Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged, causing the current pandemic of acute respiratory disease known as COVID-19. Liver injury due to COVID-19 is defined as any liver injury occurring during the course of the disease and treatment of patients with COVID-19, with or without liver disease. The incidence of elevated liver transaminases, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), ranges from 2.5 to 76.3%. The aim of the present study was to describe the hepatic biochemical abnormalities, after a SARS-CoV-2-positive polymerase chain reaction (PCR) test, and the mortality rate in critically ill patients.
Materials and methodsA retrospective study was conducted that included 70 patients seen at a private hospital in Mexico City, within the time frame of February-December 2021. Median patient age was 44.5 years (range: 37-57.2) and 43 (61.4%) of the patients were men. Liver function tests were performed on the patients at hospital admission.
ResultsGamma glutamyl transferase (GGT) levels were elevated (p = 0.032), as were those of AST (p = 0.011) and ALT (p = 0.021). The patients were stratified into age groups: 18-35, 36-50, and > 50 years of age. The 18 to 35-year-olds had the highest liver enzyme levels and transaminase levels were higher, the younger the patient. Due to the low mortality rate (one patient whose death did not coincide with a hepatic cause), the multivariate analysis showed an R2 association of 0.689, explained by AST, GGT, and C-reactive protein levels.
ConclusionsDespite the increase in transaminases in our study population during the course of COVID-19, there was no increase in mortality. Nevertheless, hospitalized patient progression should be continuously followed.
La pandemia por coronavirus de 2019 (COVID-19), puede causar El síndrome respiratorio agudo grave (SARS-CoV-2) emergió, causando la pandemia actual de enfermedad respiratoria aguda llamada COVID-19. La lesión hepática por COVID-19 se define como cualquier daño hepático que ocurra durante el curso de la enfermedad y el tratamiento de pacientes con COVID-19, con o sin enfermedad hepática. La incidencia de transaminasas hepáticas elevadas, ALT (alanina aminotransferasa) y AST (aspartato aminotransferasa), va de 2.5% a 76.3%. El objetivo del estudio fue describir las anormalidades bioquímicas hepáticas posterior a la prueba reacción en cadena de la polimerasa (PCR) positiva para SARS-CoV-2 y mortalidad en el paciente crítico.
Material y métodosEs un estudio retrospectivo, se incluyeron 70 pacientes, la mediana de edad 44.5 (rango 37-57.2), siendo del sexo masculino 43 (61.4%), de un hospital privado de la Ciudad de México, se midieron al ingreso hospitalario las pruebas de función hepática. Período de febrero-diciembre 2021.
ResultadosEncontrando elevación de gamma-glutamil transferasa (GGT) p 0.032, aspartato aminotransferasa (AST) p 0.011, alanina-aminotransferasa (ALT) p 0.021, los pacientes se estratificaron en grupo de edad, 18-35, 36-50 y > 50 años. La mayor elevación fue de 18-35 años, entre más joven mayor elevación de transaminasas, debido a la baja mortalidad, 1 paciente, que no coincide con causa hepática, el análisis multivariado explicó una asociación R2 .689 p 0.001, explicado por AST, GGT y proteína C reactiva.
ConclusionesA pesar del incremento de transaminasas durante la infección por COVID 19, en nuestra población no aumentó la mortalidad, si bien, debe darse seguimiento continuo durante la evolución hospitalaria.
The 2019 coronavirus (COVID-19) disease pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with morbidity and mortality worldwide. COVID-19 affects the respiratory system, gastrointestinal system, and central nervous system. The risk factors associated with greater mortality from COVID-19 include advanced age, obesity, male sex, and pre-existing comorbidities, including chronic liver disease.1,2
Liver injury associated with COVID-19 is defined as any liver damage that occurs during the course of the disease and the treatment of patients with COVID-19, with or without a pre-existing liver disease. That includes a broad spectrum of possible pathogenic mechanisms, such as direct cytotoxicity resulting from active viral replication of SARS-CoV-2 in the liver, causing immune-mediated liver injury due to the severe inflammatory response/systemic inflammatory response syndrome (SIRS) in COVID-19; hypoxic changes induced by respiratory insufficiency; and vascular changes due to coagulopathy, endotheliitis, or cardiac congestion from right-sided heart failure.3
The incidence of elevated liver transaminases, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), in patients with COVID-19 ranges from 2.5 to 76.3%. In a recent meta-analysis, the pooled rate for AST and ALT outside the reference range was 20 to 22.5% and 14.6 to 20.1%, respectively. Those anomalies can be accompanied by a slight increase in total bilirubin (TB) levels in up to 35% of cases.4,5
Previous studies have reported abnormalities in liver enzymes in a considerable number of patients with COVID-19. Elevated liver enzymes have been observed more frequently in critically ill COVID-19 patients. Likewise, patients with COVID-19 that present with a rising increase in liver transaminases are reported to have more severe disease, as well as a high mortality rate.6
In a Chinese retrospective study, elevated AST and direct bilirubin (DB) levels at hospital admission were identified as independent predictors of COVID-19-associated mortality.7
The aim of the present study was to describe the biochemical abnormalities in liver function tests (LFTs) in critically ill patients with a SARS-CoV-2-positive polymerase chain reaction (PCR) test and mortality.
