Waldenström macroglobulinemia (WM), or lymphoplasmacytic lymphoma, is a type of slow-growing non-Hodgkin's lymphoma with an annual incidence of 3 cases per 1 million.1 Its presentation is varied, given its systemic involvement and chronic course. The 5-year survival rate, once treatment is begun, is 87% in low-risk patients, 68% in intermediate-risk patients, and 36% in high-risk patients.2
Gastrointestinal involvement is infrequent, at less than 5%. Seventy-two years ago, Waldenström first described 3 cases, one of which presented with intense abdominal pain and bloating.3 Later reports included symptoms of pseudo-obstruction, protein-losing enteropathy associated with duodenal lymphangiectasia, severe malnutrition, and important impact on the quality of life of those patients,4 digestive tract bleeding due to hyperviscosity or infiltration, hepatosplenic thrombosis associated with hypercoagulability, and symptoms of bowel perforation during treatment.5,6 Most cases of hemolytic anemia in WM are associated with cold antibodies, with a prevalence of 1.1%.7
A 49-year-old woman with symptoms of 1-year progression initially presented with normocytic anemia with documented indirect hyperbilirubinemia, elevated LDH, and positive Coombs test for IgM, IgG, and C3d, suggestive of mixed antibody autoimmune hemolytic anemia. She was managed with prednisolone and azathioprine and her response was adequate at the follow-up at 2 months. The patient later sought medical attention at other hospitals due to intermittent diarrhea with characteristics of malabsorption, which worsened 2 months prior to hospital admission, and was associated with a 12-kg weight loss. A new hemogram showed high volumes of leukopenia and anemia, with a MCV of 108 fL. Tests reported