The approach to and management of critically ill patients is one of the most versatile themes in emergency medicine. Patients with cirrhosis of the liver have characteristics that are inherent to their disease that can condition modification in acute emergency treatment. Pathophysiologic changes that occur in cirrhosis merit the implementation of an analysis as to whether the overall management of a critically ill patient can generally be applied to patients with cirrhosis of the liver or if they should be treated in a special manner. Through a review of the medical literature, the available information was examined, and the evidence found on the special management required by those patients was narratively synthesized, selecting the most representative decompensations within chronic disease that require emergency treatment.
El abordaje y manejo de pacientes críticamente enfermos representa uno de los temas más versátiles en la medicina de urgencias. Los pacientes con cirrosis hepática tienen características inherentes a su enfermedad, que pueden condicionar modificaciones en el tratamiento agudo urgente. Los cambios fisiopatológicos que ocurren en esta enfermedad ameritan la puesta en marcha de un análisis, acerca de si el manejo global de un paciente críticamente enfermo puede aplicarse a pacientes con cirrosis hepática de forma general o si se deben tratar de una manera especial. A través de una revisión de la literatura médica, se exploró la información disponible y se sintetizó la evidencia de forma narrativa lo encontrado acerca del manejo especial que requieren los estos pacientes, seleccionando las descompensaciones urgentes más representativas dentro de la enfermedad crónica.
Liver cirrhosis (LC) is currently one of the main causes of death in the Western world,1,2 and it is an important public health problem in Mexico. The present review deals with basic aspects of the resuscitation of patients with LC that are critically ill, with a special focus on initial emergency care. The aim was to analyze the available literature on the special management that patients with LC require, in the context of emergencies, for the most frequently encountered conditions at that stage of care, according to expert opinion. The pertinent information was collected that exclusively covered patients with LC in different emergency clinical contexts: hypovolemic shock due to variceal upper gastrointestinal bleeding (UGIB), sepsis and septic shock, hepatic encephalopathy (HE), acute kidney injury (AKI), and electrolyte alterations.
MethodologyThe present narrative evidence synthesis was formulated, according to the following steps: first, topics focusing on the initial approach to the patient with decompensated cirrhosis were selected, with respect to emergency evaluation and management; second, two of the coauthors (ESGJ and JAVRV) evaluated the information, synthesizing and codifying it into the following themes: variceal bleeding, HE, sepsis, AKI, and isolated electrolyte alterations; and third, after codifying the information, directed questions were developed, and a systematic review was carried out, utilizing the DynaMed, Google Scholar, and PubMed databases. A hierarchical pyramid model for pre-appraised evidence was utilized to obtain the information. Evidence summaries were the first (clinical practice guidelines, UpToDate, and Dynamed), then evidence syntheses/synopses (systematic reviews), and lastly, original studies (randomized controlled trials and observational studies). The information search was carried out in Spanish and English. The following keywords were used: “cirrhosis”, “decompensated cirrhosis”, “critically ill cirrhotic patient”, “fluid therapy”, “water intake”, “blood products”, “shock”, “hypovolemic shock” “sepsis”, “septic shock”, “transfusions”, “vasopressors”, “encephalopathy”, “variceal bleeding”, “hyponatremia”, and “kidney injury”, in publications from 1980 to the first three months of 2021. The results were sent to the team of coauthors who, utilizing the standardized format, extracted the relevant information to be included in the present narrative evidence synthesis. The most pertinent topics follow below.
ResultsResuscitation of the patient with cirrhosis of the liver with variceal upper gastrointestinal bleeding and hypovolemic shockSeverity status evaluation: vital signs and perfusion evaluationThe basic hemodynamic monitoring of the critically ill patient for adequate identification and treatment of the cardiopulmonary status includes a directed clinical history and physical examination, vital sign assessment (heart rate [HR], mean arterial pressure [MAP], respiratory rate [RR], temperature, and O2 saturation by pulse oximetry), and urine output. However, those primary variables and the physical examination have repeatedly been shown to be insufficient and imprecise for the hemodynamic evaluation, rapid assessment, and the identification of occult shock or compensated status, especially in previously healthy patients or when the cardiopulmonary status changes quickly.3 Those evaluations are also inaccurate and inadequate in the setting of the patient with LC and can be related to 2 contexts. The first is the occurrence of pathophysiologic changes of portal hypertension itself, such as hyperdynamic circulation, and the second is associated with the need for beta-blocker use in patients receiving prophylaxis for variceal bleeding.4,5 There are several tools for recognizing tissue hypoperfusion and predicting the response to fluid infusion in the general population, but they have not been completely studied in LC. It is known that biochemical markers for tissue hypoperfusion, such as lactate, metabolic acidosis, and central venous oxygen saturation (ScvO2) found in the state of shock, can be abnormal, indicating occult tissue hypoperfusion, even without hypotension or other manifested clinical signs of shock.3 However, applying the established parameters for identifying hypovolemic shock to patients with LC would probably not be adequate. In one of the few studies on the subject, Moreau et al. analyzed tissue oxygenation in patients with LC and reported that arterial pressure (AP) tended to be reduced, ScvO2 and HR could be skewed due to hyperdynamic circulation, and elevated lactate levels could reflect the severity of the liver failure, more than a hemodynamic status response.6 Li et al. carried out a prospective study whose aim was to validate a clinical definition of shock, with respect to 28-day mortality. It was defined as the presence of an obvious sign of tissue hypoperfusion, such as mottled skin, systolic arterial pressure (SAP) < 90 mm Hg or MAP < 65 mm Hg, lactate level ≥ 4.0 mmol/L, pH ≤ 7.1, or a base deficit ≤ −5 mEq/l.7
Administration of intravenous solutionsThe infusion of large volumes of crystalloids as replacement of lost blood worsens the patient’s condition and maintains the coagulopathy resulting from the hypoxia, acidosis, and hypothermia produced. That is because intravenous fluids dilute clotting factors and produce hypothermia and acidosis. It also causes edema, with end organ dysfunction, by altering cellular mechanisms and causing inflammation, resulting in various complications, including heart, respiratory, gastrointestinal, and immune dysfunction, with a consequent increase in mortality.8 In patients with LC, starting early resuscitation with intravenous fluids is recommended for restoring tissue perfusion and maintaining MAP > 65 mmHg9 and SAP between 90-100 mmHg.10 Patients with LC that present with hematemesis have been shown to have a worse prognosis, compared with patients that present with melena, and most likely require additional fluid resuscitation, according to the presentation. Li et al. conducted a retrospective study to compare the 5-day rebleeding rate and in-hospital mortality in patients with LC and UGIB due to hematemesis versus those that only presented with melena. The study included 793 patients, and those with hematemesis at hospital admission had a significantly higher 5-day rebleeding rate (17.4% versus 10.1%, p = 0.004) and in-hospital mortality (7.9% versus 2.4%, p = 0.001) than those with melena.11 There are no studies related to fluid resuscitation and hypovolemic shock, specifically in patients with LC, but based on those conducted on critically ill patients, knowledge on the theme can be extrapolated. Dijillali et al. carried out a multicenter randomized clinical trial (CRISTAL) stratified by case mix (sepsis, trauma, or hypovolemic shock). It was an open-label trial whose aim was to evaluate 28-day mortality, utilizing colloid therapy versus crystalloid therapy for the resuscitation of critically ill patients. The study included 2,857 patients, 1,414 of whom were in the colloid group and 1,443 of whom were in the crystalloid group. At 28 days, there was no significant difference in mortality between the two groups (relative risk [RR] 0.96, 95% confidence interval [CI] 0.88 to 1.04; p = 0.26), but at 90 days, mortality was lower in the patients that received colloids (RR 0.92, 95% CI 0.86 to 0.99; p = 0.03). In addition, renal replacement therapy (RRT) was used less in the colloid group (11.0%) than in the crystalloid group (12.5%) (RR 0.93, 95% CI 0.83 to 1.03; p = 0.19). Those authors concluded that there was no significant difference in mortality between the group that received colloids and the group that received crystalloids.12 Matthew et al. conducted a pragmatic, unblinded, cluster-randomized, multiple-crossover trial that compared the use of balanced crystalloids (lactated Ringer’s solution or Plasma-Lyte A) versus 0.9% saline solution in critically ill patients, to evaluate whether they presented with adverse kidney events and death, within the first 30 days. The study included 7,942 patients in the balanced-crystalloids group and 7,860 patients in the 0.9% saline solution group. The patients in the balanced-crystalloids group had a higher risk for adverse kidney events, compared with the saline solution group (marginal odds ratio [OR] 0.91, 95% CI 0.84 to 0.99; conditional OR 0.90, 95% CI 0.82 to 0.99; p = 0.04). In-hospital mortality at 30 days was 10.3% in the balanced-crystalloids group and 11.1% in the saline solution group (p = 0.06), the incidence of new RRT was 2.5% in the balanced-crystalloids group and 2.9% in the saline solution group (p = 0.08), and the incidence of persistent renal dysfunction was 6.4% and 6.6%, respectively (p = 0.60). The conclusion was that the use of balanced crystalloids, compared with the use of 0.9% saline solution, resulted in fewer adverse kidney events in critically ill patients.13 Myburgh et al. conducted a multicenter, prospective, blinded, randomized controlled trial that included 6,651 patients admitted to the intensive care unit (ICU) that required fluid resuscitation. Six percent hydroxyethyl starch was administered to 3,315 patients and 0.9% saline solution was administered to 3,336 patients. RRT was used in 235 (7%) patients in the hydroxyethyl starch group and in 196 (5.8%) patients in the saline solution group (RR 1.21, 95% CI 1.00 to 1.45; p = 0.04), 34.6% patients in the hydroxyethyl starch group and 38.0% in the saline solution group developed AKI (p = 0.005), and the use of hydroxyethyl starch was significantly associated with more adverse events (4.6% versus 3.3%; p = 0.006). The study showed that the use of 6% hydroxyethyl starch was related to a higher rate of RRT and adverse effects, compared with the administration of 0.9% saline solution.14 Given the information cited above, the first option in fluid therapy for patients with hypovolemic shock and LC is crystalloid therapy with Ringer’s lactate solution. Even though there are no studies that specify clinical and laboratory parameters in patients with LC that present with hypovolemic shock secondary to UGIB, different analyses conducted on critically ill patients with septic shock recommend the following goals to be reached within the first 6 hours of resuscitation: MAP ≥ 65 mmHg, ScvO2 ≥ 70%, central venous pressure (CVP) between 8 and 12 mmHg or 12-15 mmHg in patients undergoing invasive mechanical ventilation, and urine output ≥ 0.5 ml/kg/h.15
Blood products and vasopressor useIn patients with LC and UGIB, emergency action is necessary, given that they have high mortality and complication rates. Variceal bleeding leads to 10-20% mortality rates within the first 6 weeks. Medical treatment should begin as soon as possible, regardless of the endoscopic procedure, to restore tissue perfusion.10,16 During the treatment of UGIB of variceal origin, it is indispensable to administer vasopressors considered first line, such as terlipressin and octreotide, to reduce the portal venous pressure (PVP) (assessed by the hepatic venous pressure gradient [HVPG]) and bleeding. They should be started as soon as possible, before endoscopy, and continued for up to 5 days.9,17 Escorsell et al. carried out a multicenter randomized clinical trial that included 219 patients with LC and variceal UGIB; 105 of the patients received terlipressin and 114 underwent emergency sclerotherapy. The failure rate for terlipressin was 33% and for sclerotherapy was 32% (no significant difference). Rebleeding presented in 43% and 44%, respectively (p = 0.55). Side effects appeared in 20% of the patients that received terlipressin and in 30% that underwent sclerotherapy (OR 1.7; 95% CI, 0.91-3.17; p = 0.06). Both therapies were similar, with respect to the need for transfusion, length of hospital stay, and 6-week mortality. Those study results suggest that terlipressin could be a first-line treatment in acute variceal bleeding, until the definitive treatment is administered.18 Since the 1990s, a Cochrane review, through studies with adequate quality, showed that the use of terlipressin, compared with placebo, significantly reduced mortality in variceal UGIB (RR 0.66). The review was last updated in 2003, with no changes to the conclusions.19 Regarding the use of somatostatin analogues, such as octreotide, another Cochrane review, available and updated in 2008, showed no decrease in mortality, compared with placebo, but did show a reduction in the need for transfusions and a controversial decrease in the risk for rebleeding (due to the quality of the studies cited).20
With respect to blood products, blood volume resuscitation should be cautiously carried out because restoration with a liberal strategy can increase the risk for additional bleeding. In patients with variceal UGIB, it is recommended to maintain hemoglobin levels between 7-9 g/dl.16 Said recommendation has been validated and confirmed by the study conducted by Villanueva et al. Their randomized controlled trial on transfusion management for UGIB compared a restrictive strategy (transfusion when the hemoglobin level fell below 7 g/dl, with a target range for the hemoglobin level of 7-9 g/dl after transfusion) with a liberal strategy. A total of 921 patients were analyzed, 461 of whom were randomly assigned to the restrictive group and 460 to a liberal strategy. The probability of survival at 6 weeks was higher in the restrictive strategy group than in the liberal strategy group (95% versus 91%; the hazard ratio for death with the restrictive strategy was 0.55, 95% CI 0.33 to 0.92; p = 0.02), and adverse events occurred in 40% of the restrictive strategy group, compared with 48% in the liberal strategy group (p = 0.02). In the first 5 days, the HVPG significantly increased in the patients assigned to the liberal strategy (p = 0.03), but not in those assigned to the restrictive strategy. In addition, the probability of survival was greater with the restrictive transfusion strategy in patients with cirrhosis and Child-Pugh class A or B disease, but not in those with cirrhosis and Child-Pugh class C disease. Lastly, in the patients with esophageal variceal bleeding, the rebleeding rate was lower in the restrictive strategy group than in the liberal strategy group (11% versus 22%, p = 0.05).21 Based on the findings of that study, the current guidelines suggest starting transfusions for patients with variceal UGIB when hemoglobin levels fall below 7 g/dl, with a target range of 7-9 g/dl for the hemoglobin level.
Bacterial infections are frequent in patients with LC and UGIB and occur in 20% of patients in 48 hours.22 The short-term use of intravenous cephalosporine is particularly useful in patients with LC that have Child-Pugh class C disease. Therapeutic endoscopy (ligature or sclerotherapy) is the management of choice, once the patient with variceal UGIB is stabilized, and the majority of international guidelines recommend that it be carried out within 12 hours after hospital admission.16
Coagulation status correctionLiver dysfunction is characterized by presenting low plasma levels of proteins involved in coagulation (except factor VIII and the von Willebrand factor, which are increased). Those pathophysiologic phenomena explain the abnormalities in the traditional coagulation tests, such as prothrombin time and activated partial thromboplastin time, typically seen in patients with LC.23 Thrombocytopenia is the most common hematologic complication in patients with LC and the main mechanisms are reduced thrombopoietin (TPO) and splenic platelet sequestration. As the liver disease progresses, the platelet defect becomes progressive, impacting platelet adhesion, activation, and aggregation.24 In the past, correction of the international normalized ratio (INR) and platelets was considered a goal. It is currently recognized that the administration of plasma does not improve thrombin production in patients with LC that have altered coagulation tests, and it can even exacerbate portal hypertension.25 The foregoing was supported by Rassi et al. in a prospective study with 53 patients that received a standard dose of fresh frozen plasma (FFP) for treating major bleeding or was used before invasive procedures. Transfusion of FFP only improved the production of thrombin and conventional coagulation tests to normal values in a limited number of patients and slightly reduced the production of thrombin in 34% of the cases. Those results support the fact that FFP transfusion in patients with LC slightly improves coagulation test values in only a limited number of patients, and in contrast, there can be unfavorable effects in one-third of the cases, and so the transfusion of FFP should be considered on an individual basis.26 Regarding the transfusion of platelets, there is no consensus on the appropriate threshold value for the transfusion in patients with LC, but it is usually performed at a threshold of 50 x 109/l in cases of bleeding or when invasive procedures are required. Another factor complicating the recommendations for platelet transfusion is that the quantity of platelets does not reflect platelet function, which can be altered by drugs, infections, or kidney injury.27,28 Lastly, fibrinogen levels are known to be normal or slightly increased in patients with compensated LC. However, patients with decompensated LC often have reduced levels, which has increasingly been recognized as an independent risk factor for increased bleeding, with a 29% increase in the probabilities of death for every reduction of 1 g/l of fibrinogen, as demonstrated in a retrospective analysis of 1,313 patients with LC, carried out by Desborough et al.29,30 However, different medical societies and expert groups have proposed maintaining fibrinogen levels above 100-120 mg/dl, in the context of acute bleeding, with fibrinogen replacement through cryoprecipitates, instead of FFP.27,31 Currently, there are synthetic fibrinogen concentrates, whose advantages over FFP are: a lower risk of infection, precise and consistent formula-based dosage, low-volume infusions, and expedited administration because no cross-over tests are required. Nevertheless, its use has not been described in quality studies on patients with LC and there are no recommendations regarding it, albeit they could be extrapolated from contexts other than LC.32 Other tests, such as thromboelastography, have been studied at pre-procedure stages and on UGIB in patients with LC. A significant benefit has only been seen with respect to the outcome of reducing the need for transfusion, but not in mortality or rebleeding events.33 Therefore, current guidelines only refer to its use as a knowledge gap and issue no recommendations on it.34 Whether its use is actually a benefit for patients or is simply a way to preserve blood component resources, is a question that has also arisen.35
Key points- 1
Determining the patient’s vital signs is indispensable in making the diagnosis of hypovolemic shock, which is also supported by clinical signs of hypoperfusion and indirect markers, such as lactate levels. Changes derived from hyperdynamic circulation and pharmacologic treatment with beta-blockers are to be taken into account.
- 2
Starting early intravenous fluid resuscitation (crystalloids) is recommended in patients with LC and hypovolemic shock.
- 3
Restoring tissue perfusion and maintaining MAP>65mmHg and SAP between 90-100mmHg.
- 4
The pharmacologic treatment of acute variceal UGIB should include vasopressors (terlipressin, somatostatin, octreotide, vasopressin).
- 5
Transfusion therapy is recommended when hemoglobin levels are < 7g/dl, with a restrictive strategy of 7-9g/dl.
There is not enough evidence to specifically determine severity status through vital signs, in the patient with LC. Therefore, the recommendation is to evaluate other relevant variables that have been described in patients with sepsis/septic shock, without LC, i.e., MAP < 65 mmHg, tachycardia (HR >100 beats per minute), and oliguria (< 0.5 ml/kg/h). Despite the fact that no study has evaluated goal-directed therapy in LC patients with sepsis or septic shock, clinical practice suggests that early resuscitation is equally as important. However, the “goals” could differ from those in the general population, given that patients with LC usually have low AP, higher ScvO2, reduced diuresis and hematocrit, as well as altered lactate clearance.15 In LC, MAP is lower and ScvO2 is higher because of hyperdynamic circulation,36 and additionally, in initial phases of the disease, the decrease in systemic vascular resistance is compensated by the development of hyperdynamic circulation, characterized by increased HR and cardiac output (CO). In a retrospective report on alcohol-induced LC, a worsening of oxygen transport as the liver disease advanced (mixed venous saturation, Child-Pugh class A: 73 versus Child-Pugh class C: 78.7; p < 0.05) and greater lactate concentration (A: 0.79, B: 0.87, C:2.17; p < 0.05) were demonstrated. The increase in lactate in advanced LC may be the result of tissue hypoxemia or altered clearance due to liver failure.6 Despite the knowledge that those variables (MAP, ScvO2) can be different in patients with LC, no study has been published that proposes a different expected normality value in the LC population. In a 2016 article, a group of experts posited utilizing a MAP goal of 60 mmHg in LC and recommended not using ScvO2 or lactate clearance as goals, due to the differences commented on above.37–39 In another prospective study on 142 patients with LC and 14 healthy controls, in which lactate in the hepatic vein and femoral artery was determined, there were higher venous and arterial lactate levels in patients with LC, compared with healthy subjects (p < 0.001), with an increase in levels as the disease advanced.38 Additional studies corroborate the evidence that the serum lactate level in liver failure is higher in healthy controls (8.24 mmol/l versus 4.29 mmol/l, p < 0.01).39 Nevertheless, there is no established cutoff point for defining the time at which hyperlactatemia should be considered present in patients with LC. A retrospective study that included 35 patients in the ICU with chronic liver disease reported that lactate > 2.2 mmol/l was associated with a higher mortality rate (58%) and clinical evidence of shock, and so the authors concluded that lactic acidosis in patients with LC was associated with clinical evidence of shock and increased in-hospital mortality.40 Patients with LC that present with sepsis/septic shock and hyperlactatemia have been shown to have a worse prognosis. In a prospective study by Sun et al. that included 480 patients with LC in the ICU, complicated with AKI, that had one year of follow-up, elevated serum lactate levels were associated with a higher mortality rate (<1.8mg/dl, 56% mortality; 1.9-2.4 mg/dl, 62% mortality; 2.5-4.0 mg/dl, 72% mortality; and > 4.1 mg/dl, 75% mortality).41 Current evidence makes it clear that, despite not knowing the exact cutoff point for defining severity, patients that are admitted with hyperlactatemia, and clear their lactate, have a better prognosis. In a multicenter study conducted by Drolz et al. that included 678 critically ill patients with LC in the ICU and a validation cohort of 250 patients, in which arterial lactate was measured upon admission and a one-year follow-up was carried out, lactate upon admission was directly proportional to the number of organ failures and mortality at 28 days (AUROC 0.72; p < 0.001). Lactate at admission ≥ 5 mmol/l and its clearance at 12 hours were significant predictors of 1-year mortality.42
The Sepsis-3 consensus established that a sequential organ failure assessment (SOFA) score is a better scoring system for making the diagnosis and prognosis of patients with sepsis and septic shock. A score ≥ 2 points is equivalent to organ failure and is the criterion for diagnosing sepsis and greater severity.43 There is evidence that utilizing the Sepsis-3 criteria in patients with LC provides better diagnostic and prognostic yield, than the former criteria based on the systemic inflammatory response syndrome in patients with LC.44 However, with the advent of acute-on-chronic liver failure (ACLF) and its close relation to infections and sepsis/septic shock, the European Association for the Study of the Liver-Chronic Liver Failure Consortium (EASL-CLIF Consortium) changed the SOFA score, creating the “CLIF-SOFA” score, and defined ACLF according to the new score. In the CLIF-SOFA score, 6 organ systems are assessed, with specific changes made to account for the special situations in end-stage liver disease. Among the outstanding modifications were: changing the platelet count to the INR or PT and substituting the Glasgow scale with HE as the criterion for central nervous system involvement. In addition, oxygen saturation/FiO2 was added as an alternative for patients without an arterial line.45 Recent studies on patients with LC that present with sepsis and septic shock have shown the efficacy of those scores for predicting severity and mortality, such as the study by Engelmann et al. In their retrospective analysis that included 202 patients with LC and organ failure, the CLIF-C ACLF was the best score for 28-day mortality, with an area under the curve (AUC) of 0.8 and a mortality rate of 100% at 28 days in patients with a score > 70.46 In addition, in a prospective study by Silva et al. that included 192 patients, the AUC for predicting mortality at 30 days was 0.84, with 64% sensitivity and 90% specificity.47
Adrenal insufficiency (AI) is frequent in patients with LC and sepsis or septic shock (51-77%) and is associated with hemodynamic instability, kidney injury, liver failure, critical illness severity, and a higher mortality rate (80-76% versus 36.7%; p ≤ 0.001) than in patients with no AI.9,48 Fernandez et al. reported an incidence of AI of 68% in LC and septic shock. They also evaluated treatment with intravenous hydrocortisone at a dose of 50 mg every 6 hours versus patients without hydrocortisone, finding that septic shock resolution occurred in 96% versus 58% (p = 0.001) and the in-hospital survival rate was 64% versus 32% (p = 0.003), and was higher in the patients that received hydrocortisone.49 A double-blinded prospective study that included 75 patients with LC and septic shock reported a prevalence of AI of 76%. The group that received the steroid had a greater reduction of vasopressor doses, higher rates of shock reversal (RR 1.58, 95% CI 0.98-2.55; p = 0.05), with no reduction in the 28-day mortality rate (RR 1.17, 95% CI 0.92-1.49; p = 0.19). However, the steroid group had an increase in shock relapse (RR 2.58, 95% CI 1.04-6.45; p = 0.03) and gastrointestinal bleeding (RR 3, 95% CI 1.08-8.36; p = 0.02).50
Administration of intravenous solutionsThe Surviving Sepsis guidelines recommend crystalloid solutions (0.9% saline or Ringer’s lactate) as the initial solution in the resuscitation of patients with sepsis. Starting with 30 ml/kg within the first 3 hours is recommended, albeit some patients may require a higher volume. Utilizing starches as volume expanders is not recommended.15,43 The evidence on the management of intravenous solutions in the patient with sepsis/septic shock does not include patients with LC, but there are studies providing interesting evidence on the use of colloids, that should be pointed out. The multicenter SAFE study included almost 7,000 patients admitted to the ICU that needed fluid administration to maintain or increase intravascular volume; they were randomized to receive 4% albumin or 0.9% saline solution. At 28 days, there were no significant differences in mortality or new organ failures, days in the ICU, or days of mechanical ventilation, but patients with septic shock or sepsis were not analyzed, and so it cannot be inferred from that study that albumin is inferior to crystalloid substances.51 In a blinded multicenter study, Pemer et al. randomized patients with severe sepsis in the ICU for fluid resuscitation with Tetraspan (hydroxyethyl starch) or lactated Ringer’s solution. Mortality was significantly higher in the starch group (51% versus 43%, RR 1.17, 95% CI 1.01-1.36; p = 0.03). Likewise, the starch group had major kidney injury, with a need for RRT (22% versus 16%, RR 1.35, 95% CI 1.01-1.80; p = 0.04), but patients with LC were not included.52 In a systematic review and meta-analysis that included 38 studies and 10,880 patients, there was a higher mortality rate with the use of starch (RR 1.07, 95% CI 1-1.14, I2, 0%), greater kidney injury (RR 1.27, 95% CI 1.09-1.47, I2, 26%), and greater use of RRT (RR 1.32, 95% CI, 1.15-1.50; I2, 0%).53 That type of colloid is not recommended for the general population, nor, knowing the pathophysiologic aspects of the disease, is it recommended in the patient with LC.
The use of albumin is controversial and only recommended for reducing fluid overload and anasarca in the patients that require large volumes of crystalloids, given that the risk for those conditions increases in LC. Its use could be justified in that context, but there are no studies supporting it.15,43 Albumin infusions were first used in patients with LC more than 70 years ago and they are still widely prescribed for restoring normal blood volume in patients with peripheral arterial vasodilation.54 With respect to albumin use in the general population, the Surviving Sepsis guidelines recommend it in patients that received large volumes of crystalloids and require an increase in blood volume. In patients with LC, its use is not totally clear, even though there are certain precise indications in which albumin infusion is of vital importance, such as spontaneous bacterial peritonitis (SBP), whereas in the rest of the infections in LC, there is still no solid evidence for recommending its use. An unblinded randomized study comparing albumin administration with that of hydroxyethyl starch in patients with SBP documented a significant increase in AP and suppression of plasma renin activity, indicating improvement in circulatory function.55 Another prospective study that has been a reference on albumin use in SBP was conducted by Sort et al. They reported the effectiveness of albumin for preventing kidney injury (33% versus 10%; p = 0.002) and achieving a lower mortality rate (29% versus 10%; p = 0.01). Nevertheless, the study did not include patients with sepsis or septic shock.56 Despite that lack of information, it is logical to think that albumin would be an ideal resuscitation therapy in the patient with septic shock. In a study presented at the 2015 Congress of the American Association for the Study of the Liver (AASLD), Philips et al. reported the results of patients with LC and septic shock (n = 308), with 154 patients per group. One group received 250 ml of 5% albumin in bolus for 15 minutes and the other group received 30 ml/kg of 0.9% saline for 30 minutes. MAP > 65 mmHg and sustained for 3 h, with better results, was reported in the albumin group (25.3% versus 14.3%, p < 0.001, OR 1.9, 95% CI 1.08-3.42). In addition, sustained reduced HR was greater in the albumin group (94 versus 103 beats per minute; p = 0.001), the increase in urine output was similar, and improvement in lactate was greater in the albumin group (p < 0.01); survival was also better at one week in the albumin group (43.5% versus 38.3%; p = 0.03).57 Lastly, one of the most recent studies on albumin in LC, the Albumin to Prevent Infection in Chronic Liver Failure (ATTIRE) study, is a multicenter analysis conducted in the United Kingdom to evaluate the efficacy of albumin for preventing infections, kidney dysfunction, or death, in patients with decompensated LC. It reported no effect on the incidence of infections, reflected in the absence of significant differences in the incidence of new infection or end-point events in the patients that were admitted with infection or that were receiving antibiotics at enrollment. Despite the targeted regimen to increase the serum albumin level to 30 g/l or more, there were no apparent benefits of the intervention in relation to the primary end point in any of the subgroups analyzed. There were also no significant differences between groups regarding the incidence of death at 28 days, 3 months, and 6 months.54
Another current theme is preventing overhydration or the abuse of rescue therapy, given the information from a recent retrospective study with more than 300 patients with LC and ACLF in the ICU, in which positive fluid balances were shown to be associated with higher mortality (OR: 1.04, 95% CI 1.01-1.07).58
Treatment with vasopressors in septic shock and liver cirrhosisAn emergent, rapid, and scaled resuscitation established in the early stage of sepsis (the first 6 hours) improves the results in patients without LC with sepsis or septic shock, in terms of organ dysfunction and survival.9 The 2016 Surviving Sepsis guidelines recommend the use of noradrenaline (NAD) as the vasoconstrictor of choice, in patients with septic shock. Epinephrine (added to or substituting NAD) can be used when a second vasopressor is needed to maintain adequate MAP. Vasopressin in low doses (0.03 U/min), added to NAD, can be utilized to elevate MAP to the goal or reduce the dose of NAD.10,59 NAD increases MAP due to is vasoconstricting effects, with few changes in HR and a lower increase in stroke volume, compared with dopamine. Dopamine increases MAP and CO, mainly due to an increase in stroke volume and HR. Therefore, NAD is more potent than dopamine and is more efficacious for reversing hypotension in patients with septic shock.15
Alpha-adrenergic effects increase vascular tone but can reduce CO and the regional blood flow, especially in the cutaneous, splanchnic, and renal beds. Beta-adrenergic effects help maintain the blood flow through inotropic and chronotropic effects, increasing splanchnic perfusion. That beta-adrenergic stimulation can also have undesirable consequences, including an increase in cell metabolism and immunosuppressive effects.60 Currently, dopamine is only used in selected cases due to its high risk for inducing arrythmias, compared with NAD. Dobutamine administration in patients with LC and septic shock is only recommended in those with clinically significant myocardial dysfunction because they tend to have an elevated CO.10,15,60 Studies on humans and animals suggest several advantages of NAD and dopamine over epinephrine or phenylephrine due to their adverse effects on cardiac function. However, there is no clinical evidence that epinephrine produces worse clinical results and it would be the first choice as an alternative to dopamine or NAD.15 Another possible disadvantage of epinephrine, more in the context of LC, is that it can increase aerobic lactate production through the stimulation of skeletal muscle β2-adrenergic receptors, and so may preclude the use of lactate clearance to guide resuscitation.59 With its almost pure α-adrenergic effects, phenylephrine is the adrenergic agent with less probability of producing tachycardia but it can reduce stroke volume. Thus, it is not recommended in the treatment of septic shock, except when NAD is associated with serious arrythmias, the patient presents with severe arrythmias, or it is the salvage therapy chosen when other vasopressors have failed to achieve the target MAP.15 Vasopressin has been used as an amine complement for patients that have severe septic shock. The rationale for its use is the relative vasopressin deficiency in patients with septic shock and the hypothesis that exogenously administered vasopressin can restore vascular tone and AP, thus reducing the need for catecholamine use.61 Low doses of vasopressin can be efficacious for elevating AP in patients with a refractory response to other vasopressors and can have other potential physiologic benefits. Terlipressin has similar effects but is long-acting.15 In a randomized controlled trial, Choudhury et al. showed that terlipressin was as efficacious as NAD, as a vasopressor in patients with LC and septic shock. In addition, it provided an early survival benefit, with a reduced risk for variceal bleeding. Terlipressin was as efficacious as NAD for achieving a MAP > 65 mmHg at 6 and 48 hours.62 A systematic review showed that the use of corticosteroids in sepsis can result in a small absolute reduction in the mortality rate of approximately 2%.63
Key points- 1
Patients with LC usually have low AP, higher ScvO2, reduced diuresis and hematocrit, and altered lactate clearance. There is no evidence that cutoff points or goals regarding resuscitation are different in LC.
- 2
The Sepsis-3 criteria in patients with LC have better diagnostic and prognostic yield than the former criteria. The CLIF-SOFA score adequately predicts both severity and prognosis.
- 3
The administration of intravenous crystalloids at 30ml/kg of weight is recommended in the first hours of resuscitation. The use of colloids other than albumin is associated with a higher complication rate.
- 4
Vasopressor use is recommended in LC and septic shock, in accordance with the Surviving Sepsis guidelines.
- 5
AI is frequent in patients with LC and septic shock. The use of corticosteroids in sepsis can result in a small absolute reduction of mortality.
HE is a complication of portal hypertension, defined as brain dysfunction secondary to liver failure and/or portosystemic shunts that manifests as a broad spectrum of neuropsychiatric abnormalities, ranging from subclinical alterations to coma.64,65 According to guidelines proposed by the AASLD and EASL, HE should be classified according to all 4 of the following factors: underlying liver disease, clinical severity, time course, and the presence or absence of precipitating factors.64 In the first axis, 3 types of HE are considered (A, B, and C). Type A describes HE associated with acute liver failure, type B with the presence of portosystemic shunts in the absence of evidence of hepatocellular disease, and type C with LC.66 For type B and type C HE, clinical severity is determined according to the West Haven classification.65–67 In patients with an altered consciousness status, the Glasgow coma scale is useful as a descriptive tool and is less subject to interobserver variability, than mental status assessment.65,66 According to the existence of precipitating factors, HE is subdivided into non-precipitated and precipitated. Precipitated factors include infections, gastrointestinal bleeding, overdose of diuretics or other medications (such as benzodiazepines or opioids), electrolyte disorders, and constipation.64
Covert HE (minimal and grade 1)68 includes patients with no clinical symptoms of recognizable brain dysfunction and its diagnosis is based on a thorough neuropsychiatric evaluation that involves at least 2 psychomotor tests.65 Grades III and IV of HE are classified as organ failure at the neurologic level, and thus acquire greater importance in the emergency context, meriting the implementation of airway protection protocols, as well as the use of enteral tubes for treatment administration.
Administration of intravenous solutions in the context of hepatic encephalopathyVolume expansion has been hypothesized to produce suppression at angiotensin II levels, resulting in an increase in the urinary excretion of ammonia. A study by Jalan et al. showed that the infusion of 1,000 ml of 0.9% saline solution in one hour was associated with a significant decrease in plasma renin and angiotensin II activity that was associated with a significant decrease from 93 to 56 micromoles/l (p<0.05) in plasma ammonia levels in patients with compensated LC.69
Specific emergency treatment in hepatic encephalopathy. Treatment of manifest or overt HE includes supportive care, identification and correction of precipitating factors, reduction of the nitrogenous load from the gut, and secondary prophylaxis.64,66
Drug management of hepatic encephalopathyNonabsorbable disaccharides: lactulose and lactitol. Lactulose is the standard treatment in HE. It reduces ammonia levels through acidification of the colon, with the resulting conversion of ammonium to ammonia, modifying the urease-producing colonic bacteria into non-urease producers.67 The initial dose recommended by the AASLD/EASL is 25 ml (16.7 g) every 1 to 2 hours, until achieving at least 2 soft bowel movements, which would be the goal in the emergency correction effort in HE, guaranteeing its adequate administration through enteral tubes in patients whose neurologic status prevents them from swallowing adequately or in those that require airway protection.60 Nevertheless, it is an area of research opportunity, given that there are no high-quality studies for analyzing whether that maneuver truly has a favorable impact on clinical results or not. Nonabsorbable disaccharides prevent the development of HE (RR 0.47, 95% CI 0.33-0.68, number needed to treat [NNT] = 6), with no differences in their efficacy or safety between lactulose and lactitol. Nonabsorbable disaccharide effectiveness was evaluated in a systematic review and meta-analysis that included 1,828 patients and 38 randomized controlled trials, compared with that of placebo or no intervention. Nonabsorbable disaccharides had a beneficial effect on HE, with a RR of 0.63 (95% CI 0.53-0.74) and NNT of 4, as well as a decrease in mortality in patients with overt HE (RR 0.36, 95% CI 0.14-0.94, NNT = 20).70 A recent study compared the efficacy of lactulose with polyethylene glycol, reporting similar clinical outcomes, and in some cases, clinical superiority with the latter.71
Nonabsorbable antibiotics. Rifaximin is an antibiotic derived from rifamycin that binds to the β subunit of bacterial DNA-dependent RNA polymerase and inhibits RNA synthesis, altering the gut microbiota and decreasing ammonia-producing microorganisms.67,72 The latest recommendations suggest that rifaximin is effective as additive therapy to lactulose as secondary prophylaxis.64 With respect to initial management, a recent systematic review and meta-analysis showed that the combination of rifaximin and lactulose significantly increased clinical efficacy, compared with lactulose in monotherapy (risk difference [RD] 0.26, 95% CI 0.19-0.32, p < 0.00001, NNT 5), in addition to decreasing mortality (RD –0.16, 95% IC –0.20-0.11, NNT 9).73
L-ornithine-L-aspartate (LOLA). LOLA increases the metabolism of ammonia to glutamine and decreases the levels of ammonia in plasma.74 A beneficial effect on mortality (RR 0.42, 95% CI 0.24-0.72) and HE (RR 0.70, 95% CI 0.59-0.83) has been shown, upon comparing LOLA with placebo and with other active agents, in a meta-analysis of 29 randomized controlled trials, with 1,891 participants, in which LOLA had no effect on mortality or HE, with very low quality evidence.75 Intravenous administration of LOLA can be used as an alternative or additional agent in patients that are non-responders to conventional treatment, given that a study demonstrated improvement in psychometric tests and ammonia levels in patients with persistent HE.64
Treatment of the most common precipitating factors and concomitant emergency management of hepatic encephalopathyThe identification and management of precipitating factors is one of the cornerstones of treatment of overt HE. Almost 90% of patients can be treated simply through the correction of a precipitating factor.64 According to the consensus of the International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN), the management of an acute episode of overt HE involves the identification and treatment of the precipitating factors: infections (systemic antibiotics), gastrointestinal bleeding (control of bleeding), diuretic overdose (volume expansion), constipation (laxatives), alcohol (thiamine), and electrolyte alterations.76 The most frequent precipitating factors in HE, reported in a descriptive study that included 132 patients with LC, were: infection (49.2%), electrolyte alterations (41%), constipation (33.3%), and gastrointestinal bleeding (16%). Of the infections, the most frequent was SBP (18%), followed by respiratory tract infections (14.4%) and urinary tract infections (13.7%). Hyponatremia was the most common electrolyte imbalance, followed by hypokalemia. Treatment with lactulose and rifaximin was superior to treatment with lactulose as monotherapy, with shorter hospital stay (7 ± 3.6 versus 9.64 ± 5.28 days, p = 0.015). Nevertheless, the difference in mortality was not statistically significant.77
Even though HE is the most frequent cause of altered mental status in the patient with LC, the differential diagnosis is extensive. Some of the most frequent clinical manifestations reported in patients with overt HE are confusion (78%), changes in mental status (57%), disorientation to time, space, or person (48%), lethargy (46%), and asterixis (45%). Other less frequent manifestations are somnolence, forgetfulness, changes in the sleep-wake rhythm, difficulty concentrating, changes in everyday activities, slurred speech, changes in personality, irritability, inappropriate behavior, and coma.78 In a retrospective case-control study that included 349 patients with decompensated LC and altered mental status, the most frequent cause of altered consciousness was HE (47%), followed by sepsis (23%). Other less frequent causes were metabolic alteration, such as hyponatremia < 125 mEq/l or hypoglycemia < 60 mg/dl (8%); ingestion of toxic agents (7%); structural brain injury (neurologic focal deficit or convulsive crises or signs of ischemia, hemorrhage, or tumor in cranial computed tomography scans) (5%); and lastly, psychiatric alterations (1%). Compared with patients with normal mental status, the patients with altered mental status had higher levels of serum bilirubin, INR, and creatinine, a lower level of albumin, and a higher model for end-stage liver disease (MELD) score (p ≤ 0.001). Mortality in the patients with altered mental status was significantly higher than in the normal mental status patients (35% versus 16%, p < 0.0001).79 Cranial computed tomography as the initial approach to altered mental status in patients with LC has been evaluated in different studies. One reported that more than half of the patients (64%) had a tomography study at admission. Of the patients with LC, altered mental status, and no focal neurologic signs upon physical examination, none had structural injuries on the cranial tomography scan, whereas 100% of the patients with focal neurologic signs (n = 25), manifested as facial hemiparesis or cranial nerve involvement (6 patients), hemiparesis or hemiplegia (5 patients), sudden unresponsiveness to stimuli (5 patients), convulsive crises (5 patients), aphasia, decorticate posture, and the presence of Babinski’s sign, had structural injuries. The tomographic findings were cerebrovascular event,75 intraparenchymal bleeding,72 and tumor.66,80 Another study showed a low probability of intraparenchymal bleeding in patients with LC and altered mental status, with no signs of focal neurologic deficit or trauma, with an OR of 0.02 (95% CI 0.001-0.14). For patients with low-risk indications for tomography, the NNT for intraparenchymal bleeding varied according to the indication; it was 9 for focal neurologic deficit, 20 for fall/trauma, and 293 for altered mental status.81
Key points- 1
HE should be classified according to all 4 of the following factors: underlying liver disease, clinical severity, time course, and the presence or absence of precipitating factors.
- 2
Intravenous volume expansion produces angiotensin II level suppression, resulting in an increase in the urinary excretion of ammonia, leading to the proposal that crystalloid infusion can be useful in the management of HE.
- 3
The treatment of HE includes supportive care, identification and correction of precipitating factors, reduction of the nitrogenous load from the gut, and secondary prophylaxis.
- 4
Despite the fact that HE is the most frequent cause of altered mental status in the patient with LC, the differential diagnosis is extensive.
- 5
Patients with LC and altered mental status, with no focal neurologic signs upon physical examination, have been shown to rarely present with structural injuries in cranial computed tomography.
AKI occurs in up to 50% of hospitalized LC patients. That increased risk is due to the combination of impaired effective arterial blood volume secondary to arterial vasodilation, with an increase in intrarenal vasoconstriction and altered renal autoregulation.37 Infections, sudden onset of hyperbilirubinemia, gastrointestinal bleeding, increase in diuresis, or use of nephrotoxic agents produce alterations in the circulatory status with reduced kidney perfusion, precipitating AKI. Those factors should be rapidly identified and corrected to prevent the development of later renal complications.82 The different classifications of ACLF include different definitions of organ failure at the level of the kidney, scoring the creatinine level in a range as low as 0.7 mg/dl (augmented renal clearance in trauma intensive care [ARCTIC]) to reaching the need for RRT (North American Consortium on End-Stage Liver Disease [NACSELD]), resulting in a very wide definition of AKI as a medical emergency. Thus, AKI, regardless of its etiology, should always be categorized as an emergency in LC, given that it can be an inflection point in the progression of decompensation of LC that merits emergency management.
Different types of AKI can occur in patients with decompensated cirrhosis. AKI of pre-renal origin is the most frequent, presenting in 70% of the cases. In addition to parenchymal or intrinsic AKI (acute tubular necrosis), patients with LC can present with hepatorenal syndrome (HRS), that is characterized by the lack of response to volume expansion with albumin, absence of shock, and no signs suggesting renal parenchymal disease.83
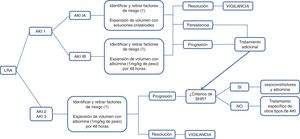
According to the International Club of Ascites (ICA), AKI is defined as an increase in serum creatinine ≥ 0.3 mg/dl in 48 hours or a 50% increase in baseline serum creatinine, established as serum creatinine in 7 days, encompassing 3 previous months, and classified in 3 stages. In stage 1, there is an increase in serum creatinine ≥ 0.3 mg/dl or 1.5 to 2-times higher than the baseline creatinine, which in turn, is classified as 1A and 1B; in the former, the serum creatinine level is < 1.5 mg/dl, and in the latter, is ≥ 1.5 mg/dl. Patients in stage 1A have a short-term mortality similar to patients without kidney injury and return to normality more frequently than those with stage 1B. Stage 2 is defined as serum creatinine 2 to 3-times higher than the baseline, and in stage 3, there is an increase > 3-times higher than the baseline creatine, or the serum creatinine level is ≥ 4.0 mg/dl, with a sudden increase ≥ 0.3 mg/dl or the start of RRT. In patients that do not have a record of their previous baseline creatinine, their creatine level at admission should be used as the reference84 (Fig. 1).
Administration of intravenous solutionsThe evaluation of intravascular volume is the first step in determining AKI etiology in patients with decompensated LC. Blood volume evaluation is difficult due to the presence of hyperdynamic circulation. According to the EASL guidelines on the management of patients with decompensated LC, regardless of AKI stage, the administration of diuretics, beta-blockers, and nephrotoxic drugs, such as nonsteroidal anti-inflammatory drugs and vasodilators, should be suspended and bacterial infections should be recognized and treated early. Volume expansion should be carried out according to the cause and severity of fluid loss. In the classification proposed by the ICA, stage 1A AKI, the stage at which hypovolemia is the most frequent cause, volume expansion can be carried out using crystalloid solutions, in cases of volume loss due to diarrhea or increased diuresis or red blood cell transfusions in patients with gastrointestinal bleeding.37,69,85,86
Volume expansion, with approximately 1.5 liters of normal saline solution or 1 g/kg of albumin (maximum of 100 g/dose), should be administered as soon as AKI is identified in a patient with LC. The reversal of AKI in 48 hours with bland urine sediment is suggestive of pre-renal injury.87
In advanced LC, the response to volume administration is abnormal, free-water clearance is reduced due to an increase in sympathetic nervous system activity, low glomerular filtration rate, sodium retention, and alterations in water absorption in the distal tubules and collecting ducts. Volume expansion with saline solution increases stroke volume and CO, reduces systemic vascular resistance, and can result in plasma and interstitial space expansion. The majority of the intravascular fluid is retained in the veins of the splanchnic system. Patients with LC also have sodium retention and an increased load of sodium will not result in increased sodium excretion by the kidneys.88
Intravascular volume expansion in the patient with decompensated LC can potentially produce the worsening of ascites, pleural effusion, or heart failure. The clinical evaluation of blood volume is difficult, given that the intravascular volume can be depleted, despite the existence of ascites and peripheral edema. The usefulness of the noninvasive evaluation of blood volume in the patients with cirrhosis has previously been described. A study that utilized echocardiography, measuring the size and collapsibility of the inferior vena cava, and multi-frequency bioimpedance showed that the patients with compensated and decompensated cirrhosis had increased fluid retention in the trunk and legs, even though ventricular filling pressures were normal and there was no peripheral edema. The patients with decompensated cirrhosis had extracellular volume expansion due to increased vascular permeability. None of those tools were reliable for predicting intravascular volume, and so clinical evaluation continues to be the crucial element in the evaluation of blood volume in patients with chronic liver disease.89
Usefulness of albuminHuman serum albumin is responsible for 75% of plasma oncotic pressure, and therefore, intravascular albumin administration increases the circulating blood volume, in addition to having antioxidant, immunomodulatory, and detoxifying functions.90 It is a molecule with a negative charge that attracts sodium, which in turn, retains water, increasing the oncotic pressure in the patient with LC. The synthesis of albumin in the hepatocyte is reduced, added to a reduced concentration of effective albumin.91 Albumin infusion in patients with LC initially produces the passage of interstitial space fluid to the plasma volume, with its consequent expansion due to the oncotic forces. Later, the infused albumin is then redistributed.88 In the evaluation of patients with AKI, the volume expansion property of albumin is utilized to determine whether kidney dysfunction responds to volume expansion.91
According to the ICA and EASL consensus, intravenous albumin at a dose of 1 g/kg of weight for 2 consecutive days, with a maximum of 100 g per day, should be administered to the patient with AKI at a stage > 1A. In that context, if the cause of the AKI is hypovolemia (pre-renal) it will resolve; if it does not, then HRS or acute tubular necrosis should be ruled out.68,86
Despite the fact that in critically ill patients without LC, a reduced 28-day mortality rate, comparing the administration of albumin (4% albumin) with that of crystalloids (normal saline solution), has not been shown (RR 0.99, 95% CI 0.91-1.09, p = 0.87).53 In patients with LC, albumin administration is recommended because of its anti-inflammatory and antioxidant properties.82
HRS is the consequence of an extreme circulatory dysfunction, with splanchnic arterial vasodilation and cirrhotic myocardiopathy associated with diastolic and systolic dysfunction that leads to renal hypoperfusion and kidney failure.90
The 2007 ICA consensus classifies HRS into type 1 and type 2. Type 1 is characterized by rapid decline in general kidney function secondary to a precipitating event, whereas type 2 is characterized by a moderate and slow decline of kidney function, with no identifiable precipitating event. Nevertheless, in the latest updating in 2015, the ICA proposed modifying the term type 1 HRS to HRS-AKI. It is defined as the lack of response to the administration of albumin at a dose of 1 g/kg of weight for 2 consecutive days, associated with the withdrawal of diuretics; it occurs in patients with LC, ascites, and AKI, according to the ICA-AKI criteria, in the absence of shock and nephrotoxic medication use (NSAIDs, aminoglycosides, iodinated contrast media); there are no macroscopic signs of renal parenchymal injury, defined as proteinuria below 500 mg/day, the absence of microhematuria (> 50 red blood cells per high power field), and normal kidney ultrasound study. The creatinine level > 2.5 mg/dl, necessary for making the diagnosis in accordance with the previous definition, was eliminated, for the purpose of starting early treatment with vasoconstrictors and albumin.86
Angeli et al. recently proposed the reclassification of HRS into HRS-AKI and non-acute kidney injury (NAKI), which in turn, is classified into HRS-acute kidney disease (HRS-AKD) and HRS-chronic kidney disease (HRS-CKD). They are defined as a decrease in the estimated glomerular filtration rate < 60 ml/min/1.73 m2 for < 3 months (HRS-AKD) and > 3 months (HRS-CKD), in the absence of other structural causes.84
Once the diagnosis of HRS is made, specific treatment should be started. Vasoconstrictors, particularly terlipressin, associated with albumin administration, have shown significant improvement in kidney function and survival. A systematic review and meta-analysis that included 13 randomized controlled trials, with 739 patients with type 1 HRS, reported that the use of terlipressin with albumin reduces mortality in the short term (OR 0.65, 95% CI 0.41-1.05), compared with placebo, and that terlipressin with albumin, as well as noradrenaline with albumin, are superior to midodrine plus octreotide with albumin for reversing HRS (OR 26.25, 95% CI 3.07-224.21).92
Albumin is crucial in the effectiveness of treatment of HRS. Terlipressin efficacy as monotherapy (3.2 ± 1.3 mg/day) was retrospectively evaluated in a multicenter study that included 99 patients with type 1 HRS, reporting kidney function improvement in 58% of the patients.93 The greatest efficacy obtained with the albumin-terlipressin combination, compared with terlipressin alone, could be secondary to a decrease in CO, associated with terlipressin administration, given that albumin has the capacity to maintain or increase CO, even at advanced stages of LC.84 The albumin dose recommended by the EASL is from 20 to 40 g/day, monitoring volume overload. Treatment should be maintained until the kidney injury is resolved, which is determined by creatinine < 1.5 mg/dl, or for a maximum of 14 days, in case of either a lack of response or a partial response (decrease ≥ 50%, with creatinine still >1.5 mg/dl).68
Different studies have shown that the albumin-terlipressin combination is more effective than albumin as monotherapy in the treatment of type 1 HRS. One study included 52 patients with HRS, assigned to receive terlipressin and albumin or albumin as monotherapy. Twenty-one (80%) of the patients assigned to the terlipressin and albumin group had complete response, compared with 5 (19%) from the albumin group (p ≤ 0.001).94 In another study, with 46 patients assigned to terlipressin (1-2 mg IV every 4 h) and albumin (1 g/kg single dose, followed by 20 to 40 g/day) or albumin as monotherapy, 43.5% of the patients in the terlipressin group had improved kidney function, compared with 8.7% in the albumin group (p = 0.017). There was no difference in survival at 3 months between the two groups (27% versus 19%; p = 0.7).95 The recently published phase 3 CONFIRM study was conducted to verify the safety and efficacy of terlipressin, associated with albumin, in adults with type 1 HRS. The patients were randomly assigned, at a 2:1 ratio, to receive terlipressin (1-2 mg IV every 6 hours) or placebo, with concomitant use of albumin recommended in both groups. HRS reversal occurred in 39% of the patients in the terlipressin and albumin group and in 18% of the placebo group (p > 0.001), corroborating that terlipressin is more effective than placebo in improving kidney function, albeit the use of terlipressin was associated with severe adverse effects, such as respiratory failure.96
Concomitant emergency managementElectrolyte correction: patients with severe AKI can present with fluid and electrolyte imbalances that require RRT, especially hyperkalemia.97 The management goal in acute hyperkalemia is the prevention or minimization of the electrophysiologic effects on the heart, to reduce the immediate risk for arrythmias. The therapeutic options for acute hyperkalemia include intravenous calcium gluconate, insulin/glucose, inhaled beta-agonists (salbutamol), intravenous bicarbonate sodium, and hemodialysis.98 In patients with LC, RRT should be offered after considering the prognosis, and in general, should only be used as a bridge to liver transplantation or in patients with potentially reversible AKI. The decision should be made cautiously in patients that are not candidates for transplantation.97
Acid-base status correction: patients with compensated LC maintain a normal acid-base status thanks to the development of hyperbicarbonaturia, to compensate for the decrease in carbon dioxide levels.99
Respiratory alkalosis: respiratory alkalosis is the most frequent acid-base alteration in patients with cirrhosis (64%).100 It is secondary to hyperventilation due to the effect of progesterone on the central nervous system and its potentiation by estrogens.99 Other associated factors are the decrease in functional residual capacity due to ascites, hepatopulmonary syndrome or portopulmonary hypertension.97
Metabolic alkalosis: metabolic alkalosis is generally induced by secondary hyperaldosteronism (effective hypovolemia, loop diuretics, and vomiting).100 The use of loop diuretics promotes bicarbonate retention with chloride depletion and increased aldosterone secretion, with increased potassium excretion and increased ammonia production.99 In addition, the decrease in albumin synthesis and lactate clearance can contribute to the development of metabolic alkalosis, given that albumin is a weak acid.97
Metabolic acidosis: the kidney establishes metabolic compensation of respiratory alkalosis by reducing the tubular secretion of acid, reducing absorption, and increasing bicarbonate excretion, added to the chloride ion retention produced by hyperchloremia. Up to 70% of the body’s lactic acid is metabolized by the liver and only a small portion is metabolized by the kidney. In that context, LC frequently results in the incapacity of the liver to clear systemic lactic acid.97
High anion gap metabolic acidosis (lactic acidosis): high anion gap metabolic acidosis has been reported in 20% to 30% of patients and the most frequent causes are lactic acidosis due to sepsis or shock, alcoholic ketosis, or acidosis due to exogenous toxins (methanol and ethylene glycol ingestion).100 It can be type A or type B. The former is associated with altered tissue perfusion and lactate production and the latter with normal perfusion and barely utilized lactate. Type B occurs in almost all patients with chronic liver disease, due to a decrease in lactic acid utilization via gluconeogenesis, whereas type A occurs in hemodynamic situations that produce hypotension, hypoperfusion, and lactate production. The treatment of type A lactic acidosis that presents as high anion gap metabolic acidosis includes the administration of intravenous solutions and vasopressor or inotropic support. In the case of lactic acidosis associated with infections, starting early antibiotic therapy is essential.97
Normal anion gap metabolic acidosis (hyperchloremic): hyperchloremic metabolic acidosis is generally secondary to acute tubular necrosis, phosphorus deficit, or primary urinary acidification defect in patients with cirrhosis, and it can also be induced by chronic spironolactone use.100 Unlike what occurs in acute tubular necrosis, urinary acidification is normal and often associated with hyperkalemia.97
Key points- 1
AKI occurs in up to 50% of hospitalized patients with LC. The precipitating factors must be rapidly identified and corrected (infections, liver failure, use of diuretics and nephrotoxic agents, accelerated volume loss).
- 2
Suspending the use of diuretics, beta-blockers, and nephrotoxic drugs, such as nonsteroidal anti-inflammatory drugs and vasodilators, is recommended, as well as the early recognition and treatment of bacterial infections.
- 3
Volume expansion (albumin or crystalloids) should be administered as soon as AKI is identified in a patient with LC.
- 4
The term type 1 HRS was replaced by HRS-AKI, defined as the absence of response to albumin administration, associated with the suspension of diuretics in patients with LC, ascites, and AKI, according to the ICA-AKI criteria, in the absence of shock and nephrotoxic medication use, no proteinuria, and a normal kidney ultrasound study.
- 5
The treatment of HRS is based on the concomitant administration of terlipressin and albumin.
