Surgical resection with negative margins is part of the curative treatment of gastric adenocarcinoma. Positive surgical margins are associated with worse outcome. The aim of the present study was to determine the clinical usefulness of extending the proximal surgical margin in patients undergoing total gastrectomy for gastric adenocarcinoma.
Materials and methodsA retrospective analysis of patients that underwent total gastrectomy within the time frame of 2002 and 2017 was conducted. Patients diagnosed with adenocarcinoma that underwent curative surgery were included. Patients were divided into three groups, depending on proximal surgical margin status: negative margin (R0), positive margin with additional resection to achieve negative margin (R1-R0), and positive margin (R1). Demographic and clinical variables were analyzed. The outcome measures to evaluate were recurrence, disease-free survival, and overall survival.
ResultsForty-eight patients were included in the study. Thirty-seven were classified as R0, 9 as R1-R0, and 2 as R1. Fifty-two percent of the patients had clinical stage III disease. The overall surgical mortality rate was 2% and the morbidity rate was higher than 29%. The local recurrence rate was 0% in the R1-R0 group vs. 50% in the R1 group (p = 0.02). Disease-free survival was 49 months in the R1-R0 group vs. 32 months in the R1 group (p = 0.6). Overall survival was 51 months for the R1-R0 group vs. 35 months for the R1 group (p = 0.5).
ConclusionsIntraoperative extension of the positive surgical margin improved the local recurrence rate but was not associated with improvement in overall survival or disease-free survival and could possibly increase postoperative morbidity.
La resección quirúrgica con márgenes negativos es parte del tratamiento curativo del adenocarcinoma gástrico. Los márgenes quirúrgicos positivos se asocian a un peor pronóstico. Determinar la utilidad clínica de ampliar el margen quirúrgico proximal en pacientes sometidos a gastrectomía total por adenocarcinoma gástrico.
Material y métodosAnálisis retrospectivo de pacientes sometidos a gastrectomía total entre los años 2002 a 2017. Se incluyeron pacientes con diagnóstico de adenocarcinoma operados con intención curativa. Tres grupos establecidos dependiendo del estado del margen quirúrgico proximal: margen negativo (R0), margen positivo con resección adicional para alcanzar margen negativo (R1-R0) y margen positivo (R1). Se analizaron variables demográficas y clínicas. El desenlace a evaluar fue recurrencia, sobrevida libre de enfermedad (SLE) y sobrevida global (SG).
ResultadosSe incluyeron 48 pacientes. Treinta y siete se clasificaron como R0, 9 como R1-R0 y 2 como R1. El 52% se encontraban en un estadio clínico III. Se encontró una mortalidad quirúrgica global de 2% y morbilidad mayor de 29%. La recurrencia local fue 0% en el grupo R1-R0 vs. 50% en el grupo R1 (p 0.02). La SLE fue 49 meses en el grupo R1-R0 vs. 32 meses en el grupo R1 (p 0,6). La SG fue de 51 meses para el grupo R1-R0 vs. 35 meses para el grupo R1 (p 0.5).
ConclusionesLa ampliación intraoperatoria del margen quirúrgico proximal positivo mejora la tasa de recurrencia local, pero no se asocia a una mejoría en la SG o SLE y podría aumentar la morbilidad posquirúrgica.
Around 28,000 new cases of gastric cancer are estimated to be diagnosed annually in the United States, with 10,960 gastric cancer-related deaths.1 Gastric cancer is the second cause of death by cancer in the world.2 In 2012, The World Health Organization calculated close to one million new cases of gastric cancer (6.8% of all malignant neoplasias), occupying fifth place, after lung cancer, breast cancer, colorectal cancer, and prostate cancer. More than 70% of cases occurred in cities of developed countries and half were in Asia.3
Surgical treatment, in combination with adjuvant or perioperative chemotherapy-radiotherapy, is the best option for providing patients with better survival, an approach that has been demonstrated particularly in patients with localized gastric cancer.4,5 Unfortunately, opportune diagnosis of patients with potentially resectable disease is not commonly made. It is estimated that two-thirds of the patients will be diagnosed in clinical stage III or IV disease, whereas only 10% will be diagnosed at an incipient clinical stage.6
It is well-known that curative treatment for gastric cancer is surgical resection with negative margins, accompanied by adequate lymphadenectomy (a minimum of 16 lymph nodes).7 The Japanese guidelines for gastric cancer treatment define an adequate margin for curative gastrectomy as: a) a proximal margin of fewer than 3cm for T2 tumors or those with an expansive growth pattern (types 1 and 2); b) 5cm for those with an infiltrative growth pattern (types 3 and 4); and c) intraoperative study of the margins for tumors with esophageal invasion to ensure R0 resection (a 5-cm margin is not necessarily required).8 Numerous studies have shown that leaving a microscopic margin with tumor cells, meaning R1 resection, is associated with worse outcome, especially in the comparison of cases of early gastric cancer versus late disease.9–11
Classically, it has been established that the presence of tumor cells in the proximal transection margin is the criterion for performing a more extensive surgical resection or not. Consequently, positive margins condition larger resections and different types of reconstructions that impact the risk of postoperative complications and affect patient quality of life.12 Few studies have evaluated the prognostic value of the intraoperative analysis of the proximal surgical margin and resection extension, if the study indicates there are tumor cells. Specifically, those studies suggest that patients with early-stage disease benefit from a larger resection when the intraoperative analysis reveals tumor cells in the transection margin, whereas extending those margins in the late stages of disease offers no additional benefit to the patient in relation to overall survival (OS).13–16 The attempt to achieve a negative proximal margin could lead to greater complexity in the reconstruction of the digestive tract and increased perioperative morbidity.
