A frequent task in the study of colorectal carcinomas (CRC) is to identify tumors harboring deficient DNA mismatch repair systems (dMMR), which are associated with microsatellite instability. Given that there is scant information on those tumors in Mexican patients, our aim was to describe their frequency, clinical and pathologic characteristics, and results, which are necessary for future trials.
Materials and methodsA consecutive series of CRC patients, treated and followed at a tertiary care center was performed. The clinical and pathologic variables and the risk of hereditary or familial cancer syndrome were retrieved. The original slides and hMLH1, hPMS2, hMSH2, hMSH6 immunohistochemistry were evaluated. Tumors with an absence of at least one protein were considered dMMR. Differences were contrasted, utilizing non-parametric tests.
ResultsOne hundred and forty-four patients were included, with a median age of 65 years. A total of 134/93% patients presented with sporadic CRC, 8/5.6% had a family history of CRC, and 2/1.4% met the diagnostic criteria for hereditary non-polyposis colon cancer, according to the Amsterdam and Bethesda criteria. dMMR tumors were found in 39 patients, distributed among the three groups. They were locally advanced (p<0.001), right-sided, had the mucinous phenotype, and harbored a Crohn’s-like lymphoid reaction (all three features, p<0.04). Adjuvant or palliative chemotherapy was administered to 57 (39.6%), concomitant chemoradiotherapy to 24 (16.7%), but 63 (43.8%) patients received no additional treatment to surgery. Five-year follow-up was completed in 131 of the patients and the outcomes alive-with-disease or died-of-disease were more frequently observed in the proficient (pMMR) lesions.
ConclusionsIn the present pre-FOLFOX case series, outcomes were better in dMMR CRC than in proficient lesions.
Una tarea frecuente en el estudio de los carcinomas colorrectales (CCR) es identificar tumores que presenten sistemas defectuosos de reparación de errores de emparejamiento en el DNA (dMMR, por sus siglas en inglés), los cuales están asociados con la inestabilidad de microsatélites. Dado que se dispone de poca información sobre estos tumores en pacientes mexicanos, nuestro objetivo fue proporcionar una descripción de su frecuencia, características clínico-patológicas y resultados, lo cual es necesario para futuros ensayos.
Materiales y métodosSe realizó una serie consecutiva de pacientes de CCR, que fueron tratados y se les dio seguimiento, en un hospital de tercer nivel. Se consideraron las variables clínico-patológicas y el riesgo de cáncer hereditario o familiar. Se evaluaron las laminillas originales y la expresión de las proteínas hMLH1, hPMS2, hMSH2 y hMSH6 mediante inmunohistoquímica. Los tumores con ausencia de al menos una proteína se consideraron dMMR. Las diferencias fueron contrastadas con pruebas no paramétricas.
ResultadosCiento cuarenta y cuatro pacientes fueron incluidos, edad mediana de 65 años, 134 (93%) pacientes esporádicos, 8 (5.6%) con historia familiar de CCR y 2 (1.4%) cumplían criterios diagnósticos para cáncer de colon hereditario no polipósico, según criterios de Bethesda y Ámsterdam. Los tumores dMMR se encontraron en 39 pacientes repartidos en los tres grupos. Fueron localmente avanzados (p<0.001), en colon derecho, con fenotipo mucinoso y reacción linfoide tipo Crohn (todos p<0.04). Se administró quimioterapia adyuvante o paliativa a 57 (39.6%), radioquimioterapia concomitante a 24 (16.7%) y 63 (43.8%) pacientes no tuvieron tratamiento adicional al quirúrgico. El seguimiento a 5 años se completó en 131, los desenlaces vivo con enfermedad/muerte por cáncer fueron observados con mayor frecuencia en lesiones con MMR presentes (pMMR).
ConclusionesEn esta serie pre-FOLFOX, el CCR dMMR tiene mejor desenlace que las lesiones pMMR.
Cancer mortality in the productive-age Mexican population holds second place, when placing cancers of the breast, uterine cervix, lung, stomach, and liver together, with an average of 20 deaths per 100,000 inhabitants. Cancer would outnumber diabetes as the main cause of death, if the remaining primary organs were included in that ranking (http://sinais.salud.gob.mx 2005).
