The performance of gastroenteric anastomosis (GEA) utilizing endoscopic ultrasound (EUS) and lumen-apposing metal stents (LAMSs) is safe and effective for treating malignant gastric outlet obstruction, but not in benign disease, due to unpredictable GEA closure after LAMS removal. Our aim was to evaluate different endoscopic techniques for creating a durable GEA in porcine models.
Material and methodsAn animal study in porcine models was conducted at the vivarium of a tertiary care hospital in Mexico City, between September and November 2023. Five techniques were carried out: direct technique (DT), radial cut (RC) technique, linear cut (LC) technique, absolute ethanol sclerotherapy (AES), and argon plasma coagulation (APC). Technical efficacy, safety, and lasting patency of the anastomosis at 4 weeks after the intervention and LAMS removal were evaluated.
ResultsTen porcine models, 2 per group, were included. Technical success was 100% and clinical success 0%. Procedure times were 29 min for the DT, 88 min for the RC technique, 74 min for the LC technique, 41 min for AES, and 75 min for APC. The RC technique had the largest anastomosis area (742 mm2). There was one adverse event (10%); it was mild and did not require any additional intervention.
ConclusionsAlthough clinical success was not achieved with any of the techniques, the technical modifications were safe, providing a better understanding of the mechanisms involved in GEA and paving the way for new explorations.
La realización de una gastroenteroanastomosis (GEA) utilizando ultrasonido endoscópico (USE) y prótesis metálicas de aposición luminal (PMAL) es segura y efectiva para el tratamiento de obstrucción al tracto de salida gástrico de origen maligno, pero no en patología benigna debido al impredecible cierre posterior al retiro de la PMAL, justificando la investigación en estos pacientes. Nuestro objetivo fue evaluar distintas técnicas endoscópicas para crear una GEA permanente en modelos porcinos.
Material y métodosSe trato de un estudio de investigación animal realizado en modelos porcinos en el bioterio de un hospital de tercer nivel de atención en México entre septiembre y noviembre 2023. Se realizaron 5 técnicas: técnica directa (TD), cortes radiales (CR), cortes lineales (CL), escleroterapia con alcohol absoluto (EAA) y ablación con argón plasma (AAP). Se evaluó la eficacia técnica, seguridad y persistencia de la anastomosis a 4 semanas de la intervención y retiro de la PMAL.
ResultadosSe incluyeron 10 modelos porcinos, 2 por grupo. El éxito técnico y clínico de 100% y 0%. Los tiempos de los procedimientos fueron: TD = 29 min; CR = 88 min; CL = 74 min; EAA = 41 min y AAP = 75 min; y la técnica CR presentó la mayor área de anastomosis (742 mm2). Hubo un evento adverso (10%) el cual fue leve y no requirió intervención adicional.
ConclusionesAunque el éxito clínico no se alcanzó con ninguna técnica, las modificaciones fueron seguras y permitieron entender los mecanismos de creación de la GEA, abriendo la posibilidad para nuevas exploraciones.
Endoscopic ultrasound (EUS)-guided gastroenteric anastomoses (GEAs), with the use of lumen-apposing metal stents (LAMSs), have been shown to be technically efficacious for treating conditions, such as malignant gastric outlet obstruction (MGOO), or for creating accesses for interventionist procedures, such as endoscopic retrograde cholangiopancreatography in patients with modified anatomies, with a technical success rate of 93.5% (95% CI 89.7–96%) and clinical efficacy of 90.1% (95% CI 85.5–93.4%).1 Adverse events are reported at 11.7% (95% CI 8.2–16.6%), most of which are mild or moderate.1
Perez Miranda et al. compared GEA with the LAMS technique versus the laparoscopic technique for patients with MGOO. They found no difference in technical success (88 vs 100%; p = 0.11) or clinical success (84 vs 90%; p = 0.11); but found differences of higher adverse event and cost rates for the laparoscopic group (12 vs 41%; p = 0.038 and $4515 USD vs $14,778 USD; p < 0.00001, respectively).2 Khashab et al. reported a higher technical success rate in the surgical group (87 vs 100%; p = 0.009), similar clinical efficacy (87 vs 90%; p = 0.18), and similar adverse events (16 vs 25%; p = 0.3).3
Only a few works include the evaluation of LAMS for GEA creation in benign disease, including some case reports, with no formal long-term evaluation, in which causes include stricture associated with gastric volvulus, peptic ulcers, refractory pyloric strictures, Crohn’s disease, postoperative strictures, and refractory gastroparesis.4–6 In addition, persistent patency of the anastomosis is unpredictable, once the LAMS is removed. In that regard, Krafft et al. evaluated spontaneous or secondary intention closures of anastomoses created using 20 mm LAMSs, after their removal, in patients with gastric bypass. They found persistent fistula patency in 41% of the patients during the testing time, 56% of whom presented with significant weight gain. There was a difference in median LAMS dwell time between the persistent fistula group and those with durable spontaneous fistula closure after LAMS removal (77 days; IQR 42–124 vs 35 days; IQR 26–45 days).7