The aim of the present study was to determine the clinical usefulness of extending the proximal surgical margin in patients undergoing total gastrectomy due to gastric adenocarcinoma, based on their intraoperative histopathologic analysis.
Materials and methodsAll the patients that underwent total gastrectomy within the time frame of 2002 to 2017 were analyzed. For the definitive analysis, all patients diagnosed with gastric adenocarcinoma that underwent total curative gastrectomy were included. Two scenarios were identified during the surgical procedure. The first was the site of the esophageal transection and the tumor-free margin revealed by the intraoperative proximal margin study, and the second was the site of the proximal margin of the surgical specimen invaded by neoplastic cells. In that context, there was the option of extending the margins and achieving, or not, a negative margin. Thus, the patients were divided into three groups, with respect to the status of the margins in the intraoperative study: negative margins (R0), positive margins that became negative upon extending the resection (R1-R0), and positive margins (R1).
The statistical analysis was conducted using the IBM SPSS v. 20.0 program. The quantitative variables were analyzed through the ANOVA test and the chi-square test was employed for the qualitative variables. The OS and disease-free survival (DFS) were determined through Kaplan-Meier curves.
Given the study's retrospective design, it was exempt from review by the ethics committee and was conducted according to the principles established in the Declaration of Helsinki.
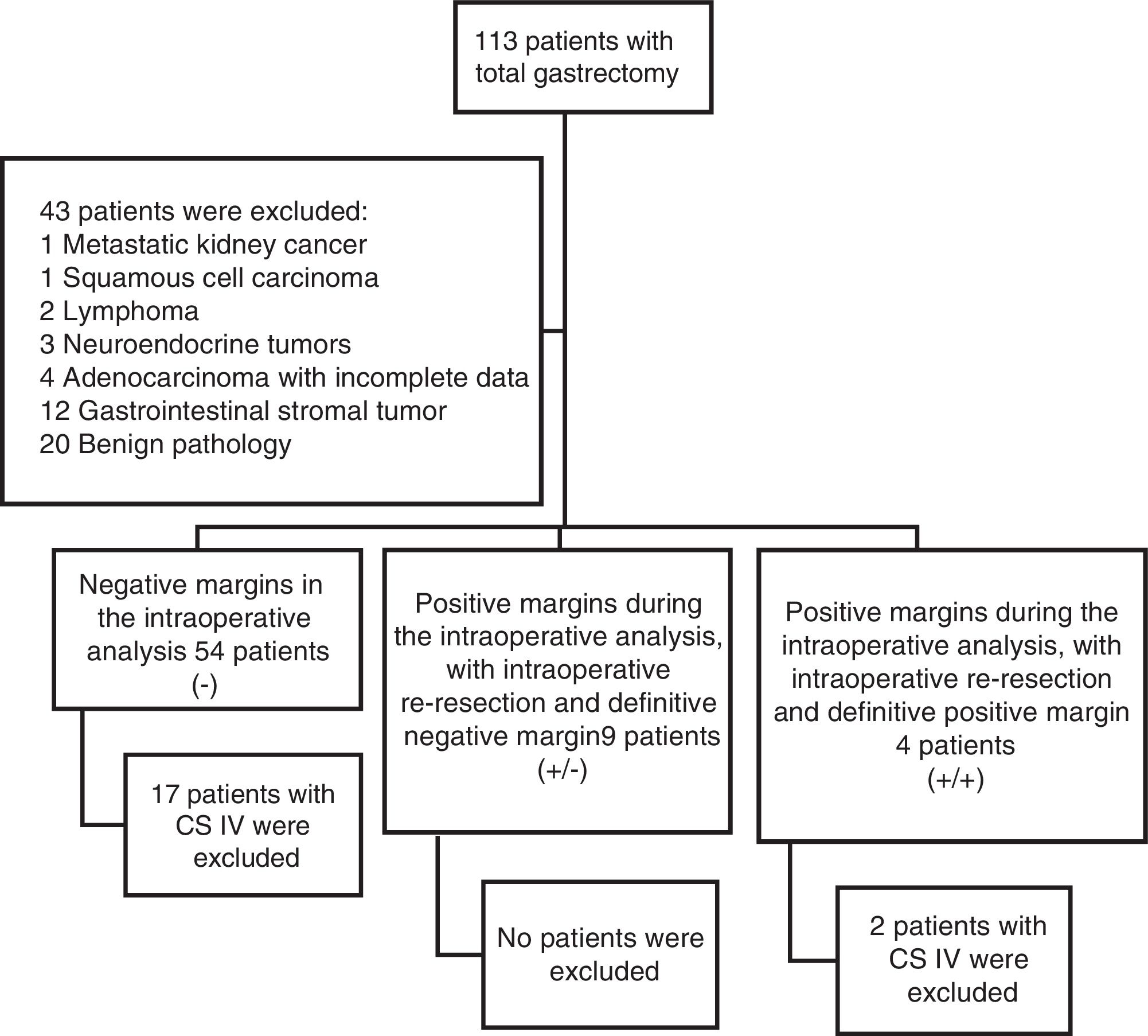
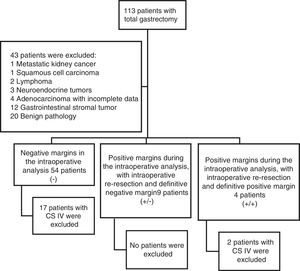
ResultsFigure 1 demonstrates the patient selection process in which 43 patients were initially excluded for the statistical analysis. A total of 67 patients were included in the study, 19 of whom were excluded because they had clinical stage IV disease, leaving a final sample of 48 patients. The negative proximal margin (R0) group was made up of 37 patients; 9 patients were in the group with a positive intraoperative margin that, when extended, resulted in a negative margin (R1-R0); and 2 patients were in the group with a positive surgical margin in the final specimen (R1).
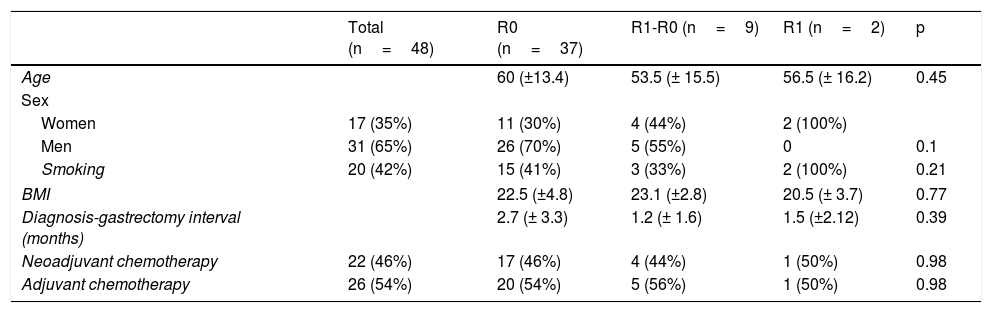
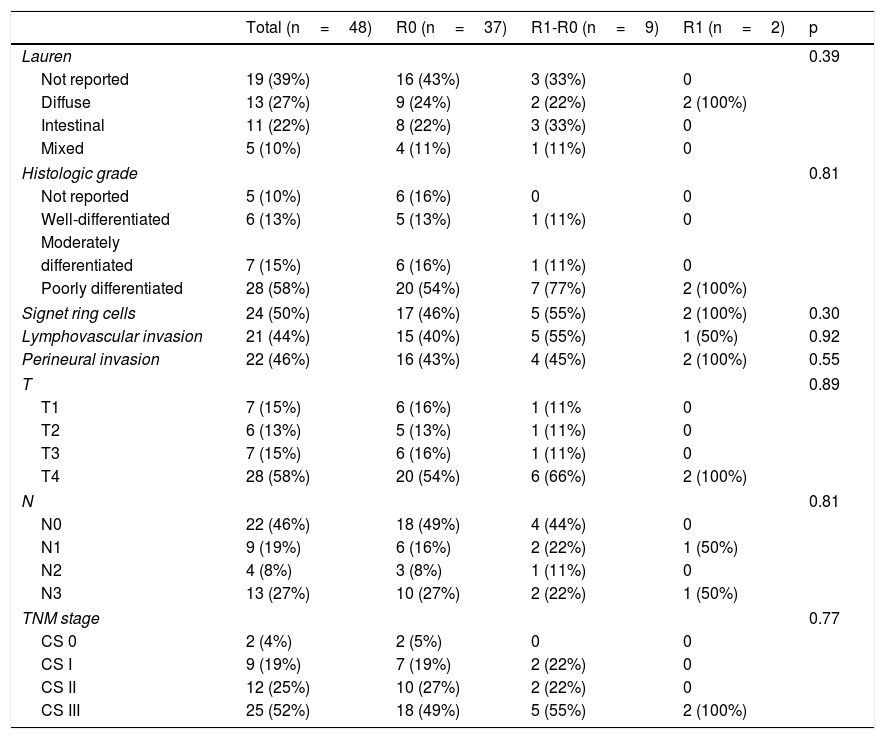
Of the total population, 35% of the patients were women and 65% were men. Forty-two percent of the patients were smokers. The interval between the diagnosis of gastric adenocarcinoma and the surgical procedure was 2.7 (± 3.3) months for the R0 group, 1.2 (± 1.6) months for the R1-R0 group, and 1.5 (±2.12) months for the R1 group. Table 1 shows the clinical characteristics of the patients.
