Fifty percent of small bowel bleeding is caused by angioectasia and the rebleeding rate due to small bowel angioectasia (SBA) is 80%. Its endoscopic treatment is difficult. Beneficial effects of octreotide on gastrointestinal angioectasia have been described, but no studies have reported its efficacy in SBA.
AimOur aim was to investigate the effectiveness of octreotide in the prevention of rebleeding due to SBA.
Material and methodsSixteen patients with bleeding caused by SBA were assigned to treatment with octreotide 100 μg/24 h SC, for at least 6 months, and compared with a non-treatment group of 36 patients. The primary outcome was the rebleeding rate, and the secondary outcomes were the number of hospital readmissions, bleeding-related death, and adverse effects.
ResultsOctreotide was administered for 10.5 ± 8.4 months. Follow-up was 12.9 ± 17.3 months and 15.3 ± 17.7 months, in the treatment and non-treatment groups, respectively (p = 0.09). At the end of follow-up, 4 (25%) treatment group patients and 26 (72.2%) non-treatment group patients presented with rebleeding (p = 0.002). In the treatment group and non-treatment group, the cumulative probability of remaining rebleeding-free at one year was 79% vs 44.2%, and 79% vs 34.6% at 2 years, respectively (p = 0.05). Through the multiple logistic regression analysis, treatment was the protective variable. Six patients presented with adverse events. One of those patients (6.25%) had a major adverse event.
ConclusionsOur results suggest that treatment with octreotide could be efficacious in the prevention of rebleeding due to SBA.
El 50% de las hemorragias del intestino delgado son causadas por angiectasias del intestino delgado (AID) y la tasa de recurrencia es del 80%. Su tratamiento endoscópico es difícil. Algunos estudios han informado efectos beneficiosos del octreótido en angiectasias del tubo digestivo, pero ninguno ha evaluado su eficacia en las AID.
ObjetivoInvestigar la efectividad del octreótido en la prevención de la recurrencia hemorrágica de las AID.
Material y métodosDieciséis pacientes con sangrado por AID fueron asignados a un tratamiento con octreótido 100 μg/24 h SC por al menos 6 meses. Esta cohorte se comparó con un grupo de 36 pacientes no tratados. El desenlace primario fue la tasa de recurrencia hemorrágica y los secundarios fueron el número de reingresos hospitalarios, muerte relacionada con el sangrado, y efectos adversos.
ResultadosSe administró octreótido durante 10.5 ± 8.4 meses. El seguimiento fue de 12.9 ± 17.3 y 15.3 ± 17.7 meses en pacientes tratados y no tratados (p = 0.09). Al final del seguimiento, el sangrado recurrente se produjo en 4 (25%) pacientes del grupo tratado y en 26 (72.2%) del grupo no tratado (p = 0.002). La probabilidad acumulada de permanecer libre de hemorragia recurrente al año fue del 79% vs. 44.2% y a los 2 años, del 79% vs. 34.6% en el grupo tratado y no tratado, respectivamente (p = 0.05). De acuerdo con el análisis de regresión logística múltiple, el tratamiento fue variable protectora. Los eventos adversos ocurrieron en 6 pacientes. En uno de ellos fueron eventos mayores (6.25%).
ConclusionesEstos resultados sugieren que el tratamiento con octreótido podría ser eficaz para prevenir las hemorragias recurrentes por AID.
Gastrointestinal angioectasia (GIA) is characterized by vascular malformations composed of dilated and tortuous arterial or venous capillaries, usually smaller than 5mm in diameter and located in the mucosal and submucosal layers of the gastrointestinal tract.1
Angioectasia can occur in any segment of the digestive tract but is more frequent in the small bowel (57 to 80%), particularly in the duodenum and jejunum, followed by the colon (44%), and the stomach (32%).2,3 It is responsible for 5% of all gastrointestinal bleeding and about 50% of small bowel bleeding.4 Rebleeding is very frequent (80%). The majority of patients become dependent on blood transfusions and parenteral iron infusions and have a significant deterioration of quality of life.5
Current treatment is very diverse due to the lack of therapy guidelines. Endoscopic argon plasma coagulation (APC) is the most common treatment.6 Selective angiographic embolization and surgical resection are used only in selected cases, especially when endoscopic treatment fails or there is hemodynamic instability.7
The diagnosis and treatment of small bowel angioectasia (SBA) are difficult due to inaccessibility. Frequently, lesions are multiple and diffusely disseminated.8,9 The long-term results of endoscopic therapy are disappointing.10,11 In a systematic review of 24 articles involving 490 patients with GIA treated with endoscopic therapy, the bleeding recurrence rate was similar to that in patients without treatment.12 Therefore, treatments that produce systemic effects on angioectasia have been explored.
