The gastric mucosa has been studied since the pre-Helicobacter pylori (H. pylori) era, but the complete descriptions of the stomach and duodenum have been anecdotal, and those of the distal duodenum, exceptional. Our aim was to evaluate the different epidemiologic characteristics and the gastroduodenal inflammatory status in patients with upper gastrointestinal symptoms.
Materials and methodsWe studied 138 patients divided into: the non-ulcer group (functional dyspepsia [n = 77] and GERD [n = 27]) and the ulcer group (peptic ulcer [n = 13] and duodenal ulcer [n = 21]). Ten biopsy samples (2 from the corpus, 3 from the antrum, 3 from the proximal duodenum, and 2 from the distal duodenum) were taken in each patient for histologic and/or microbiologic study.
ResultsThe prevalence of dyspepsia, functional dyspepsia, and H. pylori was 80.4%, 69.4%, and 82.6%, respectively. The frequency of superficial chronic gastritis in the corpus was significantly higher in the ulcer group vs. the non-ulcer group, whereas there was more chronic atrophic gastritis in the antrum in the ulcer group (P < 0.05). Duodenitis was significantly more frequent in the ulcer group vs. the non-ulcer group, in both the proximal and distal duodenum. Pangastroduodenitis was a significant finding in the ulcer group. In both groups, chronic gastritis (corpus and antrum) and duodenitis (proximal and distal) were significantly related to the presence of H. pylori. Proximal duodenitis is not an uncommon finding in functional dyspepsia (37.7%) and is twice as frequent as distal duodenitis (16.9%).
ConclusionsThe ulcer group presented with a gastroduodenal inflammatory map different from that of the non-ulcer group and was characterized by a higher frequency of superficial chronic gastritis in the corpus, chronic atrophic gastritis in the antrum, and a very high frequency of proximal duodenitis.
La mucosa gástrica ha sido estudiada desde la época anterior al Helicobacter pylori, pero son anecdóticas las descripciones completas del estómago y duodeno, y excepcionales las del duodeno distal. El objetivo fue evaluar diversas características epidemiológicas y el estado inflamatorio gastroduodenal en pacientes con síntomas gastrointestinales altos.
Material y métodosEstudiamos a 138 pacientes divididos en: grupo no ulceroso (dispepsia funcional, n = 77 y ERGE, n = 27) y grupo ulceroso (úlcera gástrica, n = 13 y úlcera duodenal, n = 21). En cada paciente se tomaron 10 biopsias (2 en el cuerpo; 3 en el antro, 3 en el duodeno proximal y 2 en el duodeno distal) para estudio histológico o microbiológico.
ResultadosLa prevalencia de dispepsia, dispepsia funcional y H. pylori fue del 80.4%, 69.4% y 82.6%, respectivamente. En el cuerpo se apreció una significativa mayor frecuencia de gastritis crónica superficial en el grupo ulceroso que en el no ulceroso; sin embargo, en el antro, el grupo ulceroso mostró más gastritis crónica atrófica (p < 0.05). La duodenitis fue significativamente más frecuente en el grupo ulceroso, tanto en el duodeno proximal como en el distal. La pangastroduodenitis fue un hallazgo significativo en el grupo ulceroso. Todos los grupos mostraron gastritis crónica (cuerpo y antro) y duodenitis (proximal y distal) relacionadas significativamente con la presencia de H. pylori. La duodenitis proximal no es un fenómeno extraño en la dispepsia funcional (37.7%), con una frecuencia que duplica a la de duodenitis distal (16.9%).
ConclusionesEl grupo ulceroso presentó un mapa inflamatorio gastroduodenal diferente al del grupo no ulceroso, caracterizado por una mayor frecuencia de gastritis crónica superficial en cuerpo, atrófica en antro y muy frecuente duodenitis proximal.
In the pre-Helicobacter pylori (H. pylori) era, Scandinavian groups carefully studied the gastric mucosa in the general population. They established the concepts of chronic gastritis (CG) of the corpus and antrum, demonstrated its increase with age (in frequency, extension, and inflammatory intensity), according to the sequence of normal mucosa, superficial chronic gastritis (SCG), and chronic atrophic gastritis (CAG), and confirmed its frequent patchy distribution and extension from the pylorus to the cardia, as well as pangastritis. They reported the prevalence of CG-corpus (41-62%) and CG-antrum (60-68%), and CAG-corpus (17-37%) and CAG-antrum (28-34%).1–4 Once H. pylori infection was discovered, it was confirmed as the main non-autoimmune cause of CG, peptic ulcer, duodenal ulcer, and gastric adenocarcinoma.1 Since then, there have been countless publications related to those findings, but very few describe a complete inflammatory map of the gastroduodenal tract. The evaluation of the duodenum is of great interest, given its recent and growing prominence in different processes, its relation to chronic antral gastritis, and the presence of H. pylori.5,6 The aim of the present study was to analyze the gastroduodenal mucosa and its relation to H. pylori in patients with upper gastrointestinal symptoms that came to our center to undergo diagnostic gastroscopy.