Clinical characteristics of the patients that underwent total gastrectomy, stratified according to margin status (R0, R1-R0 or R1).
| Total (n=48) | R0 (n=37) | R1-R0 (n=9) | R1 (n=2) | p | |
|---|---|---|---|---|---|
| Age | 60 (±13.4) | 53.5 (± 15.5) | 56.5 (± 16.2) | 0.45 | |
| Sex | |||||
| Women | 17 (35%) | 11 (30%) | 4 (44%) | 2 (100%) | |
| Men | 31 (65%) | 26 (70%) | 5 (55%) | 0 | 0.1 |
| Smoking | 20 (42%) | 15 (41%) | 3 (33%) | 2 (100%) | 0.21 |
| BMI | 22.5 (±4.8) | 23.1 (±2.8) | 20.5 (± 3.7) | 0.77 | |
| Diagnosis-gastrectomy interval (months) | 2.7 (± 3.3) | 1.2 (± 1.6) | 1.5 (±2.12) | 0.39 | |
| Neoadjuvant chemotherapy | 22 (46%) | 17 (46%) | 4 (44%) | 1 (50%) | 0.98 |
| Adjuvant chemotherapy | 26 (54%) | 20 (54%) | 5 (56%) | 1 (50%) | 0.98 |
Clinical stage (CS) distribution of the patients was 4% for CS 0, 19% for CS I, 25% for CS II, and 52% for CS III. Those proportions remained the same upon stratifying the R0 and R1-R0 groups, whereas 100% of the patients in the R1 group had CS III disease.
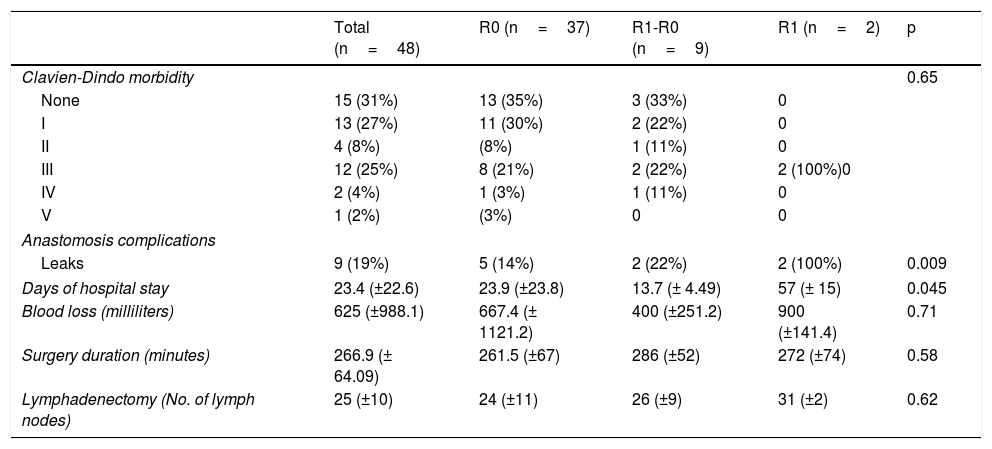
There was a 2% surgical mortality rate in the entire sample, with major morbidity (Clavien-Dindo III-IV) in 29% of the patients. In relation to tumor characteristics, the diffuse phenotype occurred in 27% of the total population, the intestinal type in 22%, and the mixed type in 10%, with no statistically significant difference between the three groups (p=0.39). The histologic grade in the majority of the tumors was poorly differentiated (58%) and was homogeneous upon group stratification (p=0.81).
The surgical procedures were performed by two oncology surgeons from the hospital where the study was conducted. In all the cases, the digestive tract was reconstructed through a Roux-en-Y esophageal-jejunal anastomosis. Circular stapling was employed for the esophageal-jejunal anastomosis and two-layer end-to-side manual suturing for the jejunal-jejunal anastomosis. Mean surgery duration was 266min. Blood loss was 667ml (±1121.2) for the R0 group, 400ml (± 251) for the R1-R0 group, and 900ml (±141) for the R1 group, with no statistical difference between the three groups (p=0.71). Tables 2 and 3 show the rest of the characteristics of the pathology analysis and the surgical procedure.
Characteristics of the pathology analysis in patients that underwent total gastrectomy stratified according to margin status (R0, R1-R0 or R1).
| Total (n=48) | R0 (n=37) | R1-R0 (n=9) | R1 (n=2) | p | |
|---|---|---|---|---|---|
| Lauren | 0.39 | ||||
| Not reported | 19 (39%) | 16 (43%) | 3 (33%) | 0 | |
| Diffuse | 13 (27%) | 9 (24%) | 2 (22%) | 2 (100%) | |
| Intestinal | 11 (22%) | 8 (22%) | 3 (33%) | 0 | |
| Mixed | 5 (10%) | 4 (11%) | 1 (11%) | 0 | |
| Histologic grade | 0.81 | ||||
| Not reported | 5 (10%) | 6 (16%) | 0 | 0 | |
| Well-differentiated | 6 (13%) | 5 (13%) | 1 (11%) | 0 | |
| Moderately | |||||
| differentiated | 7 (15%) | 6 (16%) | 1 (11%) | 0 | |
| Poorly differentiated | 28 (58%) | 20 (54%) | 7 (77%) | 2 (100%) | |
| Signet ring cells | 24 (50%) | 17 (46%) | 5 (55%) | 2 (100%) | 0.30 |
| Lymphovascular invasion | 21 (44%) | 15 (40%) | 5 (55%) | 1 (50%) | 0.92 |
| Perineural invasion | 22 (46%) | 16 (43%) | 4 (45%) | 2 (100%) | 0.55 |
| T | 0.89 | ||||
| T1 | 7 (15%) | 6 (16%) | 1 (11% | 0 | |
| T2 | 6 (13%) | 5 (13%) | 1 (11%) | 0 | |
| T3 | 7 (15%) | 6 (16%) | 1 (11%) | 0 | |
| T4 | 28 (58%) | 20 (54%) | 6 (66%) | 2 (100%) | |
| N | 0.81 | ||||
| N0 | 22 (46%) | 18 (49%) | 4 (44%) | 0 | |
| N1 | 9 (19%) | 6 (16%) | 2 (22%) | 1 (50%) | |
| N2 | 4 (8%) | 3 (8%) | 1 (11%) | 0 | |
| N3 | 13 (27%) | 10 (27%) | 2 (22%) | 1 (50%) | |
| TNM stage | 0.77 | ||||
| CS 0 | 2 (4%) | 2 (5%) | 0 | 0 | |
| CS I | 9 (19%) | 7 (19%) | 2 (22%) | 0 | |
| CS II | 12 (25%) | 10 (27%) | 2 (22%) | 0 | |
| CS III | 25 (52%) | 18 (49%) | 5 (55%) | 2 (100%) | |
CS: clinical stage.
Operation variables according to margin status (R0, R1-R0 or R1).