Recently, drugs with anti-angiogenic activity, such as thalidomide,13,14 and somatostatin analogues, such as immediate release octreotide and long-acting release (LAR) octreotide, have been used.15–20 Treatment with octreotide has been suggested to be beneficial for reducing mid-term and long-term rebleeding in GIA. However, almost all the studies that have been published so far are noncomparative analyses, with few patients, and none of them have evaluated the efficacy of octreotide, specifically in patients with SBA.
The abovementioned information prompted us to conduct a study on patients with bleeding secondary to SBA, to prospectively assess the effects of treatment with octreotide, compared with non-treatment, on bleeding recurrence.
Materials and methodsPatientsPatients referred to our unit, from January 2012 to January 2018, with acute or chronic bleeding due to SBA, and diagnosed through video capsule endoscopy (VCE), were included in the study. Angioectasia was defined as the presence of single or multiple lesions, irregular or star-shaped, that had a flat surface and a diameter greater than 2mm. The endoscopic criteria to define bleeding angioectasia were the presence of lesions with active bleeding, lesions with stigmata of recent bleeding, or the absence of other potential sources of bleeding. Angioectasia was classified as segmental, when located in one segment of the small bowel, or disseminated, when located in more than one segment, and as unique or multiple, when one or more lesions were found, respectively. Based on endoscopic characteristics, angioectasia was classified using a method previously proposed by our group: Type 1: lesions with non-pulsatile active bleeding; Type 2: lesions without active bleeding, but with stigmata of recent bleeding manifested by central ulcer, adherent clot, or adjacent blood detritus; Type 3: bright red patchy lesions; Type 4: pale-red patchy lesions.1
The medical records of the patients were reviewed. Adult patients with acute or chronic gastrointestinal bleeding, anemia (defined by plasma hemoglobin levels < 10g /dl and serum iron levels < 60μg/dl), requirements of blood transfusions or parenteral iron infusions, and occult or visible blood in stools, in whom the last bleeding episode occurred less than one week before VCE performance, were selected for the study. Patients with incomplete VCE procedures, no recorded follow-up, or angioectasia in the stomach or colon; patients treated with thalidomide, APC, selective angiographic embolization, or surgery; patients with difficult-to-treat diabetes mellitus (blood glucose > 140mg/dl or HbA1c > 7%) or asymptomatic cholecystolithiasis; and patients that refused to participate in the study were excluded.
TreatmentThe study patients were hospitalized, subsequently discharged, and divided into two groups. One group was made up of the patients treated with the subcutaneous (SC) administration of octreotide 100μg/24h. Each multi-dose ampoule of octreotide contained 1mg/5ml. The medication was administered on an outpatient basis, and the patient, or a relative, received instructions for its application. The other group consisted of patients that did not receive treatment and was used for comparison. Those patients were not treated, due to the decision of their referring physician or because medication was not available in their places of residence.
Follow-upFollow-up was carried out through subsequent visits to the hospital every month for 6 months, then every 3 to 6 months, until the end of study, which was determined by: rebleeding, major side effects, death, loss to follow-up, or exclusion from analysis.
Clinical examination and blood tests were performed (serum hemoglobin, hematocrit, glucose, creatinine, iron, and liver function tests) at each visit. When the patients did not attend their consultation, they were contacted by telephone.
OutcomesThe primary outcome was rebleeding, defined as the presence of at least one of the following parameters: decrease of hemoglobin > 2g/dl compared with the baseline values; visible blood in stools; blood transfusion requirement (serum Hb levels < 8g/dl); and iron parenteral requirement (when hematocrit was < 25%).
