The relationship between obesity and nonalcoholic fatty liver disease (NAFLD) has long been established, and the prevalence of both conditions has grown together. Recent interest in NAFLD in nonobese individuals has led to an increasing number of studies, especially in Asia. Despite the fact that the prevalence of NAFLD in Latin America is one of the highest in the world, there is a lack of information on lean NAFLD populations from the region. The aim of the present study was to assess the risk of metabolic comorbidities across the whole body mass index spectrum when nonalcoholic steatohepatitis (NASH) was first diagnosed in a Latin American population.
MethodsA single-center, cross-sectional study on Colombian patients newly diagnosed with NAFLD, within the time frame of 2010–2020, compared their metabolic biochemical profile, liver enzymes, risk of prevalent metabolic abnormalities, and liver disease.
ResultsData from 300 patients were collected. Ninety-two percent of the patients were men and the median patient age was 47 (IQR 20) years. We found no significant differences in the biochemical, metabolic profile, or liver enzyme plasma concentration between lean, overweight, and obese individuals. Obese patients had significantly higher LDL cholesterol, and a higher risk of dyslipidemia (OR 1.86, 95% CI 1.14–3.05). Every 1kg increase in body weight increased the risk of having NASH by 2% (95% CI 2–4).
ConclusionsWe evaluated the metabolic risk across the entire body mass index spectrum in a Colombian cohort with NAFLD and presented the characteristics of what we believe is the first Latin American lean NAFLD population to be described.
La relación entre obesidad y enfermedad de hígado graso no alcohólico (EHGNA) se estableció desde hace un tiempo considerable y la prevalencia de ambas condiciones ha crecido a la par. Un reciente interés en EHGNA en individuos no obesos ha llevado a un mayor número de estudios, especialmente en Asia. A pesar del hecho de que la prevalencia de EHGNA en Latinoamérica es una de las más altas del mundo, no existe información sobre poblaciones EHGNA delgadas de la región. El objetivo del presente estudio fue evaluar el riesgo de comorbilidades metabólicas en el espectro completo del índice de masa corporal (IMC) al momento de diagnóstico inicial de esteatohepatitis no alcohólica (EHNA) en una población latinoamericana.
MétodosRealizamos un estudio transversal de un solo centro en pacientes colombianos recientemente diagnosticados con EHNA, en el marco temporal de 2010 a 2020. Comparamos su perfil bioquímico metabólico, enzimas hepáticas, riesgo de anormalidades metabólicas prevalentes y enfermedad hepática.
ResultadosSe obtuvieron datos de 300 pacientes. Noventa y dos por ciento de los pacientes fueron hombres y la edad mediana de los pacientes fue 47 (RIQ 20) años. No encontramos diferencias significativas en el perfil bioquímico, metabólico o de concentración de enzimas hepáticas en plasma entre sujetos delgados, con sobrepeso y obesos. Los pacientes obesos tuvieron colesterol LDL significativamente más elevado y un mayor riesgo de dislipidemia (OR 1.86, IC 95% 1.14–3.05). Cada incremento de 1kg. de peso corporal incrementó el riesgo de presentar EHNA en 2% (IC 95% 2–4).
ConclusionesEvaluamos los riesgos metabólicos en el espectro completo del índice de masa corporal en una cohorte colombiana con EHGNA y presentamos las características de la que creemos es la primera población delgada latinoamericana con EHGNA en ser descrita.
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide, and its prevalence is rising. It used to be considered an almost exclusive condition of the overweight and obese, as the visceral manifestation of metabolic syndrome (MetS). However, recent reports suggest that the frequency with which NAFLD occurs in lean individuals is not negligible, and is also on the rise.1
Most of the data on nonobese individuals with NAFLD come from Asia, making the region particularly important for the study of this phenomenon. Reports from Japan, China, Korea, and Hong Kong suggest a high prevalence of NAFLD in nonobese Asian populations, but the highest prevalence in the general population is found in the Middle East and Latin America.1,2
NAFLD in the nonobese is probably due to a genetic predisposition, with epigenetic changes and exposure to certain ambient factors, and Latin American and Latino populations appear to have a higher risk. For example, a polymorphism in the PNPLA3 gene, rs738490[G], has been associated with an increased risk of developing NAFLD3 and is also more common in lean individuals4 and Latino populations.5,6 In addition, the high-risk phenotype of "metabolically-obese" normal-weight individuals with increased insulin resistance has been reported more frequently among Latinos.7
Metabolic conditions, such as diabetes mellitus (DM), dyslipidemia, and MetS, are common in obese persons with NAFLD. Nonobese NAFLD patients seem to be at greater risk, especially for developing DM, than those without NAFLD. But compared with obese NAFLD patients, the results are mixed, with some reports showing a similar risk profile and others a lesser frequency of metabolic comorbidities.8
The prevalence of certain metabolic conditions in the Latino population with NAFLD in the United States has been reported in multiethnic studies.9,10 However, those results cannot be generalized to all Latin American populations, and heritage-related differences in the prevalence of NAFLD have been described.11 Therefore, the differences in terms of Hispanic, African, and Native American ancestry among the Latin American countries could reflect an essential evolutionary trait related to the risk for NAFLD. Ancestrality differences could also be associated with metabolic conditions, such as insulin resistance.12
Latin American reports on NAFLD patients are scarce, and there is no available data on nonobese patients and their metabolic risk. The aim of the present study was to assess the risk of metabolic comorbidities and nonalcoholic steatohepatitis (NASH) in a large NAFLD population from Latin America at NAFLD diagnosis and explore its relation to the body mass index (BMI) continuum. This information adds to that existing on Latin Americans and Latinos with NAFLD, as well as to their baseline metabolic profile, and could help to formulate more specific screening and treatment strategies for Latin American populations.