There are several proinflammatory factors present during the initial phase of the surgical creation of a GEA that directly relate its stability to the surgical mechanism utilized. The proinflammatory factors include collagenases and metalloproteins that weaken adhesion, before the formation of new collagen by fibroblasts and smooth muscle cells of the muscularis mucosa and the muscularis propria. Thus, the anastomosis withstands only 50% of the intraluminal pressure in the first 2–3 days but then resists 100% at 7 days after the GEA. The formation and durability of the GEA depends on local (tissue perfusion, anastomotic tension, adequate apposition of the edges, absence of local infection, etc.) and systemic (malnutrition, hypovolemia, medications, sepsis, immunodeficiencies, or diabetes mellitus) factors.8 However, the mechanism of EUS-guided GEA is different, because by using a LAMS, specifically the Hot AXIOS Stent (Boston Scientific, Marlborough, Massachusetts), a direct monopolar coagulation-assisted puncture is made, to connect the two walls, through which a 10.8 Fr caliber probe is passed for stent placement. The stent is then deployed, and the two walls expand to the designated diameter size. The full-thickness cut in mechanical suturing or with surgical staples used in conventional surgery is avoided. Circular (21–33 mm) or linear (30–60 mm) anastomoses are obtained, restructuring the gastric and enteral walls, favoring low tension between the perianastomotic tissues.9
Thus, the creation of a EUS-guided GEA with a LAMS for patients with benign disease depends on the patency of the GEA once the LAMS is removed, and so the study of modifications to the original technique is needed. Our aim was to carry out a pilot exploratory project to evaluate 4 modifications of the original technique and a control group, in biologic models using live pigs, in an effort to produce a durable GEA.
Material and methodsA clinical trial was conducted, utilizing an experimental, exploratory, prospective, comparative, and longitudinal pilot model, on porcine animal models, in vivo, according to the CONSORT guidelines for clinical trials. This study was developed at the vivarium of a tertiary care hospital between September and November of 2023. The procedures were performed, complying with the institutional statutes and norms for the adequate management of animal models.
Inclusion criteriaTen female Landrace porcine models were included. The pigs were between 6 and 10 weeks old and weighed between 24 and 45 kg. All the models were certified as healthy by a veterinarian from the Mexican Health Commission.
Exclusion criteriaAll models that could not complete the correct preparation for the GEA, that had complications during anesthesia or that were not attributable to the procedure, and those that could not complete the follow-up time were excluded. If such were the case, they would be replaced by other models, so as not to compromise the contemplated sample size.
Equipment utilizedThe following equipment was utilized: a 5/7.5/10/12 MHz multifrequency linear echoendoscope, model EG-580UT (Fujifilm, Tokyo, Japan), with a 3.8 mm working channel; a conventional gastroscope, model EG-530FP (Fujifilm, Tokyo, Japan), coupled with a video processor and light source, Eluxeo 7000 (Fujifilm, Tokyo, Japan) model, and an SU-1 (Fujifilm, Tokyo, Japan) endoscopic ultrasound processor. An ERBE VIO® 300D (ERBE, Tübingen, Germany) electrosurgical unit was used, with the monopolar highcut mode at 120 W cutting power in effect 1. The materials for creating the GEA were: a 20 mm diameter LAMS (Hot AXIOS™) (Boston Scientific, Marlborough, Massachusetts), which is a tubular stent in the shape of a “yo-yo”, made of nitinol and completely covered in silicon; an I-type HybridKnife® (ERBE, Tübingen, Germany); Resolution™ hemoclips (Boston Scientific, Marlborough, Massachusetts); a 23 G or 25 G Interject™ injection needle (Boston Scientific, Marlborough, Massachusetts); an 18−20 mm CRE™ radial expansion balloon (Boston Scientific, Marlborough, Massachusetts); a 20 to 30-watt SoftCoag coagrasper forceps, effect 2 (Olympus, Tokyo, Japan), in case of bleeding, and an argon plasma APC® 2 unit coupled with a FiAPC® Axial probe (ERBE, Tübingen, Germany) in the forced argon plasma coagulopathy (APC) modality at 50 W.