The secondary outcomes were the number of hospital readmissions, bleeding-related death, treatment compliance, and major and minor adverse effects.
Rebleeding and drug tolerance were taken into account for assessing the effectiveness of the treatment.
Statistical AnalysisContinuous variables were expressed as means and standard deviations and discontinuous variables as medians, ranges, and relative proportions. The differences between groups were analyzed by the Student’s t test for the quantitative variables, and the chi-square test and Fisher’s exact test for the non-quantitative variables. The cumulative probability of remaining rebleeding-free was calculated by the Kaplan-Meier curve and the differences were analyzed by the Mantel-Cox log-rank test.
The proportional multivariate logistic regression method (Cox regression) was used to determine the independent predictive variables of bleeding recurrence. The 95% confidence intervals were calculated and a two-sided p value ≤ 0.05 was considered statistically significant. The statistical analysis was performed using the SPSS version 25.0 statistical package.
Ethical considerationsThe protocol was approved by the Ethics Committee of the Faculty of Medicine of the Autonomous University of Nuevo León. Informed consent was obtained from all patients and no pharmaceutical industry sponsored this study.
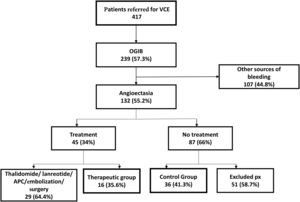
ResultsPatientsOf the 132 patients with SBA, 16 were assigned to treatment with immediate release octreotide; 36 non-treatment patients were used as controls. The remaining 80 patients were not included, for some of the following reasons: 29 had received other treatments (thalidomide, lanreotide, APC, selective angiographic embolization, or surgical resection) and 51 untreated patients did not meet the inclusion criteria or have follow-up (Fig. 1). Nevertheless, the excluded patients were similar demographically and clinically to those that participated in the study.
Patient characteristicsMean age of the treated patients was slightly higher than that of the untreated individuals (73.5±13.3 vs 66.1±6.6 years, respectively). By sex, there were no significant differences between the two groups in relation to the number of individuals > 70 years of age, time of bleeding evolution and its clinical manifestations, and number and type of comorbidities. The endoscopic characteristics of angioectasia were also similar (Table 1). Octreotide was administered for a mean time of 10.5±8.4 months (range of 6-24 months).
Demographic and clinical characteristics of the patients before inclusion in the study.
| Characteristics | Control group | Treatment group | *p value |
|---|---|---|---|
| n=36 | n=16 | ||
| Age | 66.1±13.3 [42-95] | 73.5±6.6 [64-84] | 0.01 |
| > 70 years of age | 15 (42.8) | 10 (62.5) | 0.16 |
| Females | 20 (55.6) | 8 (50) | 0.38 |
| Comorbidities | |||
| Chronic kidney disease | 2 (5.6) | 1 (6.3) | 0.67 |
| Chronic liver disease | 1 (2.8) | 0 | 0.69 |
| Valvular heart disease | 0 | 0 | 1 |
| Ischemic heart disease | 7 (19.4) | 3 (18.8) | 0.63 |
| Diabetes mellitus | 10 (27.8) | 6 (37.5) | 0.52 |
| Use of NSAIDs/antiplatelet drugs | 10 (27.8) | 3 (18.8) | 0.73 |
| More than one comorbidity | 16 (44.4) | 11 (68.7) | 0.10 |
| Clinical characteristics of bleeding before VCE | |||
| Time of evolution | 29.1±38.9 [1-186] | 28.3±31.1 [2-108] | 0.47 |
| Overt bleeding | 20 (55.6) | 11 (68.8) | 0.54 |
| Hemoglobin plasma levels (g/dl) | 6.72±1.76 | 6.9±2.1 | 0.69 |
| Number of transfused patients | 29 (80.6) | 13 (81.3) | 0.63 |
| Number of transfused blood units/patient | 4.4±4.7 | 3.8±4.3 | 0.38 |
| Number of IV iron-infused patients | 14 (38.9) | 10 (62.5) | 0.14 |
| Number of EGDs/patient | 2±1.6 | 1.6±0.7 | 0.94 |
| Number of colonoscopies/patient | 1.5±1.4 | 1.3±0.4 | 0.79 |
| VCE findings | |||
| Multiple lesions | 36 (100) | 16 (100) | 1.0 |
| Diffuse distribution | 13 (36.1) | 6 (37.5) | 0.58 |
| Type 1 | 7 (19.4) | 2 (12.5) | 0.70 |
| Type 2 | 4 (11.1) | 4 (25) | 0.23 |
| Type 3 | 16 (44.4) | 8 (50) | 0.76 |
| Type 4 | 9 (25) | 2 (12.5) | 0.46 |
EGD: esophagogastroduodenoscopy; NSAIDs: nonsteroidal anti-inflammatory drugs; VCE: video capsule endoscopy.