Hyponatremia is defined as a serum sodium level < 130 mmol/l. It is a marker of poor prognosis and is associated with increased morbidity and mortality.68 In managing hyponatremia, distinguishing between hypovolemic hyponatremia and hypervolemic hyponatremia is important.101
Hypovolemic hyponatremia: caused by a prolonged negative sodium balance, hypovolemic hyponatremia accounts for less than 10% of cases.101 It is frequently associated with diuretic use and paracentesis, given that acute volume depletion produces the release of the antidiuretic hormone, with an increase in sodium reabsorption in the proximal nephron.68,97 It should be treated when serum sodium is < 130 mmol/l, through volume expansion with saline solution and the suspension of diuretics.68
Hypervolemic hyponatremia: hypervolemic hyponatremia is the most frequent type of hyponatremia and is characterized by extracellular volume expansion, with ascites and edema. It is secondary to the activation of the renin-angiotensin-aldosterone system by the decrease in the effective circulating volume associated with splanchnic vasodilation.97 Treatment is based on a negative fluid balance, with fluid restricted to 1,000 ml/day.68 If there is no improvement within the first 24 to 48 h, other options should be considered.101 The administration of hypertonic solutions with sodium chloride can improve natremia, but increases volume overload and worsens ascites and edema.68 Therefore, its management is limited to severely symptomatic hyponatremia with life-threatening manifestations, such as convulsive crises and coma, or in cases of profound hyponatremia (Na <110 mEq/l). In acute hyponatremia (< 24 h of evolution), 100 ml of 3% saline solution is administered for 15 to 30 minutes, which can be repeated up to 3 times, with a total of 300 cc. The goal is to raise serum sodium by 4 to 6 mEq/l in the first 6 hours. In cases of symptomatic or severe chronic hyponatremia (Na <110 mEq/l), saline solution can be administered through continuous infusion at a rate of 15 to 30 ml/h.101 The sodium level should not be increased more than 8 mmol/l per day, to prevent neurologic sequelae, such as osmotic demyelination.68
One of the therapeutic options in the correction of hyponatremia is the administration of albumin, which produces an increase in serum sodium by increasing urinary free-water clearance secondary to intravascular volume expansion.101 The impact of albumin on hyponatremia resolution was evaluated in a retrospective study that included 1,126 patients with decompensated cirrhosis and hyponatremia (Na < 130 mEq), 777 of whom received intravenous albumin. The patients that received albumin with a mean total quantity utilized of 225 g (IQR 100-400) had a higher hyponatremia resolution rate, regardless of kidney function and baseline sodium levels, compared with the patients that did not receive albumin (85.41% versus 44.78%, p = 0.0057, OR: 1.5 95% CI 1.13-2.0), and showed improvement in survival at 30 days, as well.102
HypokalemiaThe presence of sarcopenia in the patient with cirrhosis is associated, together with the use of diuretics, with low body potassium reserves that predispose to the development of hypokalemia.100 In those patients, the total body potassium levels can be reduced by up to 30% to 40%.97 Hypokalemia frequently has a multifactorial origin and its main causes are loop diuretic use, gastrointestinal losses (vomiting/diarrhea), respiratory alkalosis, secondary hyperaldosteronism, renal tubular acidosis, and hypomagnesemia due to chronic malnutrition.99,100 Magnesium acts as a transport inhibitor of the renal outer medullary potassium (ROMK) channel, which is the main channel of potassium in the distal nephron, and its deficiency increases potassium excretion.99 On the other hand, terlipressin use in patients with variceal bleeding can induce hypokalemia through marked arterial vasoconstriction with an increase in the glomerular filtration rate and potassium excretion, in addition to potentiating the effect of aldosterone on potassium secretion in the collecting tubules.100 It can precipitate the develop of HE due to the increase in ammonia in the renal vein and the increase in the permeability of the blood-brain barrier caused by alkalosis.82 Hypokalemia induces intracellular acidosis in the proximal tubular cells that produces an increase in reabsorption and glutamine metabolism that leads to ammoniagenesis.99 Regarding management, potassium correction should be slow, particularly when using the intravenous route, given that patients with LC have a reduced buffering capacity of the potassium loads, with the risk for developing rebound hyperkalemia.99 According to the latest EASL guidelines on the management of the patient with decompensated LC, if serum potassium is < 3 mmol/l, furosemide administration should be suspended.68
HyperkalemiaPatients with LC can develop hyperkalemia, with a prevalence of 12% to 14%.20 It is generally secondary to the use of aldosterone antagonists and other potassium-sparing diuretics (amiloride, eplerenone).82 The patients at a higher risk for developing hyperkalemia are those with creatinine levels above 1.3 mg/dl and patients with spironolactone doses above 100 mg/day.99 In addition to the use of diuretics, patients with advanced LC receive other potassium-sparing medications, such as the angiotensin-converting enzyme (ACE) inhibitors, angiotensin II-receptor blockers (ARBs), and beta blockers. The ACE inhibitors and ARBs interact in renin-angiotensin system activity, with an increase in risk, not only for hypotension and kidney failure, but also for HE and hyperkalemia, and so are not recommended in patients with LC and ascites.103 Other factors associated with the development of hyperkalemia are potassium-rich foods, such as bananas, oranges, and ginger.100
The clinical importance of hyperkalemia in LC has been previously evaluated, with a study reporting that serum potassium levels were significantly associated with creatinine (p < 0.001) and urea (p < 0.001), and inversely with sodium (p < 0.001). There was also an increase in the mortality rate of the patients with hyperkalemia (HR 1.3, 95% CI 1.11-1.57), and a “K model” predicted both short-term and long-term mortality, with a concordance index of 0.80 in a validation cohort.104
According to the latest EASL guidelines on the management of patients with decompensated cirrhosis, if serum potassium is > 6 mmol/l, the administration of spironolactone should be suspended.68
Key points- 1
Electrolyte imbalances can be the precipitating factors of other complications in LC, such as HE.
- 2
Hyponatremia management is based on distinguishing hypovolemic hyponatremia from hypervolemic hyponatremia.
- 3
Hypokalemia has a multifactorial origin: loop diuretics, gastrointestinal losses, respiratory alkalosis, secondary hyperaldosteronism, renal tubular acidosis, and hypomagnesemia due to chronic malnutrition.
- 4
Hyperkalemia tends to be secondary to the use of aldosterone antagonists and other potassium-sparing diuretics, with a higher risk in patients with creatinine levels above 1.3mg/dl.
The present review deals with the fundamental aspects of resuscitation in critically ill patients with LC, with a special focus on initial emergency care. The aim was to analyze the available literature on the special management that patients with LC require, in the emergency context, for the most frequent conditions found at that stage of care, according to expert opinion. An attempt was made to collect the pertinent information that exclusively covered patients with LC in different clinical emergency contexts, such as hypovolemic shock due to variceal UGIB, sepsis, septic shock, HE, AKI, and isolated electrolyte alterations (Table 1).
Evaluation and resuscitation of the patient with liver cirrhosis.
| Evaluation of severity | Fluid therapy | Specific therapy | |
|---|---|---|---|
| Variceal UGIB and hypovolemic shock | - Signs of tissue hypoperfusion | Crystalloids Ringer’s lactate | Vasopressors |
| - SAP<90mm Hg or MAP<65mm Hg | - Terlipressin, somatostatin, octreotide, vasopressin | ||
| - Lactate ≥ 4.0mmol/l, | Blood product transfusion | ||
| - pH≤7.1 or base deficit ≤ −5 mEq/l | -Hb goal between 7 to 9g/dl | ||
| In cases of acute bleeding, maintain fibrinogen ≥ 120 | |||
| Sepsis and septic shock | CLIF-C ACLF | 0.9% saline solution or Ringer’s lactate | Vasopressors |
| - Noradrenalin first choice | |||
| - Additional therapy: epinephrine, vasopressin, dopamine | |||
| - Dobutamine, consider in myocardial dysfunction | |||
| Albumin in specific cases | |||
| Antibiotics | |||
| Hepatic encephalopathy | West Haven Classification | 0.9% saline solution or Ringer’s Lactate | Supportive care |
| Glasgow Coma Scale | Identification and removal of precipitating factor | ||
| - Infections, GIB, diuretics, constipation, alcohol, malnutrition, electrolyte alterations | |||
| - Nonabsorbable disaccharides (lactulose and lactitol) | |||
| - Nonabsorbable antibiotics (rifaximin) | |||
| Additional drug treatment | |||
| - BCAAs (isoleucine, leucine, and valine) | |||
| - LOLA |
BCAAs: branched-chain amino acids; CLIF-C ACLF: chronic liver failure consortium acute-on-chronic liver failure; GIB: gastrointestinal bleeding; Hb: hemoglobin; LOLA: L-ornithine L-aspartate; MAP: mean arterial pressure; SAP: systolic arterial pressure; UGIB: upper gastrointestinal bleeding.
We have frequently asked ourselves, “Are some aspects of patients in the LC population so unique that they would need special resuscitation, in general, compared with other types of critically ill patients?” There are theoretical pathophysiologic bases that imply that patients with LC require a different type of emergency resuscitation, with respect to shock, sepsis, electrolyte disorders, neurologic alteration, and kidney injury. Despite the fact that the patient with LC is singular in various aspects that are inherent to the pathophysiology of the disease, there are no specialized guidelines for that population, in relation to several of the themes addressed herein. That does not mean that the knowledge acquired in non-LC populations cannot be applied. In fact, daily practice, as perceived in the present review, is saturated with practices that are extrapolated from populations that do not present with LC. We emphasize that the unique aspects of patients with LC must be taken into account at the time of their emergency management, given that current evidence is insufficient for asserting that standard emergency management in patients with LC should be significantly different from management in other contexts, and we also underline the fact that patients, with or without LC, must always be analyzed and managed on a case-by-case basis. Emergency complications in LC include variceal UGIB, sepsis, ascites, and HE, among others. Initial resuscitation, including airway and circulation measures, is paramount in those patients. There are several new intubation and resuscitation techniques in patients with severe LC. Knowledge of the evaluation and management of the complications associated with LC can improve the care of patients with severe liver disease.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Velarde-Ruiz Velasco JA, García-Jiménez ES, Aldana-Ledesma JM, Tapia-Calderón DK, Tornel-Avelar AI, Lazcano-Becerra M, et al. Evaluación y manejo de emergencias en el paciente con cirrosis. Rev Gastroenterol Méx. 2022;87:198–215.