In the digestive system, gastric cancer and colorectal cancer are the most frequent, with the highest morbidity and mortality rates. Over the past 25 years, there has been a gradual decrease in gastric cancer and an increase in colorectal cancer in Mexico, most likely due to variations in diet and body mass index.1–3 A similar situation has been widely reported in America and Europe, contrasting with higher gastric cancer-related deaths in Africa, Southeastern Asia, and the Asian Pacific region (www.who.int/whr/2004), suggesting that environmental, hygienic/dietary, ethnic and other unidentified conditions favor those diseases.4
Colorectal carcinoma (CRC) has traditionally been deemed a single disease, with separate clinical stages and diverse phenotypes, explained by the differentiation grade and structural growth pattern of neoplastic cells.5 In that context, patients that had surgery, with free margins, underwent standardized follow-up or chemotherapy regimens, attributed to the clinical stage of the disease.6
In the early 1990s, gene identification associated with CRC suggested the possibility of hereditary, familial, and sporadic cancers.6 The first 2 categories explain less than 5% of the cases observed in the general population, and recognized syndromes include familial adenomatous polyposis, Peutz–Jeghers syndrome, Cowden syndrome, and hereditary non-polyposis colorectal carcinoma.7 The identification of that reduced percentage of patients required a large technologic and economic investment that resulted in detection criteria and new approach tools.8–11 Said screening also covers the remaining 95% of patients with CRC that currently crowd healthcare centers.6,7
With the description of ubiquitous somatic mutations in simple repeated DNA sequences in CRC, those neoplasms were subclassified into microsatellite-stable and microsatellite-unstable cancers.6–8 The greater the microsatellite instability, the higher the proportion of hereditary/familial CRC cases.9–11 Nevertheless, the availability of monoclonal antibodies to the DNA repair proteins, hMLH1, hMSH2, hPMS2, and hMSH6, enabled the immunohistochemical detection of a greater number of cases, with high sensitivity and predictive value.12,13 That finding would be no more than simple molecular inquisitiveness, were it not for the fact that: (a) it predicts carcinogenesis through a pathway other than the adenoma-dysplasia-carcinoma sequence,6 (b) the degree of microsatellite instability appears to predict the response to 5 FU and irinotecan-based chemotherapy regimens,14 and (c) it warns about the possibility of adverse reactions to those regimens.13,14
Given the scarce information available on Mexican patients with CRC and the status of their DNA repair proteins,15,16 the aim of the present study was to identify their frequency and clinical and pathologic characteristics, in a consecutive series of cases treated and followed at a tertiary care center in Mexico City.
Materials and methodsWe performed a retrospective search of all patients with a diagnosis of CRC that received medical attention and follow-up at a tertiary care hospital, within the time frame of January 2000 and December 2004. The records of each patient were reviewed to identify demographic, clinical, and surgical variables, pathologic TNM, chemotherapy treatment (fluorouracil-based regimens), complications of neoplastic disease, and status at last consultation. A clinical or family history of cancer was recorded from medical charts or interviews with patients during follow-up. The risk of hereditary or familial cancer was defined, according to the revised Amsterdam II criteria and Bethesda guidelines.9–11 Histologic slides were re-evaluated by two pathologists with experience in gastrointestinal pathology (C-R C, G-D A). Gross features, location, number, size, associated lesions, lymph node condition, invasion level, and margin status of each tumor were identified. Based on the microscopic examination, tumors were classified according to the World Health Organization guidelines.5 In addition, we determined the presence of intratumoral lymphocytes, Crohn’s-like lymphoid reaction (CLR), medullary or cribriform growth, degree of differentiation, and lymphovascular invasion, as well as precursor lesion (polyps: adenomatous, hyperplastic, serrated, mixed or aberrant crypt foci).4,5 All patients that underwent surgical resection and had 5-year follow-up were studied. The DNA repair protein analysis was conducted with immunohistochemistry for hMLH1, hMSH6 (Clones BD and GTBP, Pharmingen), hMSH2, and hPMS2 (Clones CM219C, CM344A, Biocare Mexico, División del Norte 4274-204, Tlalpan, Mexico City), in normal and neoplastic cells. Tumors were classified as mismatch repair protein-proficient (pMMR), if all immunohistochemical reactions were present, or mismatch repair protein-deficient (dMMR), if at least one protein was absent in the neoplastic nucleus. Two-sided p was assessed, using non-parametric tests.