In vivo modelsThe 10 live porcine models were acquired from a certified supplier (SCR), and before their inclusion in the study, they were clinically evaluated by hospital veterinary personnel, in accordance with the abovementioned norms, to ensure they met the established health standards for these types of protocols in the vivarium. The porcine models were treated with care, ensuring the safety of the researchers, and avoiding stress in the animals at all times. Management before, during, and after the intervention was carried out according to the NOM-062-ZOO-1999.
Prior to receiving anesthesia, all the animals underwent a 24 h fasting period, sufficient for emptying the stomach, to prevent regurgitation or aspiration of the gastric content. Pre-medication was started with intramuscular ketamine at 15 mg/kg, after which the animals were washed with antiseptic soap and then taken to the operating room. Atrial cannulation was carried out and midazolam (5 mg/kg in bolus and then 2 mg every 10–15 min, dose-response, for maintenance) was administered. Orotracheal intubation was carried out with 5.5–6.5 mm cannulas and intraoperative anesthesia was provided by a veterinarian at all times. When the procedure was finished, the porcine model was transferred to a special area with a 28 m2 concrete surface that was adequately equipped for correct postoperative surveillance. The pigs were kept in a fasting state and in observation for 4 h, after which they were transferred to a resting and surveillance area, where they remained until their next intervention.
A conventional diet for this type of model was started at 24 h and consisted of a mixture of 16% raw protein, 6% raw fiber, 3.5% lipids, and the rest in carbohydrates per ration, with water ad libitum. Analgesics authorized by the National Health, Safety, and Agri-Food Quality Service were given to the animals for 3 days and extended only if indicated by the veterinarian. Daily clinical assessment was carried out during the entire evolution period, and if any adverse event was suspected, the model underwent endoscopic or surgical examination, given that we had no access to biochemical or imaging studies. If the integrity of the animal was compromised or there were incapacitating sequelae, the veterinarian euthanized the model with intravenous sodium pentobarbital at a dose of 200 mg/kg, in compliance with the NOM-062-ZOO-1999.
Once the protocol was completed, the corresponding euthanasia was carried out, to evaluate the characteristics of the anastomoses created, their arrangement, caliber, and perianastomotic adverse events. The waste material, cadavers, and organic material were disposed of in accordance with the NOM-087-ECOL-SSA1-2002 and the Ecologic Balance and Environmental Protection General Law.
ProceduresThe conventional technique for constructing a GEA was considered the control group and 4 new techniques were analyzed. The procedures were photo-documented and recorded. The final evaluation was made after euthanasia, carried out one month after the techniques were performed.
A. Technique 1 “Conventional gastroenteric anastomosis using the direct technique”The procedure was performed on porcine models 1 (A) and 2 (B).
Basis: It is a proven technique, with acceptable effectiveness and adverse events for enteral route palliation.1 The main disadvantage is that anastomosis permanency and caliber are unpredictable.4
- •
A.1. Hot AXIOS® with the direct technique (DT) (day 1): After washing and preparing the animal model, it was transferred to the operating room. Anesthesia was induced and antibiotic prophylaxis with enrofloxacin at 10% (100 mg/1 ml) at a dose of 2.5 mg/kg was administered. Gastric echo exploration was carried out and injectable water was instilled at the post-pyloric level for adequate segment distension. The site was checked and direct puncture with a 20 mm diameter Hot AXIOS™ stent, with AutoCut monopolar current at 120 W, was carried out. The stent was deployed, adequate placement was confirmed, and the CRE radial expansion ballon was dilated to 18 mm. The porcine model was transferred from the operating room and placed under surveillance with an analgesic regimen of flunixin meglumine 50 mg/1 ml at a dose of 2.2 mg/kg for 3 days. A 24 h total fast was programmed after which the abovementioned oral diet was started.
- •
A.2. LAMS removal (day 30): After the operating room admission protocol was carried out the GEA was checked via endoscopy for later LAMS removal with a foreign body forceps. The GEA was evaluated, and the model was transferred from the operating room, maintaining fasting and observation for 4 h, after which the oral diet was started.
- •
A.3. Final evaluation (day 60): After the operating room admission protocol was carried out, the GEA site was checked and the animal was correspondingly euthanized, to perform the laparotomy and peritoneal cavity inspection, and finally, the extraction of the gastroenteric specimen of interest. The biologic remnants were managed according to the NOM-087-ECOL-SSA1-2002.
The procedure was performed on porcine models 3 (C) and 7 (G).
Basis: In this modified technique, 1 cm radial cuts (RCs) were made to create a full-thickness cut of the gastric and enteral layers, potentially enabling better quality perianastomotic remodelling.7,8
- •
B.1. Hot AXIOS® (day 1): The stent was placed, and the model was transferred to the surveillance area, the same as the control model.