MethodsStudy design and patientsWe conducted a single-center, cross-sectional study of first-time consultations in patients with NAFLD at the Hospital Pablo Tobón Uribe in Medellín, Colombia, within the time frame of 2010–2020. Our hospital is a University Center with 450 beds and annually provides hospital care to around 18,000 people, receives approximately 80,000 visits to the emergency room, and nearly 100,000 outpatient consultations. In addition, the hospital’s Hepatology and Liver Transplant Unit carries out about 400 ambulatory general hepatology consultations per month.
Convenience sampling was used, and all patients 16 years or older with a NAFLD diagnosis that attended the ambulatory liver disease clinic for the first time were included in the study. Exclusion criteria were hepatitis C, hepatitis B, or autoimmune hepatitis diagnoses, high alcohol consumption (women more than 40g/day and men more than 80g/day), and treatment with amiodarone, methotrexate, or tamoxifen. Patients whose BMI could not be computed were also excluded.
VariablesTwo of the researchers extracted the following data from the medical records of the patients: demographic variables (age and sex), weight, height, BMI, abdominal circumference, systolic blood pressure, diastolic blood pressure, heart rate, diagnosis of NASH, diagnosis of liver fibrosis, FIB-4 score, alcohol consumption, platelet count, AST, ALT, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, blood glucose, HbA1c, and the presence of comorbidities at the time of NAFLD diagnosis (arterial hypertension, DM, dyslipidemia, and MetS).
DefinitionsNAFLD was defined as fat accumulation in the liver detected through an adequate image, excluding other possible liver steatosis or disease causes.13 The diagnosis of NASH was based on a record of liver biopsy-proven steatohepatitis, steatosis, inflammatory infiltrates, and ballooning degeneration with or without Mallory bodies, or pericellular/perivenular fibrosis. Liver fibrosis was also retrieved from the histology report.
Metabolic conditions were considered only if present at NAFLD diagnosis. The American Diabetes Association (ADA) diagnostic criteria14 were followed to determine DM. To diagnose MetS, patients had to fulfill the criteria proposed by the National Cholesterol Education Program Adult Treatment Panel (NCEP).
Patients were classified as obese when their first recorded BMI was above 30kg/m2, overweight if their BMI≥25 but <30kg/m2, and lean when their BMI was lower than 25kg/m2.
Statistical analysisThe continuous variables were reported as median and IQR, and the categorical variables as absolute and relative frequencies. We used a Kruskal-Wallis test to compare fasting glucose, total cholesterol, LDL cholesterol, triglycerides, blood pressure, AST, and ALT between the three groups, obese (BMI≥30kg/m2), lean (BMI<25kg/m2), and overweight individuals (BMI between 25 and 30kg/m2). Utilizing a Holm correction for multiple testing, we performed a post-hoc analysis using the Wilcoxon test to calculate pairwise comparisons between group levels. A logistic regression model adjusted for age and sex was carried out to estimate the risk of metabolic comorbidities and NASH at NAFLD diagnosis for lean and overweight patients, compared with obese patients, and was presented as an odds ratio (OR) with a 95% confidence interval (95% CI). Missing data for weight or height were higher than expected, so to explore the result of the analysis under alternative scenarios for the missing data, we performed a sensitivity analysis, using a multiple imputation method by bootstrapping,15 to generate various complete datasets and compare the estimated risk. All analyses were conducted using R statistical packages (R: A Computing, Vienna, Austria. URL https://www.R-project.org/).16
Ethical considerationsThe Ethics Committee of the Hospital Pablo Tobón Uribeapproved the study. Only one of the researchers could identify the patients, whereas the rest of the authors accessed only a de-identified database. The committee required no informed consent due to the study design. All authors evaluated the study data, reviewed, approved the final manuscript.