Statistical analysisWe conducted a retrospective search with the aforementioned characteristics. A two-sided p was then assessed, through non-parametric tests.
ResultsOne hundred and ninety-two CRC cases were identified, 48 were excluded due to lack of paraffin blocks or clinical charts, leaving 144 cases included in the study. Seventy-eight of the patients (54.2%) were men, median patient age at diagnosis was 65 years (18–91 years), and 33 patients (23%) were younger than 50 years of age at diagnosis. Two patients (1.4%) met the diagnostic criteria for hereditary non-polyposis colon cancer (Lynch syndrome). Eight patients (5.6%) had a family history of CRC and no diagnostic criteria for hereditary non-polyposis colon cancer. The remaining 134 (93%) patients with no family history of CRC were considered sporadic cancer cases. Most of the tumors were right-sided (53.5%) and locally advanced (66.7%), and 33.3% were stage IV lesions. Bowel resection was performed in 125 cases (86.8%) and the depth of invasion of the tumors (pT) reached the submucosa (3.2%), muscularis propria (11.2%), and subserosa (85.6 %). Tumors were poorly differentiated (42.4 %), moderately differentiated (42.4%), and well differentiated (4.9%). Precursor lesions were observed in 9 patients (6.3%). A CLR pattern was identified in 17 (11.8%) cases, and signet ring cells and mucinous differentiation were found in 5.6% and 11.1% of cases, respectively.
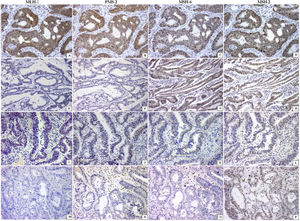
Thirty-nine patients had dMMR tumors. The majority were identified in men with locally advanced disease (p<0.04) and with right-sided adenocarcinoma (p<0.04), mucinous phenotype (p<0.04), and inflammatory reaction in the bowel wall, resembling Crohn’s disease (p<0.04), when compared with the pMMR tumors (Table 1). Twenty-seven patients had loss of 2 MMR proteins in neoplastic cells (22 MLH1/PMS2; 5 MSH2/MSH6). Tumors lacking at least one MMR protein were identified in 12 patients, PMS2 was absent in 7, MLH1 was absent in 4, and MSH2 was absent in one patient (Fig.1).
Clinical and pathologic profiles and related outcomes, according to microsatellite stability, in 144 patients.
| dMMR (n=39) (27.1%) | pMMR (n=105) (72.9%) | p | |
|---|---|---|---|
| Men | 21 | 57 | NS |
| Women | 18 | 48 | |
| Median age (range) | 64 (18−88) | 67 (20−91) | |
| Stage | |||
| Localized (I-III) | 31 (79.5) | 65 (61.9%) | <0.001 |
| Advanced (IV) | 8 (20.5) | 40 (38.1%) | |
| Treatment | |||
| Surgery alone | 19 | 44 | NS |
| Chemotherapy | 17 | 40 | |
| Chemoradiotherapy | 3 | 21 | |
| Tumor location | |||
| Right-sided | 27 | 35 | 0.04 |
| Left-sided | 12 | 70 | |
| Morphology | |||
| Mucinous | 8 | 9 | 0.04 |
| Crohn’s-like lymphoid reaction | 8 | 9 | 0.04 |
| Overall 5-year survival (%) | |||
| Stage I | 80 | 80 | |
| Stage II | 66.7 | 41.7 | |
| Stage III | 36.4 | 41.7 | |
| Stage IV | 7.1 | 5.9 |
Mismatch repair system profile in colorectal carcinoma (every line is a case). Positive nuclear reaction of all enzymes was observed in the pMMR tumors (A–D). The absence of 2 proteins (MLH-1/PMS2 or MSH6/MSH2) in line 2 (E, F) and line 3 (K, L) or the absence of 3 proteins (line 4; M–O) characterized the dMMR tumors. All are immunohistochemical reactions ×200.