- •
B.2. Performance of the RC technique (day 15): The maneuver was carried out 15 days after stent placement because that is the expected time for GEA formation. After the operating room admission protocol, the LAMS was removed and the area of the GEA examined. Four anastomotic circumference points were identified at the gastric level and RCs 10 mm in length were started on the gastric and enteral walls with the I-type HybridKnife® with the following parameters: EndoCut I, effect 1, cut duration 3, and cut interval 3. Resolution™ hemoclips, with an 11 mm opening, were placed parallelly at the selected edge, repeating the process radially at the 4 edges. The LAMS was endoscopically placed once again, as protection against any type of later perforation, corroborating adequate positioning and hemostasis. The anesthesia was reversed, and the corresponding transfer protocol was carried out.
- •
B.3. LAMS removal (day 30): After the operating room admission protocol, the LAMS was removed and the new GEA was examined again, evaluating the diameters achieved. The corresponding transfer protocol was then carried out.
- •
B.4. Final evaluation (day 60): The protocols for laparotomy, peritoneal cavity exploration, extraction of the gastroenteric specimen of interest, and the corresponding euthanasia were the same as with the DT.
The procedure was performed on porcine models 4 (D) and 9 (I).
Basis: As with the RCs, the goal was to carry out 2 full-thickness linear cuts (LCs) to widen the diameter of the anastomosis and improve the anastomotic remodelling.7,8
- •
C.1. Hot AXIOS® (day 1): The LAMS was conventionally placed using the DT and the model was transferred to the surveillance area.
- •
C.2. Performance of the LC technique (day 15): After the operating room admission protocol, the LAMS was removed and the GEA evaluated. Two transmural LCs were made with the I-type HybridKnife® Tipo I and the parameters: EndoCut I, effect 1, cut duration 3, cut interval 3. As with the RCs, a pair of clips were placed parallelly at the selected edge. A protection LAMS was put in place and the transfer protocol carried out.
- •
C.3. LAMS removal (day 30): The LAMS was removed, the GEA examined with its corresponding measurements, and the transfer protocol was carried out.
- •
C.4. Final evaluation (day 60): The protocols for laparotomy, peritoneal cavity exploration, extraction of the gastroenteric specimen of interest, and the corresponding euthanasia were carried out.
The procedure was performed on porcine models 8 (H) and 10 (J).
Basis: The aim of this technique was the injection of absolute ethanol into the tissue adjacent to the stent circumference, with the alcohol producing inflammation, tissue destruction, irreversible fibrosis, and a decrease in tissue mass due to retraction.9,10 The goal was to control the fibrosis around the LAMS, which acted as a support, maintaining lumen apposition, while the surrounding tissue was restructured.
- •
D.1. Hot AXIOS® and absolute ethanol sclerotherapy (AES) application (day 1): After LAMS placement using the DT, 4 points circumferential to the stent were identified and injection of 1.5−2 ml of ethanol at 98% with a 23 G Interject™ needle was started. The body of the stent was then dilated to 18 mm, adequate positioning and hemostasis were corroborated, and the porcine model was transferred.
- •
D.2. LAMS removal (day 30): The LAMS was removed and the GEA evaluated with the corresponding measurements. The porcine model was transferred to the surveillance area.
- •
D.3. Final evaluation (day 60): The protocols for laparotomy, peritoneal cavity exploration, extraction of the gastroenteric specimen of interest, and the corresponding euthanasia were carried out.
The procedure was performed on porcine models 5 (E) and 6 (F).
Basis: The aim was to produce tissue damage and fibrosis of the GEA to create controlled remodeling.11
- •
E.1. Hot AXIOS® (day 1): After the operating room admission protocol, the LAMS was placed using the DT.
- •
E.2. Argon plasma application (day 15): The LAMS was removed, and the argon plasma was applied using the APC® 2 unit and the FiAPC® Axial probe, in forced mode, at 50 W, 1 l/min, in the circumference of the GEA. This was done to obtain the golden tone of the surrounding tissue, extending to 5 mm. The LAMS was placed again, and the transfer protocol was carried out.
- •
E.3. LAMS removal (day 30): The LAMS was removed, the GEA and corresponding measurements were evaluated, and the porcine model was transferred.
- •
E.4. Final evaluation (day 60): The protocols for laparotomy, peritoneal cavity exploration, extraction of the gastroenteric specimen of interest, and the corresponding euthanasia were carried out.
The primary aim was to evaluate the potential creation of a durably patent GEA through the use of 4 experimental techniques and a control group. Clinical success was defined as the persistent patency of the anastomosis in the final porcine model evaluation (day 60). The secondary aim was to evaluate the safety and technical success of each of the techniques carried out.