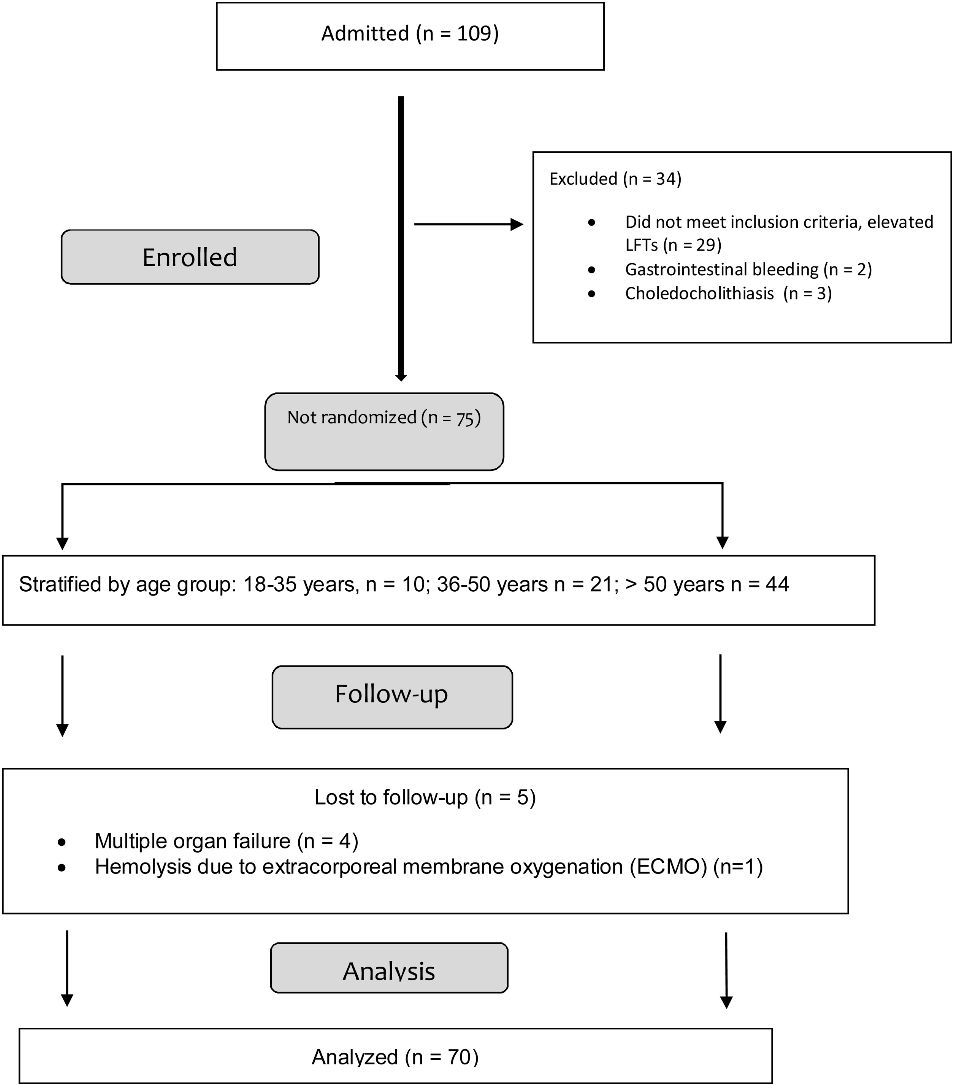
Materials and methodsStudy populationA retrospective study was conducted that included adult patients with a SARS-CoV-2-positive PCR test, seen at a private hospital in Mexico City, within the time frame of February and December 2021. A total of 109 patients diagnosed with SARS-CoV-2 were admitted to the hospital. The medical records of all patients that presented with elevated transaminases at admission were reviewed. Patients that did not have an elevated liver profile (n = 34) were excluded and those, who during their follow-up, presented with other causes of elevated LFT results, such as multiple organ failure (n = 4) and hemolysis due to the use of extracorporeal membrane oxygenation (ECMO) (n = 1), were eliminated, leaving a total of 70 study patients (flowchart, Annex 1). A clinical and laboratory parameter database was created that included the demographic variables of age, sex, and body mass index (BMI); the hospital characteristics of days of hospital stay and days on mechanical ventilation; the severity characteristics of the sequential organ failure assessment (SOFA) and the acute physiologic and chronic health evaluation II (APACHE II); the comorbidities of arterial hypertension, diabetes mellitus, hyperlipidemia, chronic liver disease, obesity, and mortality; the biochemical characteristics of alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), AST, ALT, TB, D-dimer, fibrinogen, coagulation times, procalcitonin, albumin, and C-reactive protein (CRP). The variables were evaluated by reviewing the medical records and the patients were stratified by age group (18-35.9 years, 36-50 years, > 50 years).