noninflammatory diarrhea, severe hypoalbuminemia of 1.6g/dL, and prolonged coagulation times. Studies were performed considering neoplasia in the differential diagnosis of an autoimmune disorder. Antinuclear antibodies, beta-2 microglobulin, and anticardiolipin antibodies were negative, there was complement consumption, and the lupus anticoagulant was positive.
Because of the macrocytosis, vitamin B12 and folic acid studies were carried out, with normal results. There was a decrease in iron. Active hemolysis was no longer present and the extended blood test showed the rouleaux formation phenomenon.
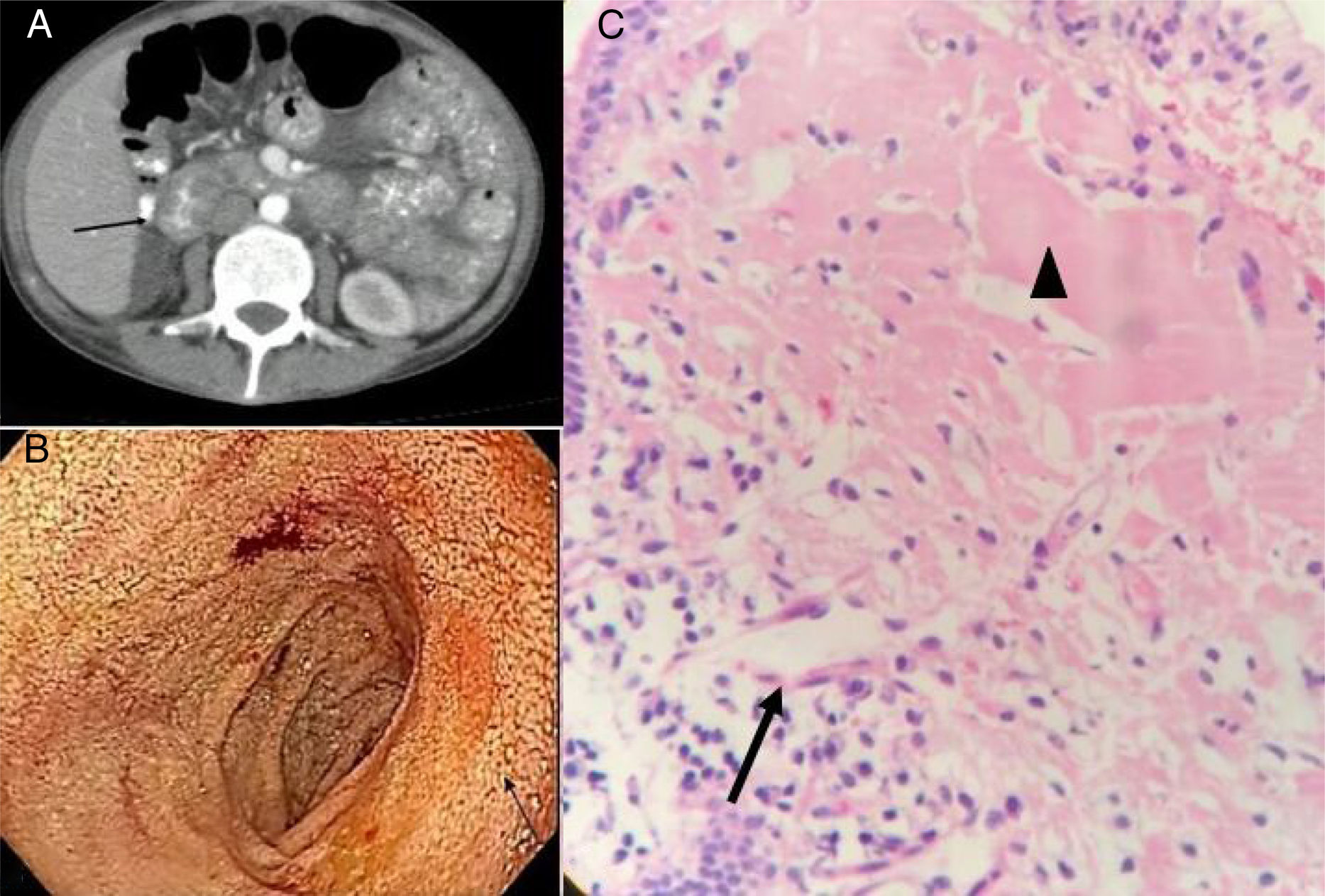
Tomography scans of the chest and abdomen revealed no masses, but identified diffuse thickening of the intestinal segments, especially at the level of the duodenum. Duodenal lymphangiectasia was found through upper gastrointestinal endoscopy, and biopsy reported anomalous matter suggestive of amyloid. However, Congo red staining was negative (fig. 1).
A) Contrast-enhanced abdominal tomography scan showing a thickened duodenum. B) Upper gastrointestinal endoscopy. The arrow indicates the area of duodenal lymphangiectasia. C) H&E-stained duodenal biopsy: the arrow indicates lymphatic vessel dilation and the arrowhead indicates the proteinaceous material suggestive of amyloid.
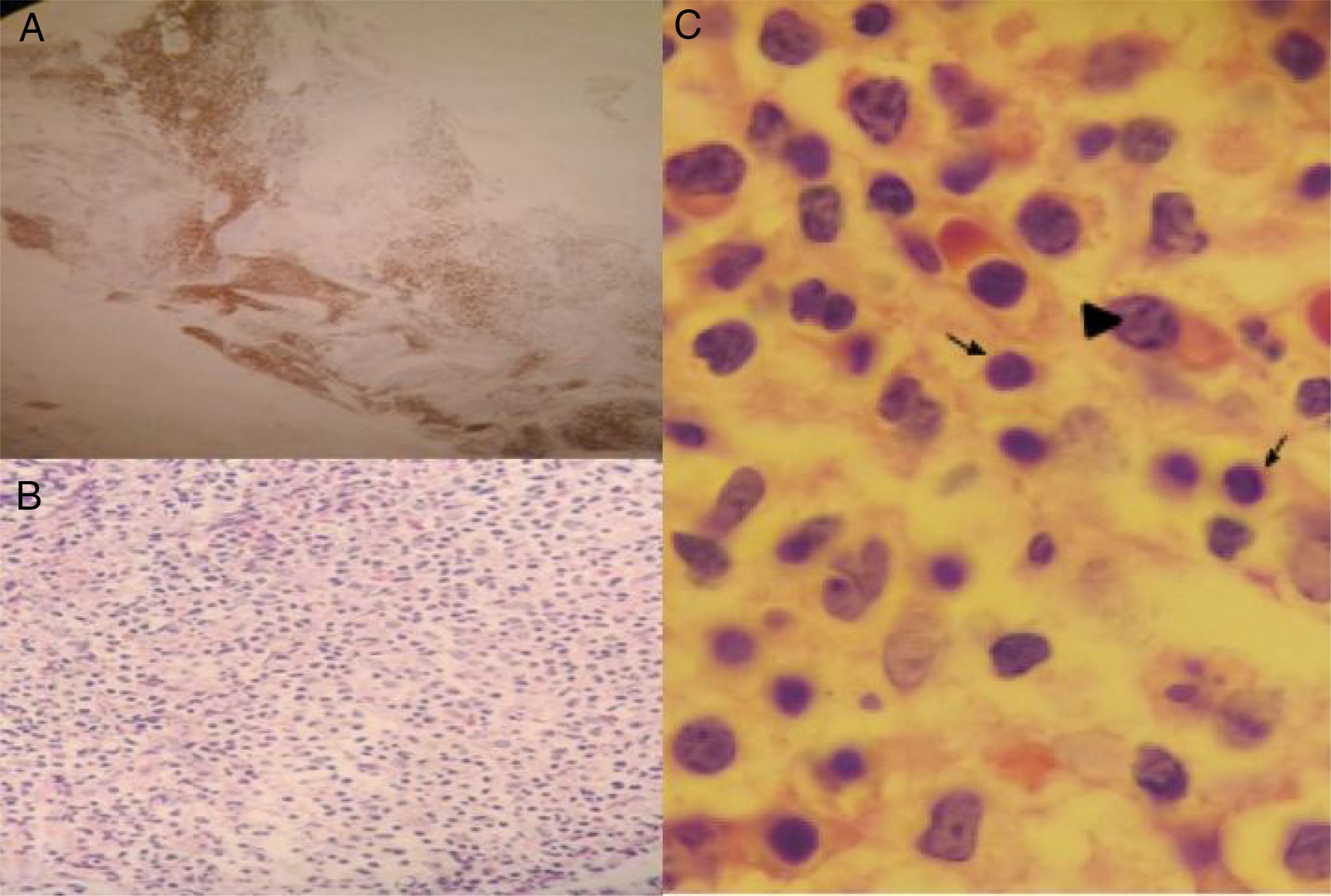
Considering a probable protein-losing enteropathy and a possible plasma cell neoplasia, protein electrophoresis and immunofixation were performed. Results included a monoclonal band in the gamma region, increased kappa light chains, IgM of 2127mg/dL, and a positive Coombs test for IgM. Bone marrow biopsy identified small B-lymphocytes with a plasmacytoid differentiation consistent with lymphoplasmacytic lymphoma (fig. 2). Chemotherapy was begun with the rituximab-bortezomib regimen, resulting in a progressive decrease of fecal output at one week and resolution of diarrhea at 15 days.