Statistical analysisWe employed convenience sampling, based on the probability of GEA patency with one of the 4 maneuvers evaluated (25%), derived from previous studies on short-term and medium-term GEA patency in benign disease that varied from 20 to 41%4–7 and the reported 93.5% technical efficacy of the GEA with LAMS,1 resulting in our sample size of 10.4 (10 models). A validated statistics program (EpiInfo, USA) was utilized. The general characteristics of the models and interventions, including technical success, clinical success, and adverse events, were documented. Descriptive statistics were carried out, expressing the quantitative variables as means and the qualitative variables as percentages.
ResultsA total of 32 endoscopic interventions were performed on 10 porcine models in vivo, within the time frame of September and November 2023. All the models were females, with a mean age of 6.9 weeks and mean weight of 23.8 kg (Table 1).
General characteristics of the models, procedures, and anastomoses created.
| Porcine model | Age (weeks) | Weight (kg) | Procedure time (min) | Technique utilized | Short GEA axis upon LAMS removal (mm) | Long GEA axis upon LAMS removal (mm) | Anastomosis area (mm2) | Technical success | Clinical success | Adverse events | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 24.3 | 32 | DT | 13.3 | 19.7 | 205.7 | YES | NO | Type I misdeployment | NO |
| 2 | 6 | 22.6 | 26 | DT | 14.7 | 18.4 | 212.4 | YES | NO | NO | NO |
| 3 | 5 | 22.5 | 98 | RC | 27.6 | 35.9 | 778.2 | YES | NO | NO | NO |
| 4 | 8 | 25.3 | 85 | LC | 19.8 | 31.3 | 486.8 | YES | NO | NO | NO |
| 5 | 7 | 25.8 | 81 | APC | 20.3 | 24.1 | 383.1 | YES | NO | NO | NO |
| 6 | 9 | 24.6 | 69 | APC | 19.8 | 21.9 | 340.2 | YES | NO | NO | NO |
| 7 | 7 | 21.7 | 78 | RC | 26.4 | 34.1 | 707.0 | YES | NO | NO | NO |
| 8 | 6 | 24.3 | 45 | AES | 17.4 | 22.3 | 303.2 | YES | NO | NO | NO |
| 9 | 9 | 23.8 | 63 | LC | 20.2 | 29.7 | 471.2 | YES | NO | NO | NO |
| 10 | 7 | 23.5 | 38 | AES | 16.7 | 19.7 | 258.3 | YES | NO | NO | NO |
AES: absolute ethanol sclerotherapy; APC: argon plasma coagulation; DT: direct technique; GEA: gastroenteric anastomosis; LAMS: lumen-apposing metal stent; LC: linear cut; RC: radial cut.
Mean procedure duration registered according to the technique implemented was as follows: DT 29 min; RC 88 min; LC 74 min; AES 41 min; and APC 75 min. DT was the fastest and the procedures with the RC technique were the longest. Technical success was achieved in 100% of the models, but clinical success was 0%, even in the control group (DT). Nevertheless, oral feeding was achieved in all the models 24 h after each of the interventions (Table 1).
The GEA was evaluated in all the models on day 15 (RC, LC, and APC) and day 30 (DT and AES). This schedule was due to the fact that there was no need to perform an initial temporary GEA after LAMS placement with the DT and AES, unlike the other techniques, in which first a temporary GEA was performed, the time at which the corresponding maneuver (RC, LC, and APC) was carried out. The LAMS was then placed again, as a support, and 15 days later was definitively removed. In all the porcine models, the LAMS was removed on day 30 and the anastomosis evaluated, finding that the greatest distance on the short axis was in the models with the RC technique, with a mean of 27 mm, and the shortest distance was with the DT, with 14 mm. On the long axis, the greatest distance was for the RC, with 35 mm, and the shortest was with the DT, with 19 mm. This was reflected in the mean total area of the anastomosis, which was greater with the RC technique (742 mm2) and the smallest with the DT (209 mm2) (Table 1, Fig. 1).
There was only one minor adverse event, which was LAMS misdeployment (type I).12 It was adequately resolved by first extracting the stent and then carrying out primary closure of the gastric perforation with an over-the-scope clip (OTSC, type “a”; Tübingen, Germany). Once the closure of the gastric perforation was corroborated, the GEA was put in place using the DT, with no immediate complications or adverse events after the procedure. There was no need for an additional GEA and there were no porcine model deaths.
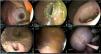
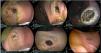
Evaluation by techniqueThe DT was the fastest technique, taking 29 min, but it obtained the smallest diameter (209 mm2, and there was poor stent placement (type I misdeployment) in one of the porcine models that was resolved endoscopically, with no complications, and the GEA could newly be put in place. In the final autopsy evaluation, GEA closure was complete in the two models, both with adherence of the omentum to the anastomosis site (Fig. 2).