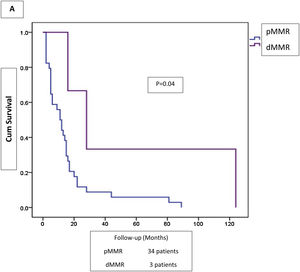
Adjuvant or palliative chemotherapy was administered to 57 (39.6%) patients, concomitant chemoradiotherapy to 24 (16.7%), and 63 (43.8%) patients received no additional treatment to surgery. Five-year follow-up was completed in 131 patients. Outcomes in dMMR versus pMMR colorectal tumors were alive-with-disease (2.5 vs 4.3%), alive-without-disease (46.2 vs 33.7%), died-of-disease (51.3 vs 58.7%), and died-of-other-cause (0 vs 3.3%). Overall survival and five-year survival rates in the dMMR tumors were 41% and 48.7%, respectively, and 32.6% and 38%, in the pMMR tumors, respectively. Disseminated disease outcome was better in the patients with dMMR tumors, although the contrasted groups were small (Fig. 2).
Discussion and conclusionsImmunohistochemistry for DNA mismatch repair system enzymes in colorectal cancer is an affordable technique that, together with the clinical identification, is a first step in recognizing the germline and familial alterations in non-syndromic sporadic CRC patients. dMMR tumors were distributed throughout the 3 groups in the present study: 2/2 patients had a pedigree consistent with Lynch syndrome (100%), 3/8 had a family history of CRC (37.5%), and 34/134 had sporadic tumors (25.3%). The overall frequency of dMMR of 27.1% in our consecutive series of CRC in Mexican patients is in line with two previous communications that identified frequencies of 34% and 21.3%,15,16 all of which were higher than the frequency observed in Latino patients living in the United States.17 Differences in the frequency of dMMR CRC could depend on the age of the population analyzed, selection bias of the study, or on unrecognized factors. However, its evaluation is important because it impacts the current standard of care of CRC with FOLFOX (folinic acid, fluorouracil, and oxaliplatin).18,19
Even though 93% of the tumors sampled were sporadic, with microsatellite instability, according to the Amsterdam and Bethesda criteria, 25.3% of them had deficient DNA mismatch repair systems. The percentage of sporadic cases with dMMR tumors was mainly associated with the absence of MLH1, which is explained by promoter methylation silencing its expression.20 The identification of dMMR CRC has been shown to be important for genetic counselling when observed in young patients and influences adjustments to systemic therapy when metastases are present.21 Most adenocarcinomas in our study were of the conventional type, but with a mucinous phenotype or an inflammatory infiltrate resembling Crohn’s disease in the dMMR lesions (both p<0.04). Aside from the right-sided colon tumor location (p<0.001), no other morphologic finding was related to the dMMR tumors. Those data were concordant with previous reports, in which there was no association of medullary/solid phenotype or dirty necrosis with protein deficiencies.22 Patients with dMMR tumors were younger than the median age of the other patients in the series (64 vs 67 years), 54% were men, and they frequently presented with stage I-III disease (p<0.04). That patient subgroup should be studied utilizing the newly proposed criteria for the study of tumor infiltrating lymphocytes, especially those depicting the Crohn’s-type inflammatory infiltrate.23 A tissue reaction that could be related to immunotherapy improvements has been observed in dMMR colorectal carcinomas.19,23
Scant information is available in Mexico on colorectal cancer incidence and mortality and none of the data provides subcellular insights.2,24–27 As expected, most of the patients analyzed had advanced (III, IV) stages of their disease (59.7%), and cancer-related death was identified in 74 (mortality rate 54.1%). That is in contrast with the low mortality rates suggested for CRC in reports from international studies, and in agreement with a high incidence/mortality pattern, as observed in Costa Rica, Colombia, and Brazil,28 but nevertheless, in line with the lack of reliable national data on cancer.29 The pMMR tumors had the worst outcome in locally advanced disease, as well as in disseminated stages of the cancer, when compared with the dMMR lesions.
The retrospective nature of the present case series and its execution in the pre-FOLFOX era are its limitations, precluding any comparison with the current standards of care.
In summary, most of the patients with CRC in our study had sporadic disease, and of the patients with dMMR tumors, the most frequent lesions were right-sided, mucinous, and had inflammatory infiltrates resembling Crohn’s disease. They were also associated with better outcomes, when compared with the pMMR tumors.
Ethical considerationsThe present work complies with the current regulations on bioethics research, was authorized by the institutional ethics committee. The authors declare that this article contains no personal information that could identify the patients. Informed consent was not requested from the patients to participate in this study.
Financial disclosureNo financial support was received in relation to this study/article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Rios-Valencia J, Cruz-Reyes C, Galindo-García TA, Rