002, based on tumor size and mitotic count.5
Statistical analysisThe storage and statistical analysis of the data were performed using SPSS version 25.0 (Illinois, USA) software. Means, ranges, and 95% confidence intervals were calculated for the quantitative variables and percentages were calculated for the qualitative variables (epidemiologic, clinical, imaging, and anatomopathologic) in each case.
Ethical considerationsThe authors declare that our work meets the current regulations in bioethical research and was approved by the institutional ethics committee (Departamento de Desarrollo Académico e Investigación and the Comité Ético Científico de la Facultad de Medicina Clínica Alemana – Universidad del Desarrollo).
The present article contains no personal information that could identify the patients, therefore, obtaining informed consent was not necessary.
ResultsFifty-one percent (n = 17) of the patients included in the study were men and the mean patient age was 59 years (range 27-92). A mean of 5.5 cases per year during the study period were reported, with a higher number (6.3 cases per year) in the years prior to the COVID-19 pandemic (2017-2019).
There was a mean of 1.76 comorbidities per person, with a maximum of three. A total of 51.5% of the patients in the study were affected by multimorbidity, the most frequent of which were those related to cardiovascular risk (47%), followed by miscellaneous diseases (36%), and tumors of a different origin (16%).
Regarding the time of diagnosis, the majority of patients were diagnosed in the asymptomatic stage in preventive studies or as incidental findings (54.5%). The most frequent symptomatic presentation was gastrointestinal bleeding (24%), followed by abdominal pain (21.2%). None of the patients presented with metastasis at the time of diagnosis.