Gastroenteric anastomosis using the direct technique. A) Initial Hot AXIOS™ placement. B) Stent removal at day 30. C and D) Anastomosis status immediately after stent removal. E and F) Final evaluation at day 60, showing total gastroenteric anastomosis closure on the gastric mucosa side and the presence of the omentum adhered to the anastomosis site.
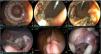
The RC technique resulted in the largest diameter (742 mm2), but the longest procedure duration, at 88 min. There were no eventualities during the cutting at the 4 cardinal points, and once the stents were placed again, as supports, there was no migration. Eight and 9 clips were employed, respectively, in each model and oral feeding tolerance at 24 h was adequate in the two models, as well as their progression. However, during the final evaluation, complete closure of the GEA was observed, with adherence of the omentum and intestinal segment in the anastomosis region, but no GEA patency. In one model, the omentum was present only on the extraluminal surface (Fig. 3).
Gastroenteric anastomosis using the radial cutting technique. A) Initial Hot AXIOS™ placement. B and C) Radial cutting with safety clip placement at day 15. D) Stent removal at day 30 and gastroenteric anastomosis status. E and F) Final evaluation at day 60, showing scar retraction on the gastric mucosa side, with adherence of the omentum and part of the intestinal segment at the level of the gastroenteric anastomosis.
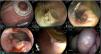
The LC technique was the fastest, at 74 min, the anastomotic diameter was the second largest, after the RC technique, and there were no complications during the procedure. Three and 4 clips were utilized in each model, respectively, and there were no adverse events after the procedure. Like all the other techniques, a closed GEA, with adherence of the omentum in the region of the anastomosis, was observed in the final evaluation of the two models (Fig. 4).
Gastroenteric anastomosis using the linear cutting technique. A) Initial Hot AXIOS™ placement. B and C) Gastroenteric anastomosis evaluation at day 15, carrying out linear cutting with clip use at the edges. D) Supportive stent removal at day 30 and gastroenteric anastomosis status evaluation. E and F) Final evaluation at day 60, showing a luminal mucosal scar, and on the extraluminal side, a scar at the level of the gastroenteric anastomosis and remnants of the omentum.
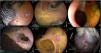
The AES technique turned out to be promising because it was the second fastest, after the DT, at 41 min, due to adding the injection time once the LAMS was placed for the creation of the GEA. The mean amount of ethanol injected was 2 ml per quadrant, encompassing both the gastric and enteral walls. No side effects after application of the alcohol were observed and the stent acted as a support during the 30 days of in-dwelling, for the formation of the GEA. When the LAMS was removed, the edges of the anastomosis were well-formed but both models presented with ulcerations at the level of the wall formed between the two organs, with no signs of gastrointestinal bleeding. In the final evaluation, the two GEAs were closed and both models had adhesions involving the stomach, omentum, and intestinal wall, as well as an important retraction at the level of the intraluminal gastric wall (Fig. 5).
Gastroenteric anastomosis using the absolute ethanol sclerotherapy technique. A and B) Initial Hot AXIOS™ placement with absolute ethanol application around and inside the stent. C and D) Endoscopic view and gastroenteric anastomosis status in both models at day 30. E and F) Final evaluation at day 60, showing a prepyloric luminal scar in the anastomosis region and gastric-omentum-enteral adherence on the extraluminal side.
The use of argon plasma for creating the GEA was shown to be a safe procedure, with adequate technical efficacy. Like the RC and LC techniques, the time involved in the ablation of the entire anastomosis circumference was added to the overall time, resulting in a total procedure duration of 75 min. When the LAMS was removed, the GEA was adequately created, with well-defined edges, and only slight ulceration in the two models. However, in the final evaluation, there was retraction caused by scarring at the luminal level, there were omental adhesions in the extraluminal fluid collection, and the anastomoses were completely closed (Fig. 6).
Gastroenteric anastomosis using the argon plasma coagulation technique. A) Initial Hot AXIOS™ placement. B and C) Argon plasma application on the gastroenteric anastomosis at 15 days. D) Gastroenteric anastomosis and support stent at day 30. E) Gastroenteric anastomosis status after Hot AXIOS stent removal. F) Final evaluation at day 60, showing complete closure at the level of the previous gastroenteric anastomosis site.
