Malignant gastric outlet obstruction is a condition that alters patient quality of life, conditioning progressive malnutrition. However, self-expanding metal stents (SEMSs) and surgical gastrojejunostomy (SGJ) are palliative options in patients with unresectable disease.
AimTo characterize patients diagnosed with malignant gastric outlet obstruction requiring SEMS placement or SGJ.
Materials and methodsSequential non-probability convenience sampling was conducted and included 68 patients, 40 of whom had SEMS placement and 28 of whom underwent SGJ.
ResultsPatients sought medical consultations for the symptoms of vomiting, abdominal pain, weight loss, and upper gastrointestinal bleeding. Ninety-five percent of the patients in the SEMS group and 64.3% in the SGJ group presented with metastasis. Technical and clinical success, patency duration, and number of patients with no complications were greater in the SGJ group. Mean survival in days was 88 (SD ± 21) in the SEMS group versus 501 (SD ± 122) in the SGJ group. The log-rank test detected a statistically significant difference between subgroups (p = 0.00).
ConclusionSGJ has greater technical and clinical success rates but SEMS placement continues to be utilized in distal gastric cancer, especially in cases in which surgery is not an option.
La obstrucción maligna al tracto de salida gástrico (OTSG) es una condición que altera la calidad de vida de los pacientes, condicionando un déficit nutricional con deterioro progresivo. Sin embargo, en aquellos con enfermedad irresecable existen opciones paliativas como los stents metálicos autoexpandibles (SMA) y la gastroyeyunostomía quirúrgica (GQ).
ObjetivoCaracterizar a los pacientes con diagnóstico de OTSG de origen neoplásico que requirieron una intervención con SMA o GQ.
Materiales y métodosMuestreo no probabilístico a conveniencia de manera secuencial. Se incluyeron 68 pacientes, de los cuales, a 40 se les realizó SMA y a 28 GQ.
ResultadosLos síntomas por los que consultaron los pacientes fueron vómito, dolor abdominal, pérdida de peso y hemorragia de vías digestivas altas. La metástasis estuvo presente en 95 y 64.3% en los grupos de SMA y GQ, respectivamente. Tanto el éxito técnico como el clínico y la duración de la permeabilidad fueron mayores en el grupo de GQ, así como la proporción de pacientes que no presentó ninguna complicación. De los casos que fueron llevados a SMA, el promedio de días que estuvieron vivos fue de 88 (DE ± 21) vs. 501 (DE ± 122) para GQ. En el análisis con la prueba de Log Rank se encontró una diferencia significativa entre los subgrupos (valor p 0.00).
ConclusiónLa GQ tiene tasas de éxito técnico y clínico mayores, sin embargo, el SMA sigue siendo útil en el cáncer gástrico distal, especialmente en casos en los cuales la intervención quirúrgica no es una opción.
Gastric cancer is a frequent pathology associated with significant morbidity and mortality and is the fifth most commonly diagnosed type of cancer worldwide. It holds third place in mortality, according to the World Health Organization1,2. Up to 50% of patients have advanced stage disease at diagnosis and only half of the patients with local or regional disease are candidates for potentially curative resection3. Distal gastric cancer is the cause of gastric outlet obstruction (GOO) in 35% of patients4 and its etiology is pancreatic adenocarcinoma in 15–25% of patients5.
By limiting or impeding the progression of the food bolus in the upper gastrointestinal tract, GOO favors the presence of altered electrolytes, malnutrition, and finally death6. Even when outcomes are not dramatic, said obstruction is associated with symptoms of significant quality of life deterioration, such as nausea and/or vomiting (92%), weight loss (63%), early satiety, and bloating7. The diagnosis of GOO depends on clinical suspicion and is confirmed through upper gastrointestinal endoscopy and diagnostic imaging, such as upper gastrointestinal radiography with water-soluble or barium-based contrast material, showing a lack of small bowel filling, or abdominal tomography, showing gastric distension, retained contrast medium and/or air-fluid levels7,8.
Intent-to-cure surgical management in GOO is an option in a very limited number of patients, and the palliative alternatives for the rest of patients do not always function, resulting in considerable morbidity and mortality associated with the procedures4. In the group of patients with GOO and the palliative option, management with self-expanding metal stents (SEMSs) is among the therapeutic possibilities. They are a minimally invasive alternative in nonsurgical palliative treatment, whose goal is to provide lasting relief from the obstructive symptoms so the patient can have a normal diet. There is also the alternative of surgical gastrojejunostomy (SGJ), in which an anastomosis between the stomach and the proximal jejunal segment is constructed. It can be an open or laparoscopic procedure, and given that it is a more invasive intervention, can increase morbidity9–12.