Patients and methodsAfter gastroscopy, 138 patients with upper gastrointestinal symptoms were selected that belonged to 2 diagnostic groups: 1) the non-ulcer group, with the following subgroups: functional dyspepsia (FD) (Rome IV criteria) (n = 77)7 and gastroesophageal reflux disease (GERD) (Montreal-Lyon criteria) (n = 27)8 and 2) the ulcer group, with the following subgroups: duodenal ulcer (DU) (n = 21) and peptic ulcer (PU) (n = 13). Patients taking proton-pump inhibitors (PPIs), antibiotics, or nonsteroidal anti-inflammatory drugs (NSAIDs)/acetylsalicylic acid (ASA) within the last 4 weeks were excluded. Ten biopsy samples were taken from each patient: 2 from the corpus (at 10 cm from the cardia, greater curvature), 2 from the antrum (at 5 cm from the pylorus, greater curvature), 2 from the proximal duodenum (bulb) (D1), and 2 from the distal duodenum (in front of the papilla) (D2), for histologic study (H&E, PAS) and H. pylori identification (Giemsa), along with one from the antrum and another from the D1, for microbiologic culture/study. The presence of CG/duodenitis was evaluated through the Whitehead et al. classification for CG9 and for duodenitis.10 In short, CG was divided into SCG (reactive changes and inflammatory infiltrate limited to the superficial epithelium and nearby lamina propria) and CAG (reactive changes and inflammatory infiltrate affecting the glandular bed, with atrophy of the tubules; the atrophy was subdivided into mild, moderate, and severe). Duodenitis was divided into 4 grades: grade 0: normal mucosa; grade 1 or mild duodenitis: normal general morphology and superficial epithelium, and only a slight increase in inflammatory cells (lymphocytes, plasma cells, and sometimes polymorphonuclear cells) in the lamina propria; grade 2 or moderate duodenitis: abnormalities in the superficial epithelium and obvious inflammatory infiltrate; and grade 3 or intense or severe duodenitis: superficial epithelial erosions and important inflammatory infiltrate with villous pattern loss. Given the scant inflammatory changes in mild duodenitis (D-1), we included it as normal mucosa and grouped moderate/intense duodenitis (D-2/D-3) in a single group (DD). A patient was considered H. pylori-positive when the bacterium was present in a histologic sample and/or the culture was positive in a biopsy sample.
Statistical analysisThe results were expressed as ± standard deviation (SD). Their relationships (differences) were statistically analyzed through the chi-square test (or Fisher’s test, when applicable) and the Student’s t test. Statistical significance was set at a p < 0.05.
Ethical considerationsThe present study was approved by the Clinical Research Ethics Committee of Granada (CEIC, Spanish acronym) and all patients signed written statements of informed consent.
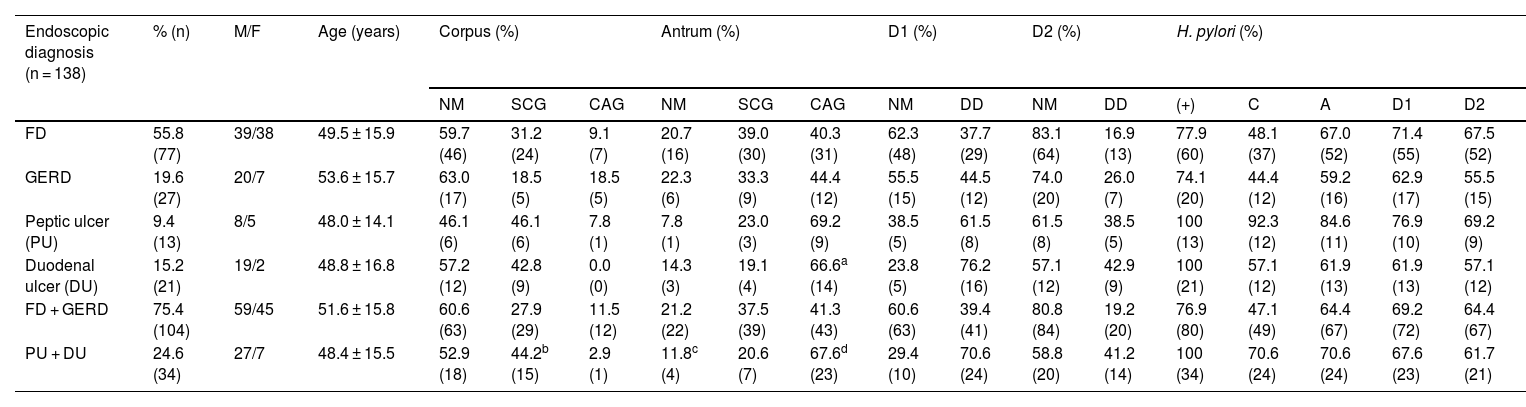
ResultsThe demographic data and the inflammatory/microbiologic status of the gastroduodenal mucosa are summarized in Table 1. In our study sample, the total prevalence of dyspepsia, FD, and H. pylori was 80.4%, 69.4%, and 82.6%, respectively.
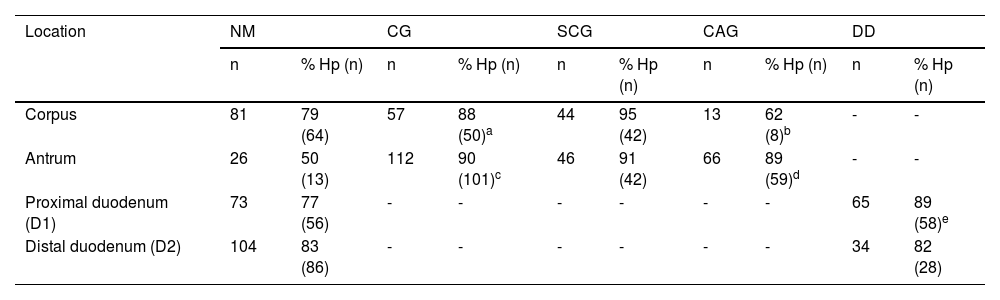
Gastroduodenal mucosa in the 138 patients.
| Endoscopic diagnosis (n = 138) | % (n) | M/F | Age (years) | Corpus (%) | Antrum (%) | D1 (%) | D2 (%) | H. pylori (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NM | SCG | CAG | NM | SCG | CAG | NM | DD | NM | DD | (+) | C | A | D1 | D2 | ||||
| FD | 55.8 (77) | 39/38 | 49.5 ± 15.9 | 59.7 (46) | 31.2 (24) | 9.1 (7) | 20.7 (16) | 39.0 (30) | 40.3 (31) | 62.3 (48) | 37.7 (29) | 83.1 (64) | 16.9 (13) | 77.9 (60) | 48.1 (37) | 67.0 (52) | 71.4 (55) | 67.5 (52) |
| GERD | 19.6 (27) | 20/7 | 53.6 ± 15.7 | 63.0 (17) | 18.5 (5) | 18.5 (5) | 22.3 (6) | 33.3 (9) | 44.4 (12) | 55.5 (15) | 44.5 (12) | 74.0 (20) | 26.0 (7) | 74.1 (20) | 44.4 (12) | 59.2 (16) | 62.9 (17) | 55.5 (15) |
| Peptic ulcer (PU) | 9.4 (13) | 8/5 | 48.0 ± 14.1 | 46.1 (6) | 46.1 (6) | 7.8 (1) | 7.8 (1) | 23.0 (3) | 69.2 (9) | 38.5 (5) | 61.5 (8) | 61.5 (8) | 38.5 (5) | 100 (13) | 92.3 (12) | 84.6 (11) | 76.9 (10) | 69.2 (9) |
| Duodenal ulcer (DU) | 15.2 (21) | 19/2 | 48.8 ± 16.8 | 57.2 (12) | 42.8 (9) | 0.0 (0) | 14.3 (3) | 19.1 (4) | 66.6a (14) | 23.8 (5) | 76.2 (16) | 57.1 (12) | 42.9 (9) | 100 (21) | 57.1 (12) | 61.9 (13) | 61.9 (13) | 57.1 (12) |
| FD + GERD | 75.4 (104) | 59/45 | 51.6 ± 15.8 | 60.6 (63) | 27.9 (29) | 11.5 (12) | 21.2 (22) | 37.5 (39) | 41.3 (43) | 60.6 (63) | 39.4 (41) | 80.8 (84) | 19.2 (20) | 76.9 (80) | 47.1 (49) | 64.4 (67) | 69.2 (72) | 64.4 (67) |
| PU + DU | 24.6 (34) | 27/7 | 48.4 ± 15.5 | 52.9 (18) | 44.2b (15) | 2.9 (1) | 11.8c (4) | 20.6 (7) | 67.6d (23) | 29.4 (10) | 70.6 (24) | 58.8 (20) | 41.2 (14) | 100 (34) | 70.6 (24) | 70.6 (24) | 67.6 (23) | 61.7 (21) |
CAG: chronic atrophic gastritis; DU: duodenal ulcer; F: female; FD: functional dysplasia; GERD: gastroesophageal reflux disease; M: male; NM: normal mucosa; PU: peptic ulcer; SCG: superficial chronic gastritis; DD: duodenitis II + III; C: corpus; A: antrum; D1: proximal duodenum; D2: distal duodenum.
Gastric corpus. The frequency of normal mucosa (NM), SCG, and CAG was similar in the 4 subgroups, with a scant atrophic component. The frequency of SCG was significantly higher than that of CAG in the ulcer group vs the non-ulcer group (p < 0.05) (Table 1).
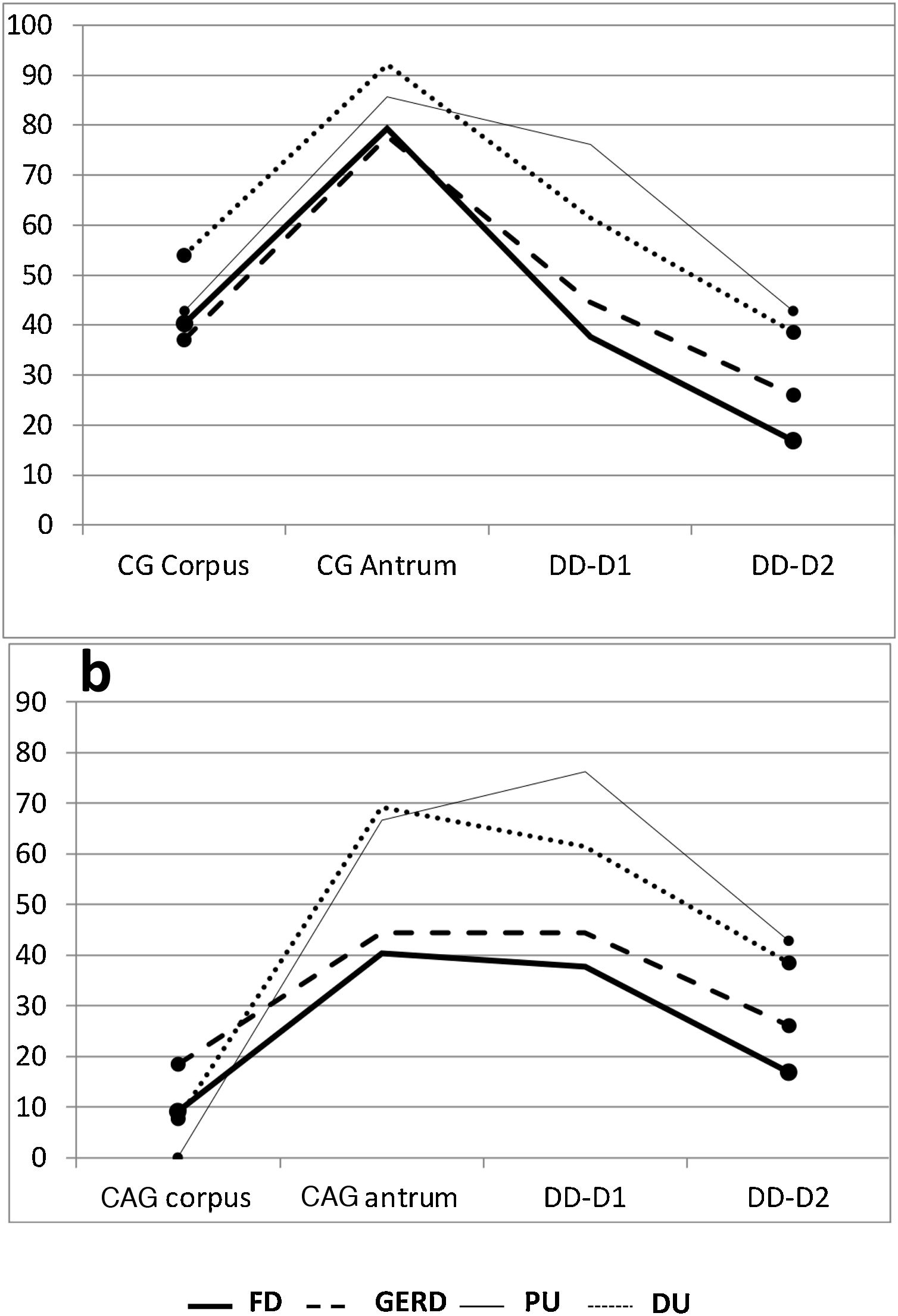
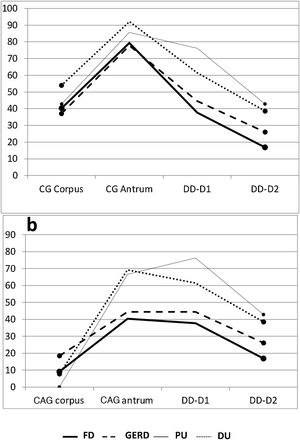
Gastric antrum. There was an increase in inflammatory phenomena in the gastric antrum, in all the subgroups, as demonstrated by the clear predominance of CG vs NM. There was also a higher frequency of atrophic phenomena (CAG) vs SCG in the antrum and all the subgroups, but that difference was significant only when the subgroups FD vs DU were compared (p < 0.05). When the ulcer group was compared with the non-ulcer group, there were differences between the frequencies of NM vs CAG (p < 0.05) and SCG vs CAG (p < 0.02) (Table 1). As shown in the graphs, the gastroduodenal inflammatory map of all the subgroups produced an inverted “V”, with greater elevation in the subgroups of the ulcer group, given the higher percentage of CG in the antrum. By considering only the presence of CAG in both the corpus and the antrum, the image in Fig. 1a was transformed into an elevation followed by a “plateau” that was descending in the PU subgroup and ascending in the DU subgroup, as a consequence of the higher percentage of DD-D1 in DU vs PU (Fig. 1b).