GOO is a condition that causes high morbidity and mortality, along with loss of patient quality of life, and the signs and symptoms are directly related to the duration and etiology of the obstruction.1,2 For malignant obstruction, surgical gastroenterostomy has been the classic treatment standard, but it is associated with numerous adverse events and not all patients are candidates for that type of treatment.3,8 Endoscopic advances have enabled a palliative alternative to be offered through the use of duodenal stents, which have good short-term technical and clinical success rates but they require multiple procedures. The advent of GEA creation through EUS has produced good results at present, with a 92% technical success rate (95% CI 86–95%), 90% clinical success rate (95% CI 85–94%), and 12% complication rate (95% CI 6–16%).1,3,8 Despite those advantages, its use in benign pathology has been limited,5,6,13,14 due to unpredictable GEA closure once the LAMS is removed. Thus, the primary aim of the present work was to evaluate the potential creation of a durable GEA, utilizing several techniques modified from the original. We could confirm the safety and efficacy of the 4 techniques evaluated but they all resulted in failed clinical success.
We employed 10 female porcine models because their anatomy is very similar to that of humans. We followed all the safety and ethics protocols for the purpose of evaluating the potential creation of a persistently patent GEA. We confirmed that the DT, carried out on two porcine models, and which we defined as the classic technique/control group, was the fastest procedure, at 29 min. It was also relatively faster than the reported 42−65 min for GEA creation in humans, using the same technique.1–3,7,14 This could be for two reasons: the first is that GEA creation in humans is usually carried out due to a disease, whether benign or malignant, initially modifying the creation or making it more difficult. The second is that x-ray use is imperative for the creation of a GEA in humans, to evaluate the anastomosis site and adequate LAMS placement after it is deployed,1,2,14 whereas in our porcine models, we only used USE. Our procedures showed that GEA creation without the use of x-rays not only is feasible, but also is safe, given that in the 10 models, we had only one misdeployment (type I). It was adequately resolved through endoscopy, closing the gastric defect with a type “a” OTSC clip, and at the same time, without aborting the procedure, the GEA was adequately placed, obtaining a post-procedure progression similar to that of the other 9 models, in which there were no adverse events, additional procedures, or deaths.
We decided to use 4 endoscopic techniques we considered reproducible and that have frequently been used in other gastrointestinal diseases. However, our aim was to seek a “more stable” remodeling and potentially persistent patency of the GEA, in relation to the original technique, based on the pathophysiology of gastroenteric anastomoses.8–11 The RC and LC techniques are derived from the endoscopic dissection of the submucosa, and we had 2 objectives. The first was to improve the diameter of the anastomosis. This was achieved in the two porcine models, with a diameter of 742 mm2 for the RC and 479 mm2 for the LC, resulting in a diameter that was 3.5 and 2.2-times larger, respectively, than that of the control group (DT = 209 mm2). Importantly, even though the final aim was not achieved, those two techniques produced the largest possible diameter via endoscopy. This could have significant clinical implications in the future, in patients with gastrointestinal tract obstruction, resulting in improved oral intake tolerance. Evaluated through an increase in the gastric outlet obstruction score (GOOS), the results could be improved and maintained in the medium term and long term, in patients in whom a durable GEA was achieved. Clinical differences have indeed been confirmed in patients with GOO, as demonstrated in the study by Bejjani et al.15 Their multicenter analysis from 19 centers included 267 patients with GOO. Clinical efficacy measured by an increase in the GOOS was compared in patients that had a 15 mm LAMS versus those with a 20 mm stent. The patients in the 20 mm group tolerated a soft solid/complete diet, compared with the 15 mm group (91.2 vs 81.2%, respectively; p = 0.04), confirming that GEA diameter is associated with clinical success in patients with GOO. At present, outcomes are limited by the diameter of the anastomosis rather than by the creation of a native, larger-diameter GEA, as we suggest in the present study. Our second objective was to create a foreign body at the level of the GEA with the use of hemoclips. This was done to improve cutting safety (in the event of a potential perforation, which did not occur in any of our models), and to promote healing and remodeling of the anastomoses while maintaining the newly achieved diameter size. As expected, the anastomotic diameter was larger in the RC group because the anastomosis involved 4 cardinal points, rather than 2, as was the case in the LC group. Even though this second objective was not attained, due to the limited capacity of the clips, it could be re-evaluated in the future using better techniques, such as endoscopic endo-suturing that would potentially enable the permanent presence of a foreign body in the constructed GEA. Regarding the use of alcohol and argon plasma, we decided to implement their use in 2 techniques, due to their facility of use and safety. These were confirmed, especially for alcohol, which had the second-best time (41 min), in relation to the DT, being only 12 min longer. It also had the advantage of being performed at the same time as LAMS placement. In contrast, the other three experimental techniques involved 2 interventions rather than one. Those 3 techniques were carried out by temporarily removing the LAMS and then repositioning it again in a second intervention. In the case of argon plasma, our aim was to create a more stable remodeling of the GEA between the gastric mucosa and the jejunal mucosa. Less ulceration was observed during the evaluation of the GEA when the stent was removed, compared with the other techniques, suggesting better epithelization. However, that did not translate into a clinical benefit in the final evaluation, at which all the GEAs demonstrated complete closure.