In total, the treated patients had lower bleeding recurrence rates than the untreated patients: 4/16 (25%) vs 26/36 (72.2%), p=0.002, respectively. The treated patients had less serum hemoglobin reduction (25% vs 58.3%, p=0.037), less requirement of blood transfusion (6.3% vs 38.9%, p=0.021), and fewer hospital readmissions (6.3% vs 36.1%, p=0.04). There were no significant differences, regarding the presence of visible blood in stools, parenteral iron requirements, or bleeding-related deaths, between the two groups (Table 2).
Characteristics of rebleeding.
| Manifestation of bleeding recurrence | Control group | Treatment group | *p value |
|---|---|---|---|
| n=36 | n=16 | ||
| Treatment duration, in months, | 10.5±8.4 [6-24] | ||
| Follow-up, in months | 15.3±17.7 [1- 78] | 12.9±17.3 [6-75] | 0.09 |
| Rebleeding rate | 26 (72.2) | 4 (25.0) | 0.002 |
| Decrease in Hb >2g/dl | 21 (58.3) | 4 (25.0) | 0.037 |
| Overt bleeding | 8 (22.2) | 1 (6.3) | 0.245 |
| Blood transfusion requirement | 14 (38.9) | 1 (6.3) | 0.021 |
| Iron infusion requirement | 12 (33.3) | 3 (18.8) | 0.340 |
| Hospital readmissions | 13 (36.1) | 1 (6.3) | 0.040 |
| Death | 3 (8.3) | 0 | 0.544 |
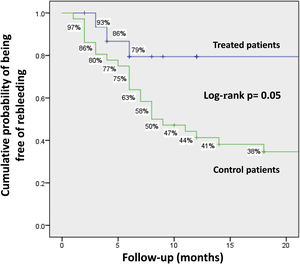
The cumulative probability of remaining rebleeding-free at 1year was 79% vs 44.2%, and 79% vs 34.6% at 2 years, in the treatment and non-treatment groups, respectively (p=0.05) (Fig. 2).
The multiple logistic regression analysis showed that treatment with octreotide was protective against rebleeding (HR: 0.013, 95% CI 0.001-0.235, p=0.003) (Table 3).
Multivariate proportional regression analysis of the independent predictive variables of rebleeding.
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Age | 0.894 | 0.774-1.033 | 0.129 |
| Body mass index | 0.856 | 0.679-1.080 | 0.190 |
| Comorbidities | |||
| Chronic kidney disease | 0.063 | 0.001-4.069 | 0.193 |
| Heart disease | 2.008 | 0.159-25.365 | 0.590 |
| Use of NSAIDs/ antiplatelet drugs | 0.425 | 0.040-4.563 | 0.480 |
| More than one comorbidity | 1.097 | 0.059-20.389 | 0.951 |
| Clinical characteristics | |||
| Number of transfused units/patient | 0.994 | 0.797-1.241 | 0.960 |
| Number of patients with IV iron infusion | 2.282 | 0.359-14.513 | 0.382 |
| Video capsule endoscopy | |||
| Bright red patchy spots | 0.322 | 0.040-2.559 | 0.284 |
| Pale red patchy spots | 0.230 | 0.024-2.210 | 0.203 |
| Treatment with somatostatin analogues | 0.013 | 0.001-0.235 | 0.003 |
CI: Confidence Interval; HR: hazard ratio; NSAIDs: nonsteroidal anti-inflammatory drugs.
Adherence to treatment was 100%. Adverse events occurred in 6 patients (37.5%). They were minor and transient in 5 of those patients (diarrhea in 2, headache in 2, and mild abdominal pain in 1), and major in one case (6.25%) (intractable abdominal pain, 4 months after starting treatment), forcing treatment suspension. That patient did not present with gastrointestinal bleeding, but the therapy was considered a failure, with respect to the analysis, and so the resulting treatment effectiveness total was 11/16 (68.7%).
Discussion and conclusionsThe results of the present study suggest that octreotide reduced rebleeding due to SBA. Overall, 25% of the treated patients had bleeding recurrence, compared with 72.2% of the control group (p=0.002). The 1 and 2-year cumulative probability of remaining rebleeding-free was significantly higher in the treated patients, despite the fact that they were older than the untreated patients. In addition, the multiple logistic regression analysis showed that octreotide treatment was protective against bleeding. The data suggest that octreotide was also effective in older subjects, which is relevant, given that those individuals are more affected by SBA.
Our study results confirm those of other published reports15–24 (Table 4). In a noncomparative study that included 17 patients treated with 300μg/d of octreotide, SC, for 6 months, 82.3% had a significant reduction in treatments for anemia.15 In other studies, doses of 10 to 20mg/month of octreotide-LAR, IM, for 3 to 12 months, produced a complete response in 50% to 70% of the patients, defined as a decrease in bleeding episodes, in low blood hemoglobin levels, transfusion requirements, and the number of hospitalizations.17–20,25 In a recent phase II double-blind, randomized, noncomparative study, 60mg of pasireotide (a somatostatin analogue with a 40-fold increased affinity for somatostatin receptor 5, compared with octreotide), IM, every month, significantly decreased the transfusion requirements in patients with recurrent bleeding due to GIA, compared with placebo.26
Prospective and controlled studies evaluating the efficacy of somatostatin analogues in patients with gastrointestinal bleeding due to angioectasia.
| Author (year) | Design | N | Treatment | Follow-up (months) | Results |
|---|---|---|---|---|---|
| Nardone (1999)15 | Cohort | 17 | SC Octreotide 100μg/8h for 6 months | 12 | Rates of complete response, partial response, and non-response were 59%, 23% and 18%. Non-significant side effects. |
| Junquera (2007)16 | Cohorts, | 65 (Rx:30 vs C:35) | SC Octreotide 50μg/12h for 12-24 months | 13 (12-36) | Bleeding recurrence: Rx=23% vs C=48% (p=0.04). Major adverse effects: Rx=3.1% vs P=2.6% |
| Rx vs P | |||||
| Scaglione (2007)17 | Cohort | 13 | IM octreotide 10mg/month for 12 months | 33 (12-60) | Rates of complete response, partial response, and non-response were 69%, 8%, and 23%. Non-significant side effects. |
| Molina (2009)18 | Cohort | 11 | IM octreotide 20mg/month | 15 (5-48) | Patients with serious comorbidities. Treatment reduced transfusion requirements and bleeding-related hospitalizations. |
| Bon (2012)19 | Cohort | 15 | IM octreotide 20mg/month for 12 months | 14 (10-36) | Rx significantly reduced bleeding recurrences, transfusion requirements and increased serum Hb levels. Side effects rare. |
| Holleran (2016)20 | Cohort | 24 | IM octreotide 20mg/month for 3 months | 8 (3-17) | Rates of complete response, partial response, and non-response were 70%, 20% and 10% respectively. Adverse events: 30% |
| Benamouzig (2018)26 | DBRNC | 22 (Rx:10 vs P:12 | Pasireotide-LAR 60mg/month for 6 months | 6 (6-12) | Rx significantly decreased transfusion requirement. Rx=83% vs P=25% |
| Current Study | Cohorts, | 52 (Rx: 16 vs C:36) | SC Octreotide 100μg/day | 10.5 (1-78) | Bleeding recurrence: Rx=25% vs C=72.2% (p=0.002). Major adverse effects: Rx=10.5%. Overall effectiveness: 68.4% |
| Rx vs Non Rx | RX duration: 10.5±8.4 months |
C: controls; DBRNC: double-blind, randomized, noncomparative; Hb: hemoglobin; IM intramuscular; LAR: long-acting release; P: placebo; Rx: treatment; SC: subcutaneous.