SEMSs are indicated in patients with unresectable or recurrent tumors that have a short life expectancy (on average between 2 and 6 months) and are contraindicated for SGJ. The performance of SGJ requires a better Eastern Cooperative Oncology Group (ECOG) functional status and high Karnofsky performance status score, as well as the absence of ascites, no metastasis to the liver, and an uncompromised nutritional status13,14.
In a randomized trial on 39 patients with GOO assigned to SGJ or SEMS placement, food intake rapidly improved in the SEMS group (5 vs 8 days), but long-term symptom relief was worse (50 vs 72 days), and there were more major complications (6 complications in 4 patients vs 0). There was no difference related to survival or health-associated quality of life. In addition, observational studies suggest that SEMS placement has a similar success rate to that of palliative surgery (90% of patients with clinical improvement), is less costly, and has lower procedure-related morbidity and mortality rates13. A study utilizing a database to compare 425 SEMS placement procedures with 399 SGJs for GOO management described a shorter hospital stay in the SEMS group (8 vs 16 days) and lower cost ($15,366 USD vs $27,391 USD)12.
The present study describes the results of the experience with SEMS placement and SGJ in the management of patients with GOO due to inoperable advanced neoplasia, evaluating the utility, efficacy, and safety of those procedures.
AimThe aim of the present study was to characterize the population of patients diagnosed with malignant GOO that required SEMS placement or SGJ and to describe the technical and clinical success rates of the two interventions.
Materials and methodsA retrospective analysis was conducted, sequentially utilizing nonprobability convenience sampling, on patients above 18 years of age that underwent SGJ or SEMS placement for the management of malignant GOO at the Hospital Universitario San Ignacio, within the time frame of January 1, 2010, to July 31, 2019. Patients were identified through databases and surgery logs. The inclusion criteria were patient age >18 years, diagnosis of malignant GOO made through upper gastrointestinal endoscopy (UGIE), upper gastrointestinal tract radiography, and/or abdominal computed tomography (CT), and management procedures. Patients were excluded that underwent SGJ or SEMS placement for benign disease. The medical records were reviewed of all the patients to determine patient demographics, clinical presentation, comorbidities, cancer staging, and radiologic study results.
Antroduodenal self-expanding metallic stentAll the SEMSs (Wallflex; Boston Scientific Corp., Natick, MA) were deployed under fluoroscopic guidance, with the patient in the left lateral position and sedated by an anesthesiologist. The body of the SEMSs measured 22 mm in diameter and the ends measured 27 mm in diameter, with lengths of 2, 9, or 12 cm. Under endoscopic vision, a 0.035-in. hydrophilic guidewire was first advanced through the stricture. A 5 Fr biliary cannula used for endoscopic retrograde cholangiopancreatography (ERCP) was then advanced over the hydrophilic guidewire and water-soluble contrast medium was injected to evaluate the length of the stricture. The SEMS was advanced over the hydrophilic guidewire, the placement of the deployment device was confirmed by fluoroscopy, and the SEMS was then deployed.
Surgical gastrojejunostomyAfter making a midline abdominal incision, an incision at the posterior wall of the stomach and the jejunal segment was made with a harmonic scalpel. The jaws of an Endo-GIA (3.5 mm/60 mm; US Surgical, Norwalk, CT) stapler were inserted in the enterotomies, creating a gastrojejunostomy by firing three “shots”. The staple line was then checked to determine the presence of blood and the enterotomies were closed, utilizing continuous sutures.
Statistical analysisA descriptive analysis of the population was carried out. For the qualitative variables, the data were expressed as absolute and relative frequencies, and the continuous quantitative variables were determined through measures of central tendency and dispersion, expressed as means and standard deviation. The qualitative variables were evaluated using the chi-square test and the qualitative variables, after establishing normality, were evaluated using the Mann–Whitney U test or the Student’s t test. Clinical success prediction related to pyloric syndrome was assessed through a bivariate analysis and the results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Statistical significance was set at a p < 0.05. For the variables that showed relevant association, a multivariate analysis was performed, using the “introductory” model of the logistic regression method, and the Nagelkerke R2 was calculated. Survival was analyzed by the Kaplan–Meier method for determining mortality from the time of intervention (SEMS vs SGJ), and the groups were compared using the log-rank test. The Cox regression method was employed to evaluate the factors that could influence mortality, according to intervention. All statistical analyses were carried out with SPSS version 25 software.
Ethical considerationsThe authors declare that no experiments on humans or animals were carried out for the present research. With respect to data confidentiality, the authors declare that they followed the protocols of their work center on the publication of patient data of the patients included in the study, and that they have preserved patient data anonymity at all times, given that the article contains no data that could identify the patients.
All the patients gave their informed consent to undergo the procedures and the study was approved by the research committee of the Hospital Universitario San Ignacio. Informed consent was not requested for the publication of the present article because it contains no personal data that could identify the patients.
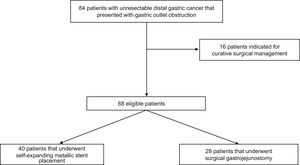
ResultsPatient characteristicsOf the 84 patients that were candidates for the study, 16 were excluded for having undergone curative surgery. A total of 68 patients were included in the study. Forty of those patients presented with poorer clinical and functional status and underwent SEMS placement, and 28 presented with better functional status (Karnofsky performance status score) and underwent SGJ. Fig. 1 describes the selection process.
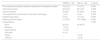
The clinical and sociodemographic characteristics of the population are presented in Table 1.
Clinical and sociodemographic characteristics.
| SEMS (n = 40) | SGJ (n = 28) | p Value | |
|---|---|---|---|
| Age (years) | 61 (±12.1) | 66 (±12.7) | 0.098 |
| Sex (males) | 29 (72.5) | 18 (64.3) | 0.471 |
| Provenance | 0.000 | ||
| Cundinamarca | 4 (10) | 20 (71.4) | |
| Bogotá | 29 (72.5) | 4 (14.3) | |
| Tolima | 3 (7.5) | 1 (3.6) | |
| Meta | 1 (2.5) | – | |
| Boyacá | 3 (7.5) | 1 (3.6) | |
| Caldas | – | 1 (3.6) | |
| No information | – | 1 (3.6) | |
| Symptoms leading to consultation | 0.000 | ||
| Vomiting | 20 (50) | 6 (21.4) | |
| Dyspepsia | 2 (5) | 1 (3.6) | |
| Abdominal pain | 4 (10) | 5 (17.9) | |
| Weight loss | 6 (15) | 1 (3.6) | |
| Upper gastrointestinal bleeding | 7 (17.5) | – | |
| Two of the previous symptoms | 1 (2.5) | 13 (46.4) | |
| Three or more of the previous symptoms | – | 2 (7.1) | |
| Time intervals of symptom progression | 0.525 | ||
| Chronic (>15 days) | 27 (67.5) | 15 (53.6) | |
| Subacute (7–15 days) | 3 (7.5) | 3 (10.7) | |
| Acute (<7 days) | 9 (22.5) | 9 (32.1) | |
| No information | 1 (2.5) | 1 (3.6) | |
| Comorbidities | 0.890 | ||
| None | 25 (62.5) | 14 (50) | |
| HBP | 4 (10) | 4 (14.3) | |
| DM2 | 3 (7.5) | 1 (3.6) | |
| HBP and DM2 | 3 (7.5) | 4 (14.3) | |
| Venous thromboembolism | 3 (7.5) | 2 (7.1) | |
| Other neoplasia | 1 (2.5) | 1 (3.6) | |
| Others | 1 (2.5) | 2 (7.1) | |
| Hemoglobin (g/dL) | 11.27 (±2.79) | 13.11 (±2.83) | 0.017 |
| Ascites | – | 6 (21.4) | – |
| Time interval from cancer diagnosis to pyloric syndrome (days) | 217 (±347) | 87 (±167) | 0.044 |
| Pyloric syndrome diagnosis based on: | 0.003 | ||
| Imaging studies (abdominal CT) | – | 1 (3.6) | |
| Upper gastrointestinal endoscopy | 5 (12.5) | – | |
| At least two of the studies (laboratory, imaging, and UGIE) | 33 (82.5) | 19 (67.9) | |
| All the studies (laboratory, imaging, and UGIE) | 1 (2.5) | 8 (28.6) | |
| Type of cancer (histopathologic diagnosis) | 0.071 | ||
| Intestinal-type adenocarcinoma (without defining differentiation) | 5 (12.5) | 1 (3.6) | |
| Well differentiated intestinal-type adenocarcinoma | 4 (10) | 2 (7.1) | |
| Moderately differentiated intestinal-type adenocarcinoma | 10 (25) | 3 (10.7) | |
| Poorly differentiated intestinal-type adenocarcinoma | 6 (15) | 1 (3.6) | |
| Diffuse-type adenocarcinoma (without defining differentiation) | 1 (2.5) | 2 (7.1) | |
| Poorly differentiated diffuse-type adenocarcinoma | 5 (12.5) | 2 (7.1) | |
| Bile duct adenocarcinoma | 2 (5) | 4 (14.3) | |
| Unclassified neoplasia | 7 (17.5) | 13 (14.3) | |
| Tumor location | – | ||
| Antrum and corpus | – | 4 (14.3) | |
| Antrum | – | 2 (7.1) | |
| Antrum and pylorus | – | 7 (25) | |
| Gastroenteric anastomosis | – | 1 (3.6) | |
| Duodenal | – | 13 (46.4) | |
| Antrum/pylorus and duodenum | – | 1 (3.6) | |
| Metastasis | 38 (95) | 18 (64.3) | 0.001 |
CT: computed tomography; DM2: type 2 diabetes mellitus; HBP: high blood pressure; SD: standard deviation; SEMS: self-expanding metallic stent; SGJ: surgical gastrojejunostomy; UGIE: upper gastrointestinal endoscopy.
Data are presented as n (%) and mean (±SD).
Mean age of the SGJ group was higher, compared with the SEMS group, but the difference was not statistically significant. The number of men was similar in the two intervention groups. Provenance and consultation symptoms were not comparable. The majority of the patients in the SEMS group came from Bogotá, whereas the majority in the SGJ group came from Cundinamarca. The symptoms that led the patients to consultation were vomiting, abdominal pain, weight loss, and upper gastrointestinal bleeding, and they were different in the two groups. Of the patients that had SEMS placement, 7.5% had a functional status, measured by a Karnofsky performance status score of 30 points, 35% of 40 points, and 10% of 50 points. In addition, 2.5% had stage IIIC disease and 97.5% had stage IV. With respect to the patients that underwent SGJ, 7.14% had a Karnofsky score of 20 points, 28.57% had 30 points, 53.57% had 40 points, and 10.71% had 50 points score. Stage IIIC disease was found in 39.29% of the patients and stage IV in 60.71%. Symptom progression was primarily >15 days (chronic) in the two groups. Approximately half of the patients that underwent either of the interventions (SEMS or SGJ) presented with no comorbidity. Hemoglobin level was slightly higher in the SGJ group, but with no significant difference. The group that had SEMS placement showed a mean of more days from cancer diagnosis to pyloric syndrome determination than the SGJ group. At least two studies (clinical, imaging, UGIE) were required to diagnose pyloric syndrome in the patients of both groups, but behavior was different in the two types of intervention. Moderate or poorly differentiated intestinal-type adenocarcinoma and unclassified neoplasia were the main histologic results of GOO etiology in the SEMS group, whereas the principal causes in the SGJ group were bile duct carcinoma and unclassified neoplasia.
Thirty-eight (95%) patients in the SEMS group and 18 (64.3%) in the SGJ group presented with metastasis. Tumor located at the level of the duodenum caused greater GOO in the patients that underwent SGJ, but no data about location or number of cases of ascites was obtained in the SGJ group.
Technical and clinical outcomes and complicationsOf the patients that had SEMS placement, a fully covered metallic stent was utilized in 38 (95%) patients and a partially covered metallic stent in 2 (5%). Of the patients that underwent SGJ, 25 (89.3%) had open surgery and 3 (10.7%) had the laparoscopic procedure. Table 2 shows the results related to outcomes and complications. The mean number of days from the diagnosis of pyloric syndrome to intervention (SEMS or SGJ) was similar in the two groups. Clinical and technical success rates, patency duration, and the number of patients that presented with no complications were greater in the group that underwent SGJ, but hospital stay was longer.
Clinical outcomes and complications after the intervention.
| SEMS (n = 40) | SGJ (n = 28) | p Value | |
|---|---|---|---|
| Time interval from pyloric syndrome diagnosis to intervention (days) | 13 (±16) | 13 (±10) | 0.541 |
| Technical success | 39 (97.5) | 28 (100) | 0.399 |
| Clinical success | 36 (90) | 27 (96.4) | 0.318 |
| Time interval from intervention to oral diet onset (days) | 1 (±0) | 3 (±4) | 0.000 |
| Hospital stay (days) | 5 (±5) | 15 (±12) | 0.000 |
| Patency duration (days) | 67 (±78) | 197 (±263) | 0.044 |
| Complications | 0.030 | ||
| None | 28 (70) | 26 (92.9) | |
| Bleeding | 1 (2.5) | – | |
| Displacement | 7 (17.5) | – | |
| Occlusion | 4 (10) | – | |
| Fistula | – | 1 (3.6) | |
| Infection | – | 1 (3.6) |
SD: standard deviation; SEMS: self-expanding metallic stent; SGJ: surgical gastrojejunostomy.