Lesions were diagnosed from the esophagus to the rectum and the most frequent location was the stomach (n = 19) (Table 1). Mean tumor size was 2.5 cm (95% CI 3.6-5.4 cm) and 70% of the tumors were smaller than 5 cm.
Frequency of GIST location in different segments of the gastrointestinal tract.
| Tumor location | Number | Percentage |
|---|---|---|
| Gastric | 19 | 58% |
| Duodenal | 3 | 9% |
| Intestinal | 9 | 27% |
| Colonic | 1 | 3% |
| Rectal | 1 | 3% |
| Total | 33 | 100% |
GIST: gastrointestinal stromal tumor.
Original table with data based on the main results of our cohort.
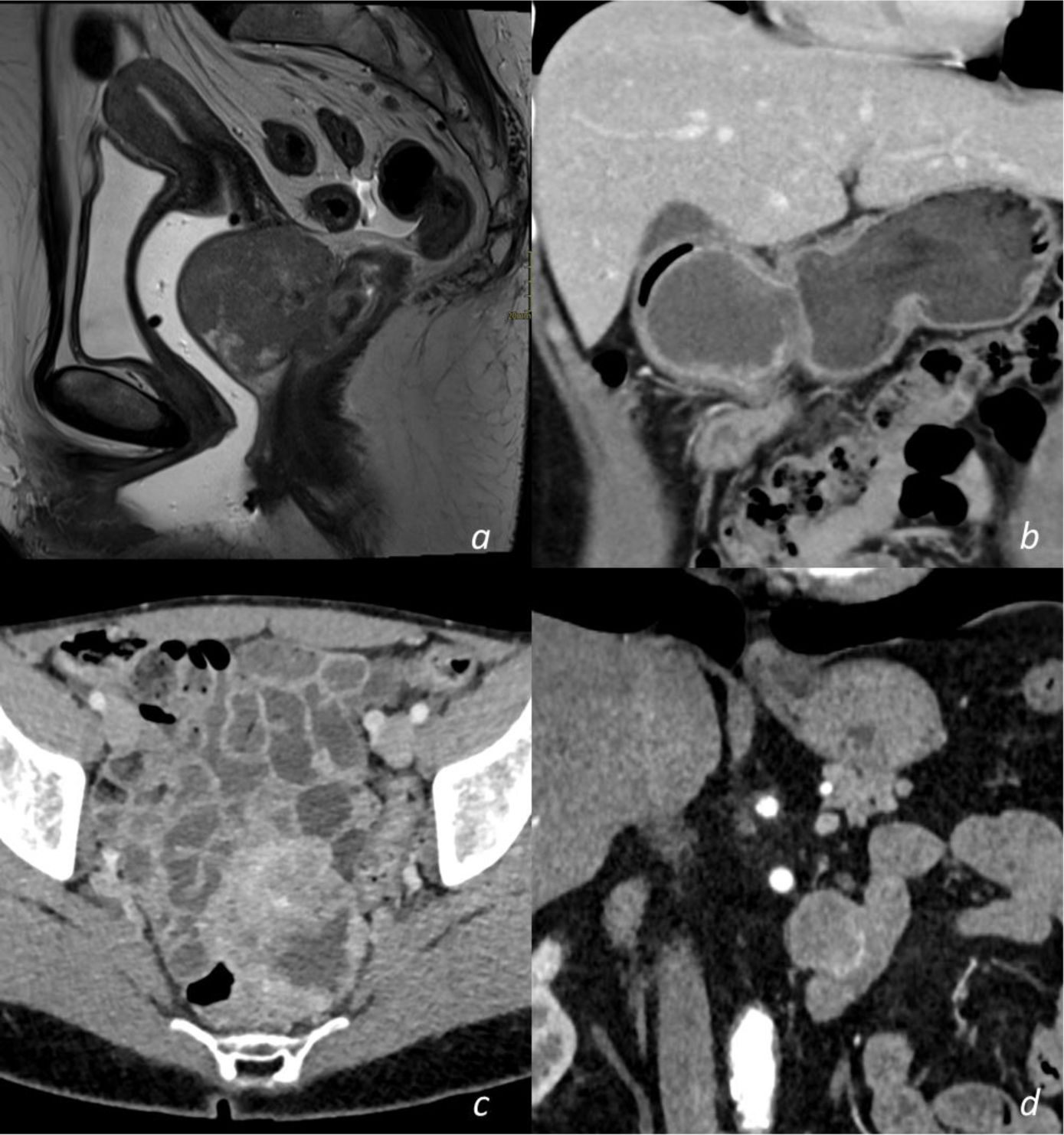
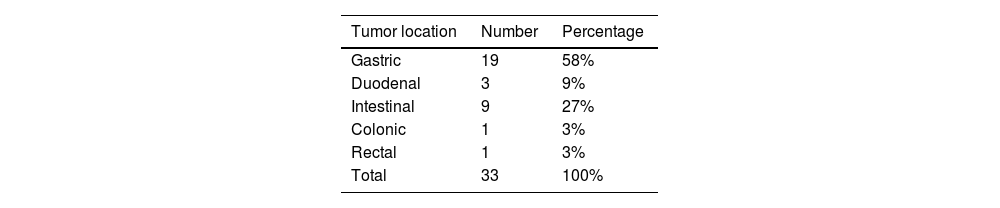
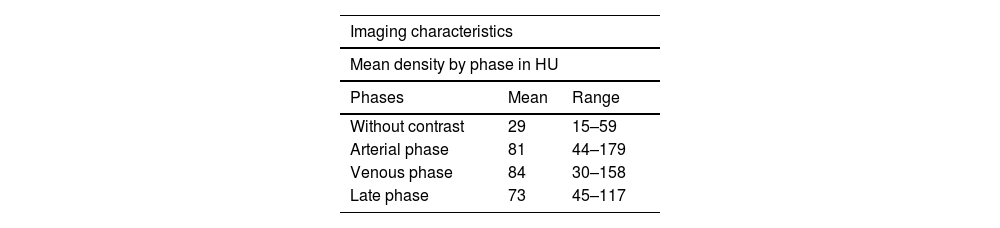
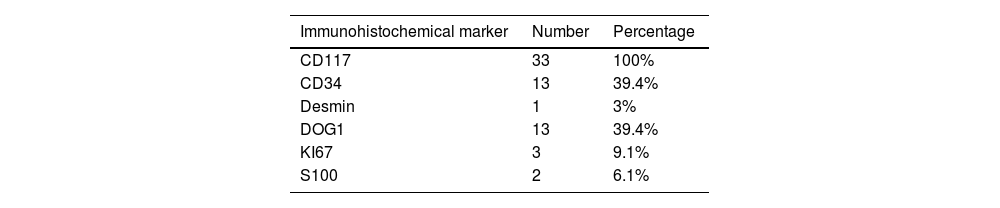
With respect to the imaging study characteristics of the GISTs, 81.8% were well-delineated, and in relation to the intestinal wall, 48.5% were exophytic, 33.3% were endophytic, and the remaining 18.2% had no predominance. With respect to contrast medium enhancement, 51.5% were heterogeneous, and in the triple phase studies (19 in total), 58% had progressive enhancement, 37% had arterial phase enhancement and venous phase enhancement maintenance, and only one case (5%) demonstrated washout (venous phase has 15 HU less than arterial phase). Mean density values measured in HU in the different tomographic phases are shown in Table 2. Intrinsic tumor characteristics were visualized in half of the cases (52%): necrosis/cystic areas (59%), calcifications (18%), cavitation (3%), and bleeding (3%). MRI was carried out in nine cases, all of them showing intermediate signal intensity in the T2 sequence and a hypointense signal in T1, with no restrictions in diffusion and a similar enhancement pattern in the CT studies. The ADC measured in the lesions was a mean 1,376 mm2/s (range: 837-2,548). No cases of local invasion or distant spread were registered. Representative cases are shown in Fig. 1.