Although numerous therapeutic studies on octreotide in patients with angioectasia have been published, most of them are noncomparative or have a small number of patients. A comparative study by Junquera et al., similar to ours, included 65 patients with GIA. Thirty patients were treated with low doses of octreotide (50μg/12h), SC, for a period of one year, and 35 patients received placebo. A reduction in rebleeding was demonstrated in the treated patients (23% vs 48%, p=0.043) and drug tolerance was good.16
Importantly, ours is the first therapeutic study to exclusively address bleeding caused by SBA, which is relevant for the following reasons: a) the small bowel is the most common location of angioectasia in the digestive tract (57-80%); b) lesions are frequently multiple and distributed in the large segments; c) bleeding recurrence is higher, compared with angioectasia in other digestive segments (80%); and d) most lesions are inaccessible to endoscopic therapy.27 Many patients become dependent on repeated blood transfusions and parenteral iron infusions, and have multiple hospital readmissions, significantly deteriorating their quality of life. Therefore, treatments with a systemic effect are greatly needed.
Octreotide and lanreotide are somatostatin analogues that are suggested to have multiple pharmacologic effects that involve the pathophysiology of angioectasia. They have hemodynamic effects on the splanchnic circulation, producing a reduction in portal pressure and mesenteric blood flow.28 They also inhibit angiogenesis by blocking biochemical factors that promote vascular proliferation, such as VEGF, b-FGF, and IGF-1k.29–31 They stimulate the relaxation of the intestinal muscle, leading to a decrease in the chronic obstruction of the submucosal veins.32,33 The disappearance or reduction in size of angioectasia has been reported during treatment with octreotide.15
Octreotide is highly resistant to enzymatic degradation and has a prolonged plasma half-life in humans.34 In the present study, low doses of octreotide appeared to be effective. The beneficial effect could be attributed to some of the multiple actions on angioectasia described above. The dose employed in the study by Junquera et al., similar to the dose used in our study, was reported as effective.16 We used a low dose to reduce treatment costs because octreotide is expensive in Mexico. Furthermore, we did not increase the dose of octreotide in patients with rebleeding. Dose scalation might have rescued some non-responder patients. In general, the drug was well tolerated. Most of the adverse events were transient and treatment suspension was necessary in only one case.
Octreotide requires daily SC administration, which may hamper treatment compliance. However, octreotide-LAR (extended-release octreotide), administered IM every month, can resolve that disadvantage.
Limitations of our study include the fact that it was a single center study; it was not a randomized controlled trial; no placebo was used, and evaluations were non-blinded; in addition, the sample size was small, with insufficient follow-up in some of the treated patients. However, in our opinion, the strength of the primary outcome (bleeding recurrence) makes the results reliable.
In conclusion, the results of our study suggest that octreotide is effective in preventing rebleeding due to SBA. The drug was well tolerated and was associated with reduced hospital readmissions related to bleeding. The low doses of octreotide used in our study may increase treatment adherence, resulting in fewer adverse events, and in turn, reducing the costs of therapy. However, multicenter, randomized, double-blind, controlled trials with large samples of patients are needed to confirm therapeutic effectiveness in that setting. The cost-benefit ratio and the impact on quality of life must also be evaluated.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Del Cueto-Aguilera ÁN, García-Compeán D, Jiménez-Rodríguez AR, Borjas-Almaguer OD, Wah-Suárez MI, González-González JA, et al. Eficacia del octreótido sobre la recurrencia hemorrágica de las angiectasias del intestino delgado. Estudio comparativo. Rev Gastroenterol Méx. 2022;87:411–419.