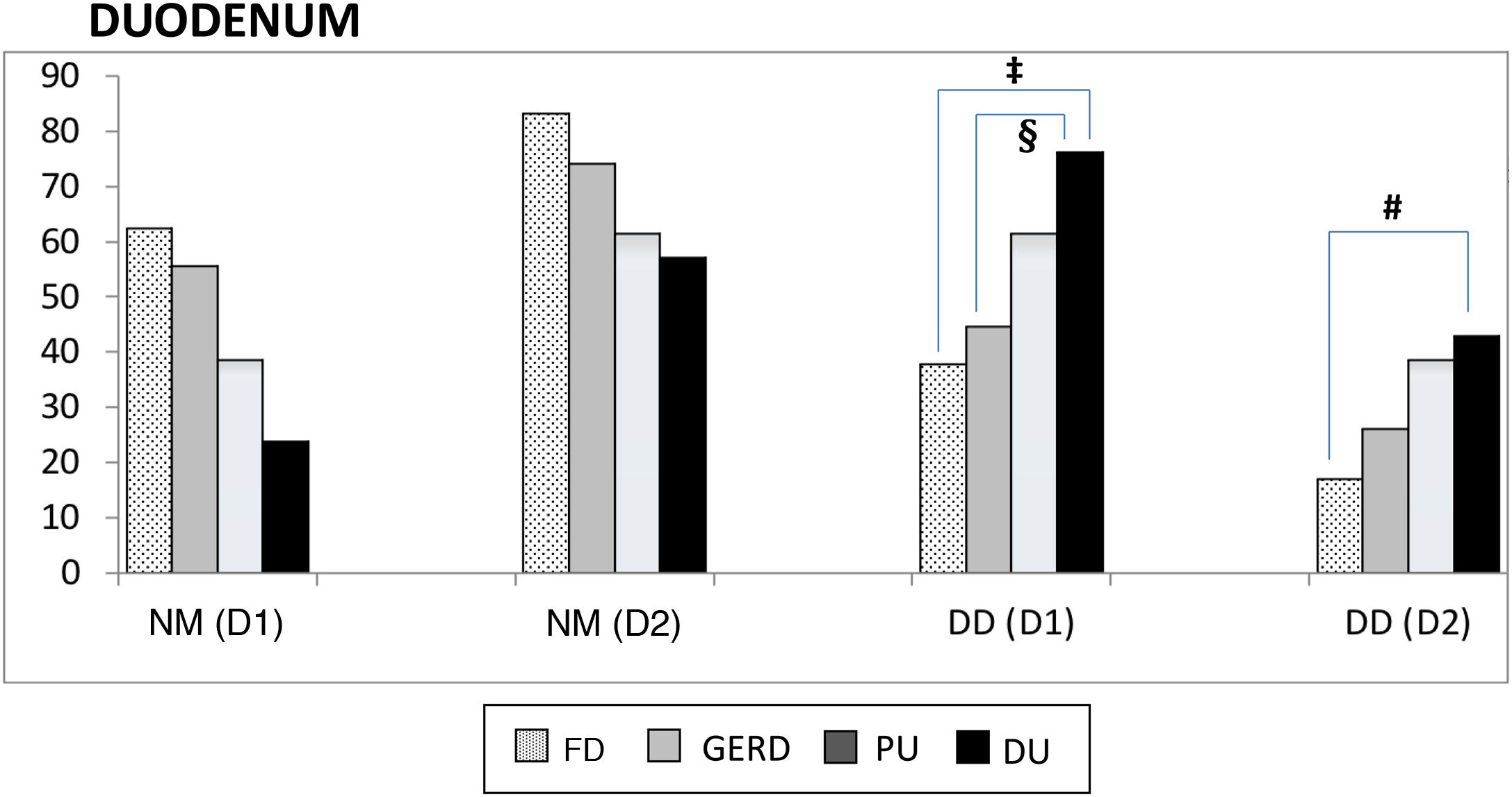
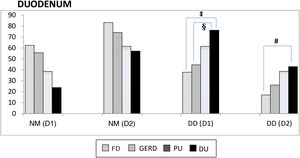
Proximal and distal duodenum. In the duodenum, at both the D1 and D2 levels, there was a higher frequency of DD vs NM in the patients with PU, albeit with no statistical significance when compared with that of the other subgroups. In contrast, in the patients with DU, there were several differences with other subgroups, with respect to the frequency of DD vs NM, at the D1 level (DU vs FD, p < 0.004; and DU vs GERD, p < 0.05), as well as at the D2 level (DU vs FD; p < 0.02) (Fig. 2). Similarly, at both the D1 and D2 levels, there was a higher frequency of DD vs NM, when comparing the ulcer group with the non-ulcer group (in D1: p < 0.003 and in D2: p < 0.01). DD was significantly more frequent in D1 vs D2 in the patients with FD (p < 0.007). That finding was also seen in the patients with DU (p < 0.05), as well as in the ulcer group and non-ulcer group (p < 0.002 and p < 0.02, respectively). Taking all 138 study patients into account, the presence of NM and DD in D1 and D2 was clearly related, in such a way that when the mucosa was normal in D1 it was also normal in D2, in 89% of the cases. Inversely, when there was DD in D1, it was also present in D2, in 43% of the cases (p < 0.0001); that finding was confirmed, as well, when the 138 patients were divided into the ulcer group and non-ulcer group (p < 0.001).
Normal duodenal mucosa and duodenitis.
DD: duodenitis II+III; DU: duodenal ulcer; D1: proximal duodenum; D2: distal duodenum; FD: functional dyspepsia; GERD: gastroesophageal reflux disease; NM: normal mucosa; PU: peptic ulcer.
‡ NM vs DD-D1 between DU and FD, p < 0.004.
§ NM vs DD-D1 between DU and GERD, p < 0.05.
# NM vs DD-D2 between DU and FD, p < 0.02.
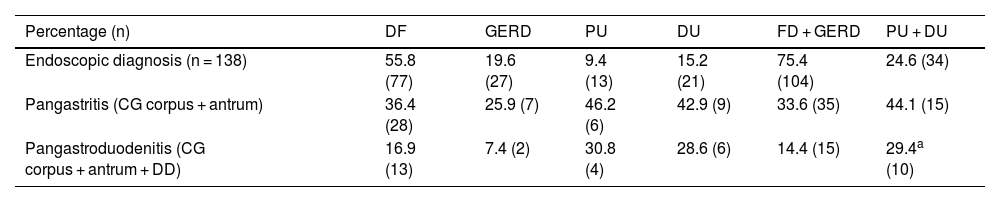
Pangastritis and pangastroduodenitis. Pangastroduodenitis was only more frequent in the ulcer group vs the non-ulcer group (p < 0.05) (Table 2).
Extension of the gastroduodenal inflammation.
| Percentage (n) | DF | GERD | PU | DU | FD + GERD | PU + DU |
|---|---|---|---|---|---|---|
| Endoscopic diagnosis (n = 138) | 55.8 (77) | 19.6 (27) | 9.4 (13) | 15.2 (21) | 75.4 (104) | 24.6 (34) |
| Pangastritis (CG corpus + antrum) | 36.4 (28) | 25.9 (7) | 46.2 (6) | 42.9 (9) | 33.6 (35) | 44.1 (15) |
| Pangastroduodenitis (CG corpus + antrum + DD) | 16.9 (13) | 7.4 (2) | 30.8 (4) | 28.6 (6) | 14.4 (15) | 29.4a (10) |
CG: chronic gastritis; DD: duodenitis II + III; DU: duodenal ulcer; FD: functional dyspepsia; GERD: gastroesophageal reflux disease; NM: normal mucosa; PU: peptic ulcer.
Helicobacter pylori. Table 3 shows the gastroduodenal inflammatory map of the 114 patients with H. pylori. In all the groups and at all the levels evaluated, the inflammatory map was closely related to the presence of H. pylori. Thus, CG of the corpus, CG of the antrum, and DD were significantly related to H. pylori colonization (Table 3), and even though it was not significantly predominant in any of the locations, it was higher in the patients with PU, both in the gastric corpus (92.3%) and the gastric antrum (84.6%). On the other hand, H. pylori colonization was less frequent when there was CAG in the corpus vs CAG in the antrum (% H. pylori+ in CAG-corpus vs CAG-antrum; p < 0.03). A relation between the presence of H. pylori, CAG, and DD-D1 was observed only between the ulcer group and the non-ulcer group at the level of the antrum (p < 0.02).
Inflammatory map in patients with Helicobacter pylori.
| Location | NM | CG | SCG | CAG | DD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % Hp (n) | n | % Hp (n) | n | % Hp (n) | n | % Hp (n) | n | % Hp (n) | |
| Corpus | 81 | 79 (64) | 57 | 88 (50)a | 44 | 95 (42) | 13 | 62 (8)b | - | - |
| Antrum | 26 | 50 (13) | 112 | 90 (101)c | 46 | 91 (42) | 66 | 89 (59)d | - | - |
| Proximal duodenum (D1) | 73 | 77 (56) | - | - | - | - | - | - | 65 | 89 (58)e |
| Distal duodenum (D2) | 104 | 83 (86) | - | - | - | - | - | - | 34 | 82 (28) |
CAG: chronic atrophic gastritis; CG: chronic gastritis; DD: duodenitis II + III; DU: duodenal ulcer; Hp: with Helicobacter pylori; NM: normal mucosa; PU: peptic ulcer; SCG: superficial chronic gastritis.
Corpus:
The prevalence of FD in our patients with dyspepsia was 69.4%, similar to other figures that have been reported,11–13 in which no gastroduodenal lesions are found in two-thirds of the patients that undergo gastroscopy.12 Logically, the prevalence of H. pylori infection in patients with dyspepsia (84.7%) and in patients with FD (77.9%) was higher than that of the general population of our study patients (52%),14 although within the reported range.15–19 According to those data, the prevalence of H. pylori in FD is ≈50% higher than in the general population, suggesting a prominence of H. pylori, in some aspect of FD pathophysiology and symptoms. That supposition, now reported,20–24 explains the fact that up to 10% of the patients with FD remain asymptomatic after eradication of the bacterium. This new dyspepsia, no longer called FD, is known as “H. pylori-associated dyspepsia”.20–22 The different prevalence figures that have been reported may be due to the different criteria utilized for defining the patients with H. pylori, the varying number of biopsies taken, or to studies conducted on geographically/culturally different populations.25
At the level of the corpus, in the 4 subgroups, ≈50-60% of the patients had NM. According to our data, approximately half of our patients around 50 years of age that present with FD/GERD have NM in the corpus. The remaining patients will present with CG, which will generally be SCG. That coincides with the hypothesis that H. pylori infection is acquired in infancy/adolescence that later develops into CG in the corpus and slowly progresses to moderate/severe grades of CG.1–4 In the corpus, H. pylori, together with CG, was present in 45-60% of all the subgroups, except PU (92.3%), confirming the great prominence of H. pylori at the level of the corpus, in the development of PU.1,3 An elevated frequency of CAG does not appear to be necessary for that to occur, given that it was found in only 7.8% of the patients with PU. When CAG of the corpus is extensive, achlorhydria is produced by the massive destruction of parietal cells, changing the habitat, resulting in the decrease/disappearance of H. pylori.1 The low frequency of CAG in PU would favor the high level of colonization found.1,2 Our findings concur with the PU pattern described by the Scandinavian groups:3,4 marked CAG in the antrum/incisura angularis with intestinal metaplasia; and in the corpus, SCG with absent/mild CAG (with no achlorhydria). At the level of the corpus, we consider that the non-ulcer group could have ≈60% NM, parallel to lower colonization (<50%), and the ulcer group could have high bacterial colonization (≥60%) and somewhat more inflammation, with SCG significantly more frequent than CAG, which is in line with results from other studies.1,26
There was an increase in CG in the gastric antrum in all the subgroups. The presence of CG was especially high in the patients with PU (92.2%) and DU (85.7%), with CAG in more than two-thirds of the cases and H. pylori in more than 60%, with no significant predominance of the bacterium in the antrum vs the corpus, as referred to in other studies.2,4,15,26 In the DU subgroup, at the level of the antrum, the high frequencies of CAG and H. pylori colonization developed parallelly to the previously described pattern at the level of the corpus (predominance of NM or SCG and normal secretion due to the absence or scarcity of atrophic phenomena).3,4 In summary, at the level of the antrum, there were non-ulcer patients with ≈20% NM, a similar proportion of SCG-CAG, and ≈65% colonization; and PU patients with very little NM (<15%), predominant CAG (65-70%), and ≈70% bacterial colonization. The elevated frequency of CAG in the antrum of the ulcer group vs the non-ulcer group is also a characteristic reported in other studies,1,3,4,15,26 suggesting that atrophy at the level of the antrum is a prior condition for the later development of a PU.3,4 Surprisingly, at the levels of the corpus and the antrum, and in the subgroups of the ulcer group, the frequencies of H. pylori infection were similar. However, the percentages of CAG were slightly higher at the level of the antrum vs the corpus. That finding has been explained by a greater immune response and consequent inflammatory reactivity in the presence of H. pylori, at the level of the antrum or by the more frequent presence of H. pylori strains (CagA gene) that would induce greater production of proinflammatory cytokines.2,26,27
There are very few histologic studies of the stomach that complement those of the duodenum, and those that include D2 are exceptional. Logically, the prevalence of DD-D1 in ulcer groups is higher than in FD, with prevalence reports between 36 and 76%18,25,26,28,29 vs 37% in asymptomatic controls.29 Our prevalence of DD-D1 in FD (37.7%) is in the lower range of that reported, similar to that of asymptomatic controls, and in line with the results of some European studies.25,28 The highest prevalences have been reported in studies that utilize semi-quantitative methods for defining DD and that are conducted on Asian populations, which are culturally different from Spanish populations,18,26 possibly explaining the resulting differences. We encountered no studies for comparing the prevalence of DD-D2 that we found in FD (16.9%), as that segment is not commonly analyzed. An explanation of its low frequency is mentioned further ahead. Therefore, the present study is the first to report the joint prevalence of DD at the D1 and D2 levels in FD. Those data should alert us to the possibility of moderate/severe inflammatory lesions in the duodenum of patients that do not present with ulcer, in those that have symptoms (FD), as well as those that do not (asymptomatic controls).29
As stated above, more than 2/3 of the patients in the ulcer group and more than 3/4 of the patients in the DU subgroup were characterized by presenting DD in D1 and ≈40% cases in D2. In the ulcer group, the presence of DD was significantly related to H. pylori and CAG in the antrum, but only in D1. That relation is controversial because it has been confirmed in some studies,17,18,27,28 but not in others.30 Regarding the antrum-duodenum conditioned relationship, the progression of the bacterial infection and inflammatory and atrophic state from the antrum to the next level (D1), reproducing the phenomena that previously occurred in the antrum seems logical. For a PU to develop, the previous presence of CAG in the antrum is needed, and for a DU to develop, H. pylori colonization of the D1, coming from the antrum, with the later development of DD, is needed. The fact that said relationship appears only at the level of D1, and not D2, could be explained by the changes in the habitat in more distal areas that would impede H. pylori infection from developing under adequate conditions. At any rate, said discrepancy could be explained by the very heterogeneous patient groups analyzed or the different methodologies employed for the histologic/bacterial classifications or determinations. In an adequate environment, H. pylori colonizes the antrum and induces CG. From there, it moves into D1, where there tends to be sufficient acid load to survive and induce DD, without an associated DU. If acid overload is chronic/intense in D1, adaptive phenomena are produced (gastric metaplasia), favoring H. pylori colonization/infection and predisposing to the development of DU. That sequence is not produced in D2 by remaining “stalled” in the DD phase, with no gastric metaplasia, and so DU is exceptional at that level.3,4,27 The “stalling” of the sequence would take place due to the dilution of the acid and/or the continuous peristalsis that would impede the persistent action of the acid on the mucosa, gastric metaplasia, and bacterial colonization. Our study data adapt well to that entire sequence of events. DD in patients with H. pylori that do not present with DU could be the step prior to the development of DU or the step following its recent healing, which would explain why those patients were classified as having FD, in the absence of duodenal biopsies that could have shown the presence of an underlying DD. Those occurrences could justify and explain why dyspeptic symptoms disappear after H. pylori eradication in some patients diagnosed with FD,19–21 and overwhelmingly suggest that the symptoms in the subgroup of patients with FD actually have a duodenal, rather than a gastric, origin.5,6,23,25,26 In D2, after the inflammatory storm in the corpus-antrum-D1, we recognized an inflammatory calm, with an increase in the frequency of NM of ≈30% (in the non-ulcer group) and ≈100% (in the ulcer group), with respect to that seen in D1. As described above, the finding of NM in D1 almost always conditioned normality in D2 (89%), but if DD was found in D1, DD only occurred in D2 in 43% of the cases. Finally, the fact that only the ulcer group showed a stomach with significantly higher frequencies of pangastritis, DD-D1, and H. pylori colonization vs the non-ulcer group, resulted in a significantly more intense gastroduodenal inflammatory map or greater pangastroduodenitis.
FinancingNo sponsorship of any kind was received for this article.
Conflict of interestsThe authors declare that they have no conflict of interest.
Please cite this article as: Caballero-Plasencia MR, Caballero-Mateos AM, Caballero-Plasencia AM. Mapa inflamatorio de la mucosa gastroduodenal en pacientes con síntomas gastrointestinales altos. Protagonismo de la infección por H. pylori. Rev Gastroenterol Méx. 2023;88:238–245.