Mean density and range in HU of GISTs in different phases of the contrasted computed tomography scans.
| Imaging characteristics | ||
|---|---|---|
| Mean density by phase in HU | ||
| Phases | Mean | Range |
| Without contrast | 29 | 15–59 |
| Arterial phase | 81 | 44–179 |
| Venous phase | 84 | 30–158 |
| Late phase | 73 | 45–117 |
GIST: gastrointestinal stromal tumor; HU: Hounsfield unit.
Original table with data based on the main results of our cohort.
Selected abdominal and pelvic magnetic resonance imaging (MRI) and computed tomography (CT) scan views showing different GIST locations. a) Sagittal T2-weighted MRI scan, showing homogeneous exophytic lesion of intermediate intensity dependent on the anterior wall of the inferior rectum. b) Abdominal CT coronal view in the venous phase, showing a heterogeneous endophytic lesion of the gastric antrum, with a necrotic center, protruding into the pylorus and first part of the duodenum. c) Pelvic CT axial view in the venous phase, showing a heterogeneous exophytic lesion with necrotic/cystic areas dependent on the wall of the ileum. d) Abdominal CT scan in the arterial phase, showing a homogeneous exophytic lesion in the fourth part of the duodenum.
Original figure based on CT and MRI images of GIST tumors carried out at our center.
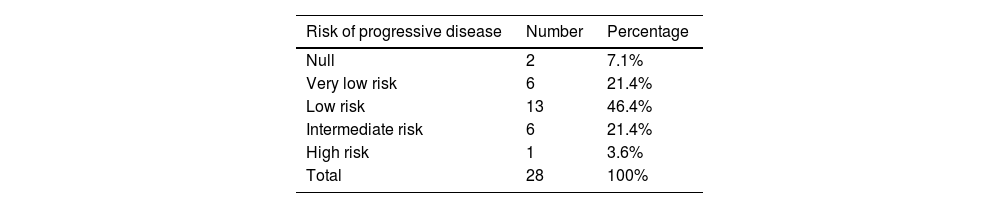
Anatomopathologically, the spindle cell variant was the most frequent histologic subtype (78.8%), followed by the epithelioid variant (12%). The CD117 marker was positive in all cases. Table 3 shows the percentage of positivity of each of the markers utilized in the samples. Ninety-four percent (n = 31) of the cases could be categorized according to the recurrence risk classification of the NIH.5 Only 6% of them corresponded to the high-risk group and Table 4 shows the distribution. The mitotic index was fewer than 5 mitoses per high power field in 84% of the cases.