Data are presented as n (%) and mean (±SD).
Table 3 describes the results of the bivariate analysis for predicting clinical success. The factors related to clinical success were age ≤60 years, having come from Bogotá or Cundinamarca, and the absence of anemia in both men and women, but none of those variables were statistically significant. A p value <0.05 was found only in the patients that had technical success and in those with a determined histologic diagnosis (i.e., not an unclassified neoplasia).
Clinical success predictors, according to the bivariate analysis.
| OR (95% CI) | p Value | |
|---|---|---|
| Age (years), ≤60 | 2.811 (0.297−26.614) | 0.349 |
| Sex, female | 0.648 (0.1−4.196) | 0.647 |
| Provenance, Bogotá or Cundinamarca | 1.472 (0.147−14.72) | 0.741 |
| Symptom time, chronic | 0.413 (0.043−3.925) | 0.429 |
| Type of cancer, any histologic diagnosis (vs unclassified) | 11.75 (1.22−113.001) | 0.010 |
| Absence of metastasis | 1.5 (0.165−13.57) | 0.718 |
| Absence of comorbidities | 0.313 (0.033−2.956) | 0.287 |
| Hemoglobin (g/dL), women >12 g/dL | 1.375 (0.074−25.433) | 0.830 |
| Hemoglobin (g/dL), men >13 g/dL | 2.889 (0.243−34.309) | 0.383 |
| Time interval from oncologic diagnosis to pyloric syndrome (days), ≥120 | 0.579 (0.061−5.528) | 0.631 |
| Time interval from pyloric syndrome diagnosis to intervention (days), ≥7 | 0.164 (0.017−1.558) | 0.079 |
| Type of intervention, SEMS | 0.333 (0.035−3.154) | 0.318 |
| Technical success | 25.6 (1.96−333.5) | 0.000 |
| OR: odds ratio; SEMS: self-expanding metallic stent. |
The results of the multivariate logistic regression analysis for predicting clinical success are shown in Table 4. The variables included in the model as potential predictors were sex, type of cancer, metastasis, comorbidity, technical success, and age ≤60 years, but none of the associated factors were statistically significant.
Clinical success predictors, according to the multivariate logistic regression analysis.
| β | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Age (years), ≤60 | 0.524 | 1.68 | 0.08−34.30 | 0.733 |
| Sex, female | 1.095 | 2.99 | 0.38−23.47 | 0.298 |
| Type of cancer, any histologic diagnosis (vs unclassified) | 1.739 | 5.69 | 0.45−71.78 | 0.179 |
| Comorbidity | 1.416 | 4.12 | 0.37−45.91 | 0.250 |
| Absence of metastasis | 0.124 | 1.13 | 0.083−15.40 | 0.926 |
| Technical success | 3.176 | 23.95 | 0.25−2233.23 | 0.170 |
OR: odds ratio; β: β coefficient; 95% CI: 95% confidence interval.
The model correctly classifies up to 91% of the cases.
Hosmer–Lemeshow test = 0.698.
Nagelkerke R2 = 0.287.
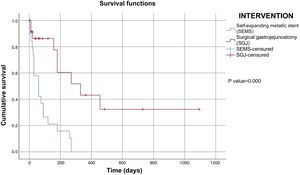
Upon finishing the data collection (04/2019), 10% (n = 4) of the patients that had SEMS placement and 54% (n = 15) that underwent SGJ were alive, whereas 47% (n = 19) of the patients in the SEMS group and 32% (n = 9) in the SGJ group had died. Those data were missing in 43% (n = 17) of the patients with the SEMS intervention and 14% (n = 4) with the SGJ intervention. Of the patients with SEMS placement, the mean number of days of survival was 88 (21 ± SD), whereas it was 501 (122 ± SD) in the patients that underwent SGJ. The mean general survival was 269 days (64 ± SD). Fig. 2 describes patient survival evaluated through the Kaplan-Meier method by intervention group (SEMS vs SGJ). The analysis was performed utilizing the log-rank test and there was a statistically significant difference between the subgroups (p = 0.00).
The results of the multivariate Cox regression model (Table 5) demonstrated that the only variable that significantly affected the risk function was the type of intervention performed, without affecting the results, when controlling for the presence of metastasis, a comorbidity, or hospital stay was done.
Discussion and conclusionThe behavior of patients with GOO due to advanced gastrointestinal cancer that had SEMS placement or underwent SGJ was described in the present study, taking into consideration the fact that said obstruction can limit or impede progression of the food bolus through the digestive tract, producing malnutrition, electrolyte alterations, and finally death4. The indications for defining one intervention or another are known to be related to the clinical condition of the patient6, which could result in the groups not being comparable. However, in the present retrospective study, we found that age, sex, progression time, comorbidities, hemoglobin level, and type of cancer were similar, with only very slight differences, between the two study groups. With the exception of provenance, the symptoms that led to consultation, the diagnostic tools utilized, the time interval from cancer diagnosis to the appearance of symptoms of GOO, and the presence of metastasis were different in the two groups, and considerably greater in the SEMS group, regarding the last two variables. Unfortunately, data on ascites and tumor location were not obtained in either group, and therefore, could not be included in the analysis, which must be kept in mind when interpreting the results13.
Technical success, as well as clinical success, were slightly higher in the SGJ group, but with no statistically significant differences, and complications appeared to be fewer in the SGJ group. However, the times for beginning oral diet and hospital stay were significantly longer in the SGJ group, which was similar to that described in other studies. Among the statistically significant predictive factors for clinical success were histologic determination of the type of cancer and technical success. However, given their wide confidence intervals, they should be cautiously interpreted, a fact that was verified by the multivariate analysis, in which no factor was a determining one for clinical success.
In clinical practice, patients with pyloric syndrome are treated through SEMS placement, if their clinical condition is more compromised or their life-expectancy is below 3 months, because it is a less invasive technique9. That was reflected in the mean number of days of survival in the patients with a SEMS, compared with those that underwent SGJ. However, the SGJ-related risk of death was lower according to the Kaplan–Meier analysis, a finding that was modified by the multivariate Cox regression analysis, when controlling for the variables of comorbidities, metastasis, or hospital stay was done. Nevertheless, those results should be interpreted with caution, when considering a greater presence of metastasis and a longer time from the diagnosis of cancer in the SEMS group.
Important limitations of the present study were that probability sampling was not performed and the data were sequentially collected by convenience from a single center. Given the low frequency of the type of interventions studied, the sample size was small, which could also have limited the results. In addition, a descriptive analytic study is not the best design for establishing association, which is why we suggest that new studies, with better methodological quality for defining the best type of intervention in relation to efficacy and survival, should be conducted to confirm the findings of the present study. Because the present analysis was a retrospective study, some type of information and measurement bias is inevitable, given that all information is gleaned from what is written in the clinical history. Nevertheless, the information was thoroughly reviewed in an effort to avoid that type of bias as much as possible.
In conclusion, palliative alternatives for managing GOO in cases of distal gastric cancer, such as antroduodenal SEMS placement or SGJ, should be kept in mind. Enteral SEMS placement continues to be useful in distal gastric cancer, especially when surgery is not an option, as is the case of patients with more advanced disease and lower functional status. SGJ is considered in patients with better functional status that would potentially have greater technical and clinical success rates, as well as longer patency intervals. However, the extent of the clinical benefit provided by those procedures and their real impact on quality of life must be evaluated. Another important factor to be contemplated is expense, given that the cost of a SEMS on average is $735 USD, which does not include the additional charges for the resources required and hospital stay. The utility of those procedures is important to evaluate, not only for the rapid resolution of gastroduodenal obstruction, but also to determine whether the general health status of the patient with advanced-stage cancer will be improved or worsened. The possible complications must be assessed, and the risk-benefit of the procedures carefully analyzed12.
The results of the present study were insufficient for making recommendations on the theme, but they do illustrate the need to conduct a multicenter study for stronger results, with a closer follow-up of the patients. Ideally, it should also be a prospective study, to determine the effect the selection of a given intervention has on patients with pyloric syndrome, as well as to establish prognostic factors.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Ovalle-Hernández AF, Vargas-Rubio RD. Experiencia del manejo de la obstrucción al tracto de salida gástrico de origen neoplásico en pacientes del Hospital Universitario San Ignacio (HUSI) en Bogotá, Colombia. Revista de Gastroenterología de México. 2022;87:35–43.