As mentioned above, gastrointestinal involvement due to diarrhea as a malabsorption symptom, with a frequency of 1-3%, is described in WM, as well as protein-losing enteropathy associated or not with intestinal lymphangiectasia that manifests as unexplained chronic diarrhea.4 Amyloid deposits8 and IgM accumulation in the intestinal wall have been described as pathophysiologic mechanisms that cause intestinal villi loss, stasis, and lymph canal obstruction, with the consequent formation of lymphangiectasia.9,10 In the present case, Congo red dye was negative and the deposits in the mucosa were identified as IgM, which was confirmed by immunoperoxidase staining.5 Added to the bone marrow findings, WM diagnosis was made.
In conclusion, the indolent nature of WM makes it difficult to diagnose. Given its systemic involvement, WM should be considered in the differential diagnosis of patients with chronic diarrhea.
Ethical disclosuresThe procedures carried out in the present study were performed according to the norms of the Research and Ethics Committee of our institution (Pontificia Universidad Javeriana and Hospital Universitario San Ignacio), the Council for International Organizations of Medical Sciences (CIOMS), and the Declaration of Helsinki.
Confidentiality of data.The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent.The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Amado Garzón SB, Villanueva Ortega MM, Botero Bahamón JD. Diarrea crónica: un caso de macroglobulinemia de Waldenström. Revista de Gastroenterología de México. 2018;83:467–469.