| Total (n=48) | R0 (n=37) | R1-R0 (n=9) | R1 (n=2) | p | |
|---|---|---|---|---|---|
| Clavien-Dindo morbidity | 0.65 | ||||
| None | 15 (31%) | 13 (35%) | 3 (33%) | 0 | |
| I | 13 (27%) | 11 (30%) | 2 (22%) | 0 | |
| II | 4 (8%) | (8%) | 1 (11%) | 0 | |
| III | 12 (25%) | 8 (21%) | 2 (22%) | 2 (100%)0 | |
| IV | 2 (4%) | 1 (3%) | 1 (11%) | 0 | |
| V | 1 (2%) | (3%) | 0 | 0 | |
| Anastomosis complications | |||||
| Leaks | 9 (19%) | 5 (14%) | 2 (22%) | 2 (100%) | 0.009 |
| Days of hospital stay | 23.4 (±22.6) | 23.9 (±23.8) | 13.7 (± 4.49) | 57 (± 15) | 0.045 |
| Blood loss (milliliters) | 625 (±988.1) | 667.4 (± 1121.2) | 400 (±251.2) | 900 (±141.4) | 0.71 |
| Surgery duration (minutes) | 266.9 (± 64.09) | 261.5 (±67) | 286 (±52) | 272 (±74) | 0.58 |
| Lymphadenectomy (No. of lymph nodes) | 25 (±10) | 24 (±11) | 26 (±9) | 31 (±2) | 0.62 |
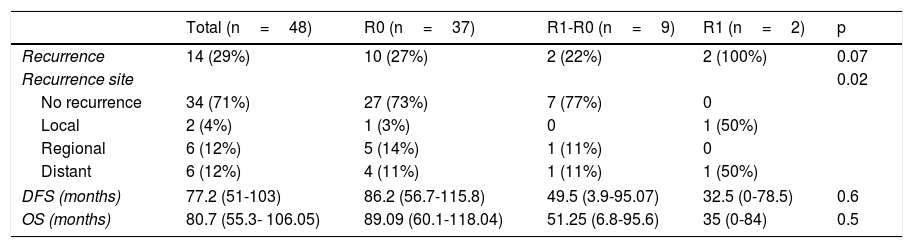
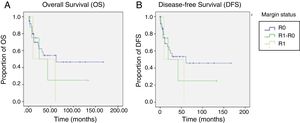
Regarding OS, DFS, and recurrence (Table 4), we found an overall recurrence of 29%. Upon analyzing the data per group, recurrence was 27% in the patients with R0 surgical margins, 100% in the patients with the R1 surgical margins, and 22% in those with the R1-R0 margins (p=0.07). To report recurrence, it was classified by site as local (defined as esophageal-jejunal anastomosis), regional (defined as lymph nodes or perianastomotic lesions), and distant (defined as liver, brain, or lung metastases or peritoneal carcinomatosis). The R1 group had the highest percentage of local recurrence and distant recurrence, with 50% at each of those sites.
Analysis of OS and DFS of the patients that underwent total gastrectomy stratified by margin status (R0, R1-R0 or R1).
| Total (n=48) | R0 (n=37) | R1-R0 (n=9) | R1 (n=2) | p | |
|---|---|---|---|---|---|
| Recurrence | 14 (29%) | 10 (27%) | 2 (22%) | 2 (100%) | 0.07 |
| Recurrence site | 0.02 | ||||
| No recurrence | 34 (71%) | 27 (73%) | 7 (77%) | 0 | |
| Local | 2 (4%) | 1 (3%) | 0 | 1 (50%) | |
| Regional | 6 (12%) | 5 (14%) | 1 (11%) | 0 | |
| Distant | 6 (12%) | 4 (11%) | 1 (11%) | 1 (50%) | |
| DFS (months) | 77.2 (51-103) | 86.2 (56.7-115.8) | 49.5 (3.9-95.07) | 32.5 (0-78.5) | 0.6 |