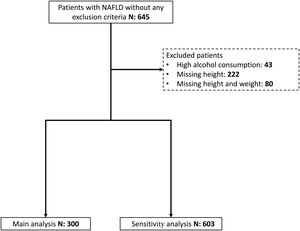
ResultsFrom 2010 to 2020, 603 patients with NAFLD without any exclusion criteria were seen at the hepatology section for the first time, and 300 had data to calculate their BMI. Most of the patients were men and the median patient age was 47 years (IQR 20). Sixteen percent of the patients were lean individuals, with lower median blood fasting glucose and AST levels. Fig. 1 shows the patient selection, and Table 1 includes their characteristics.
Population characteristics.
| Lean (n=48) | Overweight (n=115) | Obese (n=137) | Overall (n=300) | |
|---|---|---|---|---|
| Age (y) | 47 [23.3] | 46 19 | 48 20 | 47.0 20 |
| Sex (men) | 45 (93.8%) | 109 (94.8%) | 122 (89.1%) | 276 (92.0%) |
| Body weight (kg) | 62.0 [12.3] | 74.5 [12.0] | 90.6 [18.0] | 80.0 [21.0] |
| Height (m) | 1.64 [0.143] | 1.66 [0.120] | 1.65 [0.130] | 1.65 [0.130] |
| Body mass index (kg/m2) | 23.5 [1.69] | 27.3 [2.35] | 32.6 [4.91] | 29.0 [6.26] |
| High blood pressure | 14 (29.2%) | 45 (39.1%) | 64 (46.7%) | 123 (41.0%) |
| Diabetes mellitus | 7 (14.6%) | 20 (17.4%) | 37 (27.0%) | 64 (21.3%) |
| Dyslipidemia | 27 (56.3%) | 68 (59.1%) | 100 (73.0%) | 195 (65.0%) |
| Metabolic syndrome | 3 (6.3%) | 6 (5.2%) | 15 (10.9%) | 24 (8.0%) |
| Liver biopsy | 9 (18.8%) | 16 (13.9%) | 21 (15.3%) | 46 (15.3%) |
| Steatohepatitis | 5 (10.4%) | 5 (4.3%) | 11 (8.0%) | 21 (7.0%) |
| FIB4 score | 0.725 [0.431] | 0.825 [0.593] | 0.985 [0.841] | 0.871 [0.685] |
| Systolic blood pressure (mmHg) | 120 [6.00] | 120 [10.0] | 125 [20.0] | 120 [10.0] |
| Dyastolic blood pressure (mmHg) | 70.0 [10.0] | 70.0 [10.0] | 80.0 [18.0] | 78.0 [10.0] |
| Aspartate aminotransferase (U/mL) | 33.0 [30.5] | 38.0 [27.0] | 43.5 [30.8] | 40.0 [30.5] |
| Alanine aminotransferase (U/mL) | 47.5 [56.5] | 54.0 [60.0] | 63.5 [53.3] | 58.0 [58.0] |
| Total cholesterol (mg/dL) | 221 [68.0] | 211 [56.0] | 203 [45.0] | 209 [55.0] |
| LDL cholesterol (mg/dL) | 157 [19.5] | 140 [35.0] | 104 [24.0] | 118 [51.0] |
| HDL cholesterol (mg/dL) | 35.0 [8.00] | 42.0 [17.0] | 41.5 [13.8] | 41.0 [15.8] |
| Triglycerides (mg/dL) | 209 [122] | 183 [134] | 198 [121] | 195 [124] |
| Serum glucose (mg/dL) | 94.0 [16.0] | 96.0 [27.5] | 100 [24.0] | 98.0 [23.0] |
We found no significant difference between the groups in their fasting plasma glucose (p = 0.057), total cholesterol (p = 0.9), HDL cholesterol (p = 0.74), or triglycerides (p = 0.95). LDL cholesterol was significantly different between the groups (p = 0.008). The post-hoc analysis revealed significantly lower LDL cholesterol levels in lean individuals, when compared with obese ones (median difference –37mg/dL (95% CI –55 to –16).
Liver disease markersNASH was found in 7% of the patients at the time of NAFLD diagnosis, but the number of patients with a liver biopsy was relatively low (15%). The median FIB4 score at the time of NAFLD diagnosis was 0.87 (IQR 0.68), with no significant difference between obese patients and those in the other groups (p = 0.07).
No differences were found between the groups regarding AST (p = 0.11) or ALT (p = 0.24) concentrations.
Metabolic and liver disease riskWe found no differences in metabolic comorbidities for NASH in lean or overweight patients, compared with obese patients (Table 2).
Metabolic Comorbidities and risk for NASH at NAFLD diagnosis.
| Lean vs. obese | Overweight vs. obese | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Diabetes mellitus | 0.58 (0.20–1.42) | 0.76 (0.40–1.43) |
| Metabolic syndrome | 0.73 (0.17–2.30) | 0.53 (0.19–1.33) |
| Dyslipidemia | 0.66 (0.35–1.27) | 0.68 (0.42–1.11) |
| NASH | 1.64 (0.38–7.59) | 0.40 (0.10–1.38) |
NAFLD: nonalcoholic fatty liver disease; NASH: nonalcoholic steatohepatitis; OR:odds ratio; 95% CI: 95% confidence interval.
Patients with a BMI of 30 kg/m² or more exhibited a greater risk for presenting with dyslipidemia (OR 1.86, 95% CI 1.14–3.05). Using the mean weight as the reference, for every 1 kg increase in body weight, the risk of NASH increased by 2% (95% CI 2–4).
Sensitivity analysisIn the 603 patient cohort with imputed data, the estimated risk remained unchanged in the sensitivity analysis (Appendix B supplementary Table 1).
DiscussionNAFLD is the leading risk factor for developing cirrhosis worldwide, and its prevalence has increased alongside the obesity pandemic. The relationship between obesity and NAFLD is unquestionable. However, individuals within the normal weight range are also at risk. Diagnosis is delayed, potentially resulting in complications, in at risk lean individuals due to the fact that BMI is the most common surrogate measure of body composition.
Nonobese NAFLD patient metabolic profiles appear to differ from those of obese patients and the general population. A recent meta-analysis showed that the worldwide prevalence of DM is 13.6% (95% CI 8.6–21.0) in nonobese individuals with NAFLD, which is higher than the percentage in the general population of most regions.17 We found similar results in our study. Insulin resistance is crucial in NAFLD and MetS, and the relationship appears to be bidirectional. In severely obese patients, hyperglycemia markers, such as C-peptide and insulin resistance, correlate with NASH18 but not body weight. Furthermore, NAFLD and elevated hepatic enzyme levels are associated with incident DM.19
We aimed to estimate the risk of metabolic comorbidities in obese, overweight, and lean individuals with NAFLD. As previously reported, the risk of having MetS and dyslipidemia in lean individuals with NAFLD is no different from that of overweight and obese patients.8,20 We also found the risk for DM to be the same, but other authors have reported a lower risk.9
In a multiethnic study conducted in the United States, Younossi et al. reported that lean NAFLD patients had lower ALT and AST levels,9 something not reported in Asian and European populations.20–22 We found no difference in ALT or AST concentrations between obese and nonobese (lean and overweight) patients and we speculate that most of our patients, as a newly diagnosed population, had not developed any liver damage.
In other reports, NASH is not unusual in nonobese NAFLD patients; frequencies of 40% have been reported, compared with 53% in the obese.8 We found lower NASH rates than those reported in the literature because not many patients are offered a liver biopsy on their first consultation, given that biochemical biomarkers primarily influence that decision. One interesting finding was the 2% increase in the risk for NASH with every 1 kg increase in body weight. A linear relationship between an increase in BMI and the risk for NAFLD has previously been established in prospective studies.23
With respect to the limitations of our study, first, our data came from a highly specialized center, which could introduce selection bias, making our results not generalizable to other settings. Second, the higher-than-expected missing body weight data made us cautious about our conclusions, but most of our findings remained unchanged in the sensitivity analysis with multiple imputations. Third, the changes in recommendations and increased participation in clinical trials for NAFLD treatment in recent years at our institution could have introduced bias regarding liver biopsy indications.
ConclusionsOur study assessed metabolic risk across the entire body weight spectrum, in a Colombian NAFLD population. It adds to the evidence provided by previous reports on characteristics found in other ethnicities, especially Asians, such as comparable MetS and dyslipidemia between lean and obese NAFLD patients. Knowing the risk of metabolic comorbidities and liver disease may help increase awareness of the growing problem of lean NAFLD.
Financial disclosureThe authors received no financial support for the research, authorship, or publication of this article.
Conflict of interestThe authors declare that they have no affiliation or involvement with any organization or entity with any financial interest (such as fees, financial aid for education, shares, employment contracts, consulting work, or any other type of interest) or non-financial interest (such as personal or professional relationships, affiliations, or beliefs) in the topic of the present article or any material discussed herein. Carlos E. Builes-Montaño has received consulting or speaker fees from Sanofi, Novo Nordisk, Novartis, and Boehringer Ingelheim.