Importantly, even though clinical success was not reached, there are 4 factors that could have influenced that result: first, we were operating on a healthy gastrointestinal structure that had no pathophysiology of a true GOO, unlike the study by Krafft et al.4 Those authors described fistula persistence in 41% of the anastomoses after LAMS removal in patients who underwent previous gastric bypass. The anastomosis was performed between the gastric pouch and the remnant, which conditioned other physical circumstances, such as an increase in the intraluminal pressure at the level of the pouch, which in turn, could impede closure of the created anastomosis. That is an effect similar to the one evaluated in benign or malignant GOO. On the other hand, gastrogastric anastomosis, because it involves the same organ, could create a more stable anastomosis with no intermediate structures. Second, we believe the presence of a foreign body could be an important factor because when surgical anastomoses have a permanent foreign body, such as a suture or mechanical suture, the foreign body interferes with the complete closure of the anastomosis. That differs from the endoscopic technique, in which there is gastric and enteral edge apposition. Upon LAMS removal, the edges remain well-irrigated, with no foreign body that could interfere in healing and closure. In our study, the use of clips served as a foreign body, but not to the extent that it affected patency. Third, the in situ dwell time of the LAMS has been an important factor in clinical studies. For evaluating the permanence of the anastomosis, it has not been a determining factor or well-studied. In our study, LAMS dwell time was 15 days for the RC, LC, and APC techniques and 30 days for the DT and AES. That is a short time, compared with the 85 days in the study by Krafft et al.,4 who reported patency in 41% of patients, but which may not necessarily be attributed to dwell time or the performance of a gastrogastric anastomosis instead of a GEA. In addition, a recent study by Abel et al.13 that evaluated the use of LAMS for GEA in patients with benign disease showed adequate long-term patency of the stent that remained indwelling up to 286 days, with 3 patients passing the 900-day mark (944, 1408, and 1444 days), which, even though it confirms that a LAMS could remain in situ for up to 4 years, is not necessarily related to a GEA still being patent, following LAMS removal after such time points. Fourth, in the final evaluation of all the porcine models analyzed herein, we can confirm that the omentum was interposed between the gastric and enteral tissues, regardless of the technique employed. The omentum is a source of multiple growth factors, containing pluripotential stem cells that can differentiate into different types of cells. This prevents or limits sepsis, promotes angiogenesis, and provides vascular support. Thanks to their efficient influence on tissue repair, the omentum’s biologic properties have been utilized in multiple surgical procedures.16 Even though said properties may prevent anastomotic leaks and other complications, they could also play a fundamental role in anastomosis closure by apposition. This occurred in our study, regardless of the technique employed, even when the goal was to produce transmural cuts (RC, LC) or controlled tissue damage (AES, APC).
Among our study’s limitations are the short follow-up period, which could potentially be important in relation to permanent anastomosis creation; the absence of a histologic evaluation, which could have provided valuable information for exploring premature closure of the anastomosis, regardless of the technique employed; and sample size, which although calculated based on convenience sampling for this study, could be considered “borderline” in statistical terms, but sufficient for being used as a basis for planning a future project with similar aims, incorporating variables, such as longer follow-up and additional modifications to the techniques employed herein. These limitations, although important, do not invalidate our results, which suggest there are multiple factors that intervene in the creation of a permanent GEA.
In conclusion, although the present study did not achieve the clinical aim of creating a permanent GEA with any of the techniques employed, likely due to a complex multifactorial pathophysiology, it was possible to create better-caliber GEAs, compared with the original technique, without a great difference in procedure time and with an adequate safety profile. This opens the door to new and needed endoscopic examinations, with the ultimate goal of creating a minimally invasive therapeutic alternative that is safe, effective, and durable, for the treatment of patients with benign GOO.
Ethical considerationsThe present protocol was approved by the hospital ethics and research committee, with number R-2023-3601-004. All the procedures carried out on animals complied with the norms for the performance of studies on animal models and in accordance with the NOM-062-ZOO-1999 and NOM-087-ECOL-SSA1-2002 official Mexican norms, as well as the Ecologic Balance and Environmental Protection General Law. The ARRIVE guidelines regarding experimentation on animal models were followed, along with the recommendations given by the EU 2010/63/EU for experimentation on animal models, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications No 8023, revised 1978).
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this study.
The authors declare that there is no conflict of interest.