All parameters were assessed utilizing standard laboratory tests. Normal ranges were those employed by the hospital where the study was conducted.
Statistical analysisThe categorical variables were expressed as number (n) and percentage (%) and the continuous variables as median and interquartile range (IQR). Normality tests were carried out and the Shapiro-Wilk test was utilized to prove normal distribution. The Mann-Whitney U test was employed to compare the continuous variables that were not distributed normally between 2 groups and the Kruskal-Wallis test to compare the continuous variables that were not distributed normally between 3 groups. The Pearson chi-square test or Fisher’s exact test were utilized to compare the categorical variable groups. Lastly, a multivariate analysis, through logistic regression, was carried out to calculate the adjusted effect of the associated variables. The variables entered were defined a priori, based on the known literature, and statistical significance was introduced into the model. Interaction with the covariables was proved, adjusted odds ratios (ORs) were obtained, and the goodness-of-fit model was evaluated through the Hosmer-Lemeshow test. Sample size was based on proportions, with an outcome prevalence of 76.3%4; n = [EDFF*Np(1 − p)]/ [(d2/Z21-α/2*(N − 1) + p*(1 − p)], resulting in 63 patients that was increased to 70 to compensate for lost patients.
IBM SPSS® software was employed for the statistical analysis and statistical significance was set at a p < 0.05.
Ethical considerationsThe present study meets the current regulations for bioethical research and follows the good clinical practice guidelines. The study was authorized in writing by the ethics committee of the Hospital Ángeles Mocel. The lead author is in possession of the original document and it can be sent to the journal, if necessary.
Due to the retrospective design of the present study, in which the information was retrieved from medical records, informed consent was not requested. Nevertheless, no vulnerable data that could identify the patients were described, maintaining patient anonymity and privacy. Thus, the present study contains no personal information that could identify the patients.
ResultsA total of 70 patients with a SARS-CoV-2-positive PCR test were included. Forty-three percent of the patients were men and 27% were women. Median patient age was 44.5 years; 9 (12.8 %) patients were between 18-35.9 years of age, 20 (28.5%) were between 36-50 years of age, and 41 (58.5%) were > 50 years of age. None of the patients had a history of liver disease. As age increased, so did the prevalence of comorbidities (arterial hypertension, diabetes mellitus, dyslipidemia, cardiovascular disease, and chronic kidney disease). The rate of obesity was highest in the patients from 40 to 61 years of age. Table 1 shows the patient characteristics.
Patient characteristics of the study population.
| Variables | n = 70 |
|---|---|
| Age n, median (IQR) | 44.5 (37-57.2) |
| Sex n | |
| M (%) | 43 (61.4) |
| F (%) | 27 (38.6) |
| Height n, mean (±SD) | 1.68 (±.072) |
| Weight n, median (IQR) | 78 (70-84.2) |
| BMI n, median (IQR) | 26.4 (24.3-30.1) |
| Days of hospital stay n, median (IQR) | 17 (14-24.2) |
| Days on ventilation n, median (IQR) | 4.19 (2.5-7.25) |
| Days of decrease in transaminases n, median (IQR) | 8.4 (7-16.4) |
| SOFA n, median (IQR) | 10 (9-15.25) |
| APACHE II n, median (IQR) | 22 (18-29) |
| Intubated n, yes (%) | 29 (41.4) |
| HFNC n, (%) | 37 (52.9) |
| TNC n, (%) | 4 (5.7) |
| Comorbidities n, (%) | |
| SAH | 20 (28.5) |
| DM | 25 (35.7) |
| Dyslipidemia | 10 (14.2) |
| Mortality n, (%) | 1 (1.4) |
APACHE II: acute physiology and chronic health evaluation II; BMI: body mass index; DM: diabetes mellitus; F: female; HFNC: high-flow nasal cannula; IQR: interquartile range; M: male; SAH: systemic arterial hypertension; SD: standard deviation; SOFA: sequential organ failure assessment; TNC: traditional nasal cannula.