| OS (months) | 80.7 (55.3- 106.05) | 89.09 (60.1-118.04) | 51.25 (6.8-95.6) | 35 (0-84) | 0.5 |
DFS: disease-free survival; OS: overall survival.
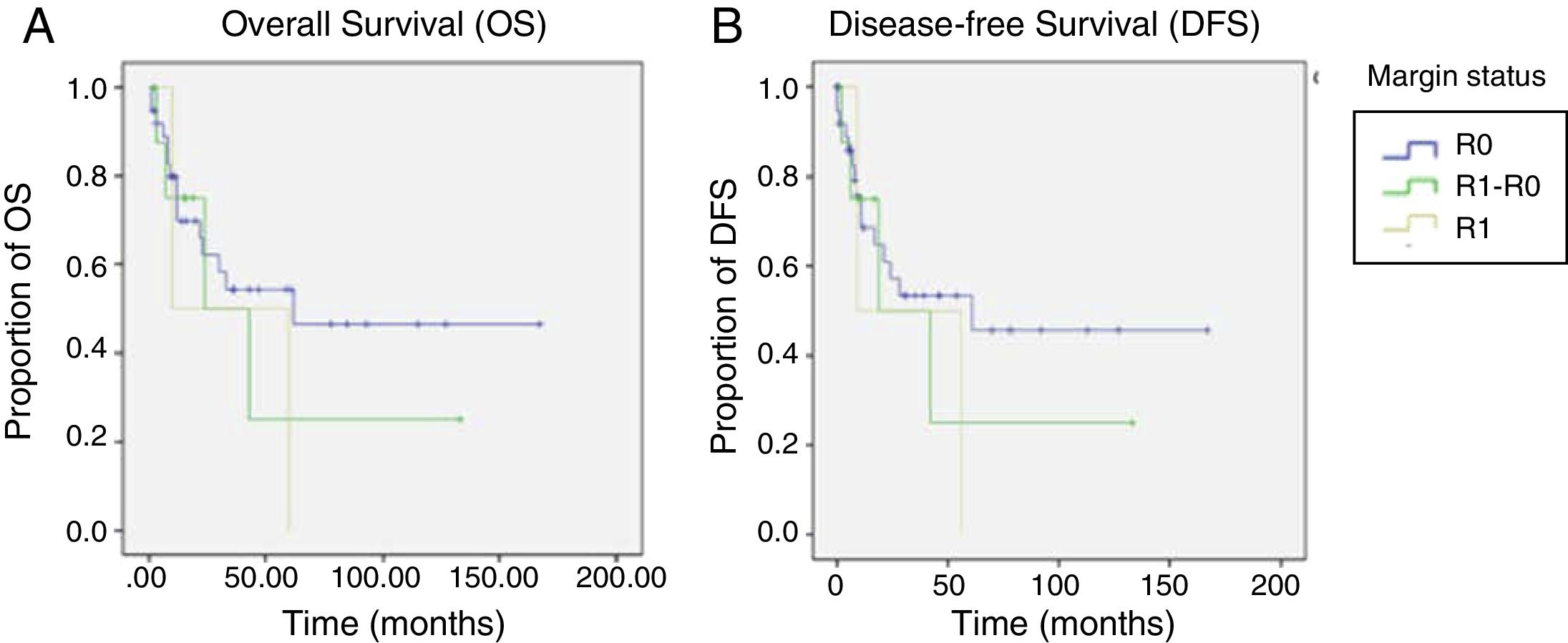
In general, the mean DFS was 77 months. It was 86.2 months for the R0 group, 49.5 months for the R1-R0 group, and 32.5 months for the R1 group (p=0.6). General OS was 80.7 months. It was 89.09 months for the R0 group, 52.25 months for the R1-R0 group, and 35 months for the R1 group (p=0.5) (Table 4 and figure 2).
Discussion and conclusionsThe present analysis is one of the few studies that evaluates the clinical implication of proximal margin status in patients that underwent total gastrectomy due to gastric adenocarcinoma. In 2014, Squires et al.13 analyzed the usefulness of the intraoperative study of the proximal surgical margin in patients that underwent gastrectomy due to gastric cancer, including distal, subtotal, and total gastrectomies. Of the 520 patients included in that study, 288 (44%) underwent total gastrectomy. It should be pointed out that the technical difficulty involved in extending the proximal surgical margin during subtotal or distal gastrectomy is much lower than extending the proximal surgical margin during total gastrectomy. Thus, in the present study, we decided to include only patients that underwent total gastrectomy.
Nine of the 11 patients with a positive surgical margin were successfully converted to R0 status through additional intraoperative resection, bringing the final positive surgical margin rate to 4.1% (n=2), comparable to that reported in other studies.9,10,13,17
Survival compared between the R0 group and the R1 group was 89 months vs. 35 months, respectively (p=0.25). In contrast, OS compared between the R1-R0 group and the R1 group was 51 months vs. 35 months, respectively (p=0.82). Even though those results were not statistically significant, most likely because of the sample size, the clinical inference is that performing a more extended proximal margin resection does not influence OS in patients that undergo total gastrectomy due to gastric adenocarcinoma.
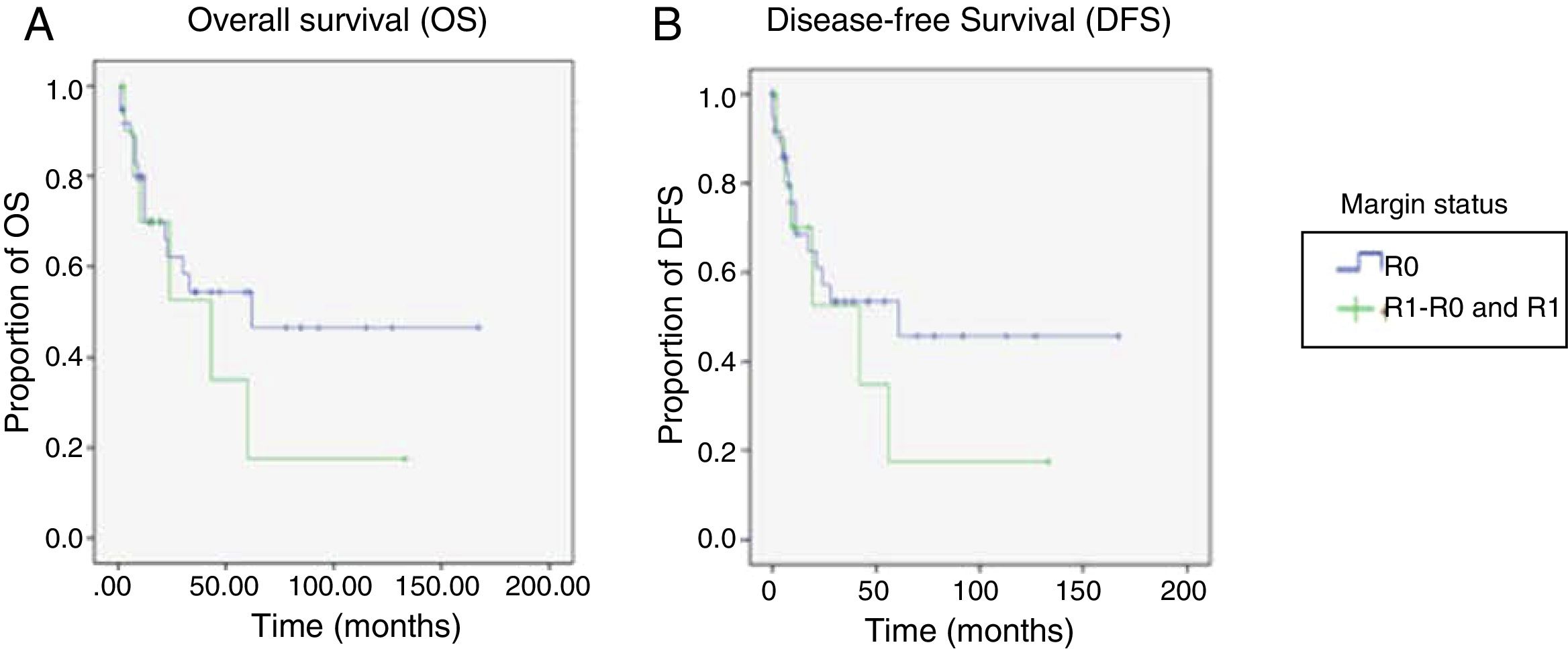
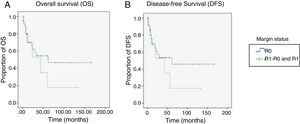
For the OS and DFS comparison, the R1-R0 and R1 patient groups were combined and compared with the R0 group, resulting in an OS of 89 months (60.1-118.04) for the R0 group vs. 47 months (14.68-80.32) for the combined R1-R0 and R1 groups (p=0.37) (Figure 3A). DFS was 86.2 months (56.7-115.8) for the R0 group vs. 45.4 months (11.9-78.9) for the combined R1-R0 and R1 groups (p=0.43) (Figure 3B).
The overall recurrence rate of 28% was similar to the 30% reported by Squires et al. Local recurrence was 3% for the R0 group, 0% for the R1-R0 group, and 50% for the R1 group (p=0.02). Those data suggest that extending the proximal surgical margin improved the local recurrence rate.
Finally, in analyzing the major morbidity in the groups (Clavien-Dindo III, IV, or V), it was 27% in the R0 group, whereas it was 45% in the combined R1-R0 and R1 groups (p=0.46). Itemizing the specific complications of the esophageal-jejunal anastomosis, the incidence of leaks was 19%, being higher in the R1 group (100%) and the R1-R0 group (22%), compared with the R0 group (15%) (p=0.009). In the patient follow-up, the incidence of anastomotic stricture was 14% in general, with no significant difference between the three groups. We did not identify any significant differences in relation to surgery duration, blood loss, or number of lymph nodes harvested.
In conclusion, the intraoperative analysis of the proximal surgical margin in patients that underwent total gastrectomy due to gastric adenocarcinoma was a useful tool for guiding the oncology surgeon during the operation. The present study found no improvement in OS in the patient groups, but there was a trend towards an increase in major morbidity. Additional resection of the proximal surgical margin to achieve negative surgical margins was associated with a decrease in the local recurrence rate. The sample size of the study limited its statistical power and we suggest conducting a study with a larger number of cases and adequate stage stratification to define the true value of extending positive proximal margins. Therapeutic conduct should be cautious with respect to the intraoperative status of the proximal margin in gastrectomized patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this study/article.
Authorship/collaboratorsAll the authors contributed equally to the realization of the present study, which included writing the manuscript, editing the images, discussing the case, and commenting on all stages of the manuscript.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Clemente-Gutiérrez U, Sánchez-Morales G, Santes O, Medina-Franco H. Utilidad clínica de la ampliación del margen proximal en gastrectomías totales por adenocarcinoma gástrico. Revista de Gastroenterología de México. 2019;84:136–142.