Frequency of immunohistochemical marker expression in the case series.
| Immunohistochemical marker | Number | Percentage |
|---|---|---|
| CD117 | 33 | 100% |
| CD34 | 13 | 39.4% |
| Desmin | 1 | 3% |
| DOG1 | 13 | 39.4% |
| KI67 | 3 | 9.1% |
| S100 | 2 | 6.1% |
CD117: cluster of differentiation 117; CD34: cluster of differentiation 34; DOG1: discovered on gastrointestinal stromal tumor 1.
Original table with data based on the main results of our cohort.
Frequency of categories of risk for progressive disease in GISTs, according to size and mitotic count.20
| Risk of progressive disease | Number | Percentage |
|---|---|---|
| Null | 2 | 7.1% |
| Very low risk | 6 | 21.4% |
| Low risk | 13 | 46.4% |
| Intermediate risk | 6 | 21.4% |
| High risk | 1 | 3.6% |
| Total | 28 | 100% |
GIST: gastrointestinal stromal tumor.
Original table with data based on the main results of our cohort.
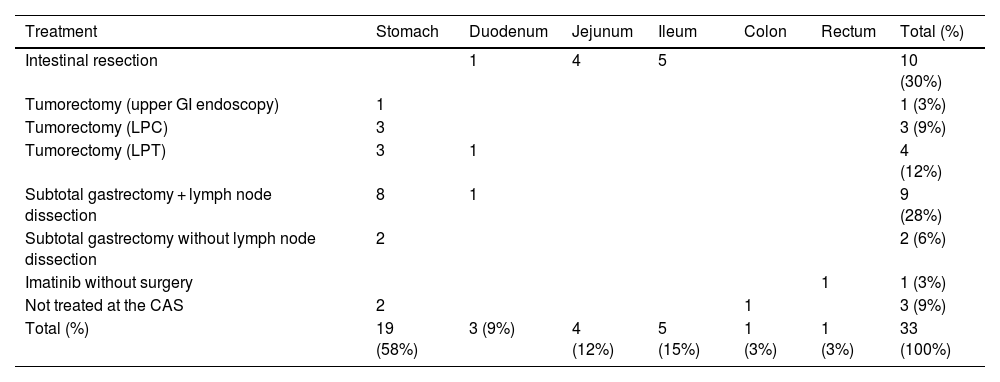
Thirty patients were treated at our institution. Seventy-seven percent were treated only surgically, 3% only with imatinib (one case), and 20% were treated with both. The most frequent type of surgery was resection of a segment of the small bowel and enteroenteric anastomosis. Table 5 describes the distribution in detail.
Management summary of patients with GIST at our center.
| Treatment | Stomach | Duodenum | Jejunum | Ileum | Colon | Rectum | Total (%) |
|---|---|---|---|---|---|---|---|
| Intestinal resection | 1 | 4 | 5 | 10 (30%) | |||
| Tumorectomy (upper GI endoscopy) | 1 | 1 (3%) | |||||
| Tumorectomy (LPC) | 3 | 3 (9%) | |||||
| Tumorectomy (LPT) | 3 | 1 | 4 (12%) | ||||
| Subtotal gastrectomy + lymph node dissection | 8 | 1 | 9 (28%) | ||||
| Subtotal gastrectomy without lymph node dissection | 2 | 2 (6%) | |||||
| Imatinib without surgery | 1 | 1 (3%) | |||||
| Not treated at the CAS | 2 | 1 | 3 (9%) | ||||
| Total (%) | 19 (58%) | 3 (9%) | 4 (12%) | 5 (15%) | 1 (3%) | 1 (3%) | 33 (100%) |
CAS: Clínica Alemana de Santiago; GI: gastrointestinal; GIST: gastrointestinal stromal tumor; LPC: laparoscopy; LPT: laparotomy.
Our original table with data based on the main results of our cohort.
During the data collection and processing, only two deaths were registered, none of which were directly caused by tumor spread.
DiscussionOur GIST case series showed similar results in the epidemiologic variables to other Chilean case series and Latin American studies, highlighting a similar mean age and no clear trend by sex.12,18,19
With respect to comorbidity, several studies have reported cardiovascular causes as the most frequent, as in our case series, but a history of a previous or even synchronous cancer is an important condition in the subjects with GIST in different case series, also coinciding with our study.20 We included the concept of multimorbidity, which is a recently added measure for characterizing populations, and has been associated with tumors of the entire digestive tract in Chile. Our distribution of multimorbidity is similar to population values reported in the literature.21
Patients with stromal tumors usually present with the symptomatology of gastrointestinal bleeding or abdominal pain. Our case series differs in the number of asymptomatic cases, which were the majority, but within the symptomatic group, follows the same distribution pattern as reported in international case series.22,23 Within the studies included in a broad systematic review on the clinical and anatomopathologic characteristics of GIST, the main difference in the series with a higher or lower number of patients diagnosed incidentally, was the mitotic index and the number of patients with high-risk GIST, according to the NIH classification. Our study population had a higher number of high-risk tumors.23,24 In those patients, there was no important difference regarding tumor size or the number of patients with extraintestinal location that would justify said difference. In our group, we believe it is necessary to consider local factors and methodological study limitations to explain those phenomena, keeping in mind that our cohort is from a private healthcare institution that treats a population with high economic resources and healthcare plans that are strongly focused on prevention.
There is agreement that the detection of GISTs is determined by the presence of CD117 (c-kit), present in the majority of lesions (95%), as well as other frequently positive markers that complement the diagnosis, such as CD34 (60-70%), smooth-muscle actin (30-40%), and S-100 (5%), among others.5,18 In our case series, 100% of the patients presented with positive CD117, and positive CD34 (39%) in second place, concurring with the literature.
Imaging study characteristics have been simply described in descriptive studies with a greater epidemiologic or anatomopathologic focus. We found concordant results with a case series whose focus was predominantly based on imaging studies and data described in international guidelines, characterizing lesions as exophytic or endophytic tumor with heterogeneous enhancement in the majority of cases, with cystic or necrotic areas, and less frequently, calcifications (3%).1 However, we have provided objective GIST attenuation values in the different phases and tumor behavior in triple-phase CT, predominantly revealing progressive enhancement. The high ADC values are concordant with weak restriction to mobility of the protons in MRI, consistent with the low tumor aggressivity in our case series.
Our results agree with the treatment focus defined in the international guidelines and described in other descriptive works on GIST management strategies. Complete surgical resection continues to be the therapy of choice in patients that do not have metastatic disease.1 However, therapy with adjuvant imatinib, or alone, in cases of high-risk localized disease and advanced or metastatic disease, respectively, has shown a decrease in recurrence and an increase in survival, in addition to being used in several studies on the same subgroup of patients.7,19 The higher number of patients with localized disease in our study, with respect to other case series, justified the higher number of patients treated only with surgery (77%), but strikingly, up to 20% of them were treated with surgery plus adjuvant imatinib. Even though a European guideline on the treatment of GIST suggests said management in patients with high-risk localized disease, it recommends an individualized decision in patients with intermediate risk.25 The trend in our case series is possibly related to the similar percentage of patients with intermediate or high risk (21.4 and 3.6%, respectively).
Among the limitations of our study are its single center and descriptive character, as well as the incomplete follow-up in some of the patients.
ConclusionGISTs are infrequent tumors but awareness of their distinctive clinical, immunohistochemical, imaging study, and management characteristics is very relevant. A contribution of our case series is the description of multiple tumor aspects in our study population, distinguished by low tumor aggressivity, as well as the provision of applicable data in determining imaging characteristics for GIST detection.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.