Upon admission to the emergency room, LFTs were requested, after the first SARS-CoV-2-positive PCR test. The parameters of hepatocellular damage (AST/ALT) were elevated in 97.1% (n = 68/70) of the patients and the parameters of cholestatic liver injury (ALP/GGT) were elevated in 40.0% (n = 35/70). Transaminase levels were 3-times > the upper limit of normal. Albumin levels decreased in all age groups, but particularly in the group > 50 years of age.
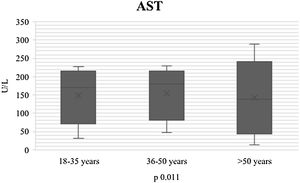
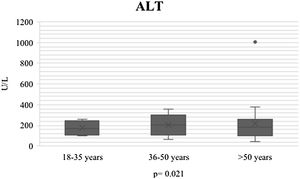
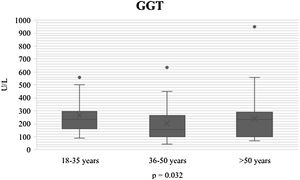
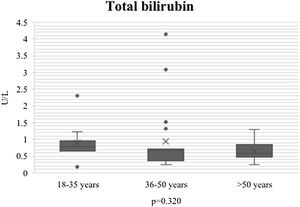
During the course of SARS-CoV-2 infection, the plasma level parameters of hepatocellular damage were compared by age group (AST: p = 0.011; ALT: p = 0.021) (Figs. 1 and 2). Regarding cholestatic damage, ALP (p = 0.416) and GGT (p < 0.032) were not statistically significant (Fig. 3), and there were no significant changes in TB (p = 0.320) (Fig. 4).
Table 2 shows COVID 19-related liver injury by age group. The number of patients with COVID-19-related liver injury was particularly high in the 18 to 35-year age group, and the lower the age, the greater the inflammation or liver injury. In contrast, with respect to albumin, the higher the age > 50 years, the lower the level of albumin. All the patients presented with a high level of CRP, indicating inflammation due to COVID-19. Those data confirmed the fact that, despite being admitted with an inflammatory response, some patients between 18 and 35 years of age presented with a higher level of transaminases than older patients.
Characteristics and comparison of patients by age group.
| Characteristics | n = 70 | Age | p value | ||
|---|---|---|---|---|---|
| 18-35 years (n = 9) | 36-50 years (n = 20) | > 50 years (n = 41) | |||
| Intubated n, (%) | 29 (41.4) | 4 | 10 | 15 | 0.65 |
| HFNC n, (%) | 37 (52.8) | 7 | 18 | 12 | 0.72 |
| TNC n, (%) | 4 (5.7) | 2 | 1 | 1 | 0.21 |
| Albumin n, median (IQR) | 2.77 (1.9-3.0) | 2.7 (1.6-2.2) | 2.8 (1.4-2.9) | 2.3 (1.4-4.3) | 0.112 |
| Globulins n, median (IQR) | 3.2 (2.5-3.7) | 3.4 (1.6-4.8) | 3.2 (2.1-4.3) | 2.9 (1.2-4) | 0.083 |
| A/G ratio n, mean (SD) | .8 (.35) | .76 (.53) | .86 (.82) | .59 (.21) | 0.705 |
| TB mg/dl n, median (IQR) | .58 (3.96) | .68 (.18-2.3) | .55 (.25-4.1) | .56 (.26-1.30) | 0.320 |
| IB mg/dl n, median (IQR) | .27 (.22-.34) | .30 (.10-1.20) | .27 (.11-1.43) | .29 (.14-.60) | 0.189 |
| DB mg/dl n, median (IQR) | .28 (.22-.40) | .39 (.08-1.10) | .28 (.11-2.80) | .28 (.12-.70) | 0.200 |
| AST U/l n, median (IQR) | 165 (89-200) | 170 (34-220) | 160 (15-230) | 150 (45-270) | 0.011* |
| ALT U/l n, median (IQR) | 178 (100-240) | 172 (58-260) | 178 (30-355) | 180 (48-310) | 0.021* |
| GGT U/l n, median (IQR) | 189 (107-270) | 200 (91-320) | 167 (149-244) | 139 (142-283) | 0.032* |
| ALP U/l n, median (IQR) | 90 (78.7-109) | 95-6 (75-126) | 94 (92-124) | 87 (82-99) | 0.416 |
| LDH U/l n, median (IQR) | 300 (228-400) | 299 (238-534) | 306 (278-381) | 302 (264-369) | 0.913 |
| Amylase U/L n, mean (SD) | 49.7 (21.5) | 53 (14.3) | 43 (14.1) | 54 (29.1) | 0.161 |
| Lipase U/L n, mean (SD) | 49.5 (30.2) | 50 (17.9) | 48 (11.8) | 61 (44.9) | 0.627 |
| PT n, mean (SD) | 13.4 (.85) | 13.4 (.36) | 13.6 (1.0) | 13.2 (.79) | 0.285 |
| PTT n, mean (SD) | 31.8(1.34) | 31.5 (1.3) | 31.7 (1.5) | 32.1 (1.0) | 0.123 |
| Fibrinogen n, mean (SD) | .970 (360) | 941 (309) | 1043 (381) | 901 (357) | 0.171 |
| DD ng/ml n, mean (SD) | 1.418 (720) | 1576 (1098) | 1422 (723) | 1337 (467) | 0.985 |
| Ferritin ng/ml | 1636(1278-2422) | 1760 (1470-2953) | 1616 (1670-2589) | 1602 (1444-1937) | 0.530 |
| Cholesterol mg/dl | 180 (132-230) | 178 (118-293) | 189 (164-217) | 180 (156-203) | 0.846 |
| Triglycerides mg/dl | 245 (174-289) | 250 (181-338) | 222 (206-330) | 266 (200-282) | 0.905 |
| Total lipids mg/dl, mean (SD) | 598 (.335) | 695 (403) | 500 (308) | 573 (332) | 0.559 |
| CPK U/l, median (IQR) | 88 (64-2509) | 100 (81-971) | 84 (402-2782) | 80 (92-382) | 0.365 |
| Procalcitonin ng/ml, mean (SD) | .54 (.63) | .34 (.27) | .56 (.68) | .61 (.70) | 0.538 |
| CRP mg/dl n, median (IQR) | 218 (76-367) | 172 (122-222) | 213 (188-238) | 245 (216-274) | 0.022* |
| Uric acid mg/dl n, mean (SD) | 2.8 (1.6) | 2.63 (1.3) | 2.9 (1.8) | 2.77 (1.6) | 0.890 |
A/G ratio: albumin/globulin ratio; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CPK: creatine-phosphokinase; CRP: C-reactive protein; DB: direct bilirubin; DD: D-dimer; GGT: gamma-glutamyl transferase; HFNC: high-flow nasal cannula; IB: indirect bilirubin; IQR: interquartile range; LDH: lactate dehydrogenase; mg/dl: milligrams/deciliter; ng/ml: nanograms/milliliter; PT: prothrombin time; PTT: partial thromboplastin time; SD: standard deviation; TB: total bilirubin; TNC: traditional nasal cannula; U/l: units/liter.
Of the 68 patients that had elevated transaminases at hospital admission and the 2 that had high levels after 48 h of hospitalization, none had abdominal pain in the right hypochondrium, nor did they present with jaundice. A total of 29 (41.4%) patients required mechanical ventilation and only one (1.4%) patient died due to COVID-19-related kidney disease, with a hospital stay of 28 days. Thirty-seven (52.9%) patients had high-flow nasal cannulas (HFNCs) and 4 (5.7%) required traditional nasal cannulas (TNCs).
By age group, the rate of intensive care unit (ICU) admission, intubations, and median hospital stay was higher in the patients > 50 years of age. None of the patients had a history of liver disease. Median hospital stay was 17 days (14-24.2), median number of days on mechanical ventilation was 4.19 (2.5-7.25), 20 (28.5%) patients had arterial hypertension, 25 (35.7%) had diabetes mellitus, and 10 (14.2%) had dyslipidemia. In the present study, cholestatic and liver injury parameters increased after SARS-CoV-2 infection, which resulted in a high mortality rate. The only patient that died was a 48-year-old man, with 2 comorbidities, and his death was related to kidney disease. The multivariate analysis described an R2 association of 0.628 (95% CI 14.1-22.3; p = 0.008), as well as ALT (OR 1.50, 95% CI .91-1.71; p = 0.695), AST (OR 1.39, 95% CI 1.1-1.9; p = 0.049), GGT (OR 1.79, 95% CI 1.3-2.5; p = 0.001), and CRP (OR 1.57, 95% CI 1.3-2.6; p = 0.012), which shows a moderate association between patient age and elevated LFTs, causing transitory liver injury during the course of COVID-19.
DiscussionIn the 2020 retrospective study by Yang et al. that included 710 patients, 52 were admitted to the ICU and underwent mechanical ventilation. A majority of that study population had damage to other organs that was associated with severe COVID-19 pneumonia; 29% presented with liver dysfunction, 29% had acute kidney injury, and 23% presented with cardiac injury. In our study, the cause of death in the patient that died involved the kidney, not the liver.8
According to the 2022 retrospective cohort study by Hartl et al., liver disease due to COVID-19 had no impact on liver-related mortality, coinciding with our results. Despite our smaller sample size, there was only one fatal outcome and it did not involve the liver. Liver injury that presented at admission after a positive diagnosis of SARS-CoV-2 infection did not represent greater mortality.9
Initially, elevated ALP and GGT cholestatic liver enzymes were considered somewhat rare, but recent systematic reviews report elevated ALP and GGT levels in 6.1% and 21.1% of patients with COVID-19, respectively. In our study, only GGT was elevated at admission, with a p = 0.032. ALP was not elevated, nor was it statistically significant (p = 0.416).10
Wang et al. (2020)11 reported prolonged prothrombin time (PT) (13.0 s) in 80/138 patients (58%) and Zhou et al. (2020),12 in their study on 191 patients, found no prolongation. Only 5 patients in our study had PT > 13.5 (7.14%), with no statistical significance (p = 0.285).
In the 2020 study by Chao et al., they stated that GGT had not been reported in the existing case studies on COVID-19, but they found that it was elevated in 30 (54%) of the 56 patients during their hospitalization. The level of ALP was elevated in one of their 56 patients (1-8%), concurring with our study results of little or hardly any increase in ALP.13
An increase in ferritin, CRP, and lactate dehydrogenase (LDH) levels reflects the consequences of inflammatory cytokine release. However, severe cases are associated with a hyperinflammatory condition. A massive release of interleukin-6 (IL-6) has been reported in patients with COVID-19. In a retrospective series on 150 patients in China, elevated levels of IL-6 and serum ferritin were associated with mortality. In our study, all the patients had high levels of ferritin, CRP, LDH, and fibrinogen, but IL-6 per se was not measured or compared.14
Other causes of liver injury in patients with COVID-19 can be hypoxic hepatitis, when there is a sudden increase in aminotransferases in patients in the ICU. An increase in AST can be related to myocarditis. A case report described a patient with respiratory distress syndrome that was autopsied. The procedure revealed microvesicular steatosis with mild inflammation in the liver, albeit it was not clear whether the condition was related to the virus or to other conditions.15,16
In their 2020 original article, Harsh et al. found that inflammatory markers predicted elevated LFT results. Hyperbilirubinemia predicted mortality on admission and at maximum peak. AST and ALT had the opposite impact on mortality, with AST positively associated with death and ALT negatively associated with death. Thus, in addition to hyperbilirubinemia, the AST/ALT ratio emerged as the best mortality marker of the LFTs.17
TB ≥ 1 mg/dl at admission and the AST:ALT ratio were significant mortality predictors, together with age, myocardial injury, and chronic medical illness. Our study results coincide with those, in which the younger population presented with a higher AST level. However, in our study, the bilirubin level did not increase on admission or at peak, with a later decrease, which we agree could explain the low mortality we found, related to the lack of increase in bilirubin.17
ConclusionIncreases in LFT results are very common in patients with COVID-19, albeit the majority are slight. The liver profile should be monitored from the time of admission, and the increase in bilirubin should be monitored using the AST/ALT ratio, because those aminotransferases are predictors of mortality in COVID-19 disease.
The pathogeny of COVID-19 is clearly multisystemic, with multifactorial mechanisms of liver injury that rarely progress to acute liver failure. Even if an increase in transaminase levels has been shown to be a predictor of mortality in other case series, the early identification of severity and opportune multiorgan support can modify those outcomes. Whether there is an effect on liver function, in the long term, in patients with COVID-19 and no liver injury, is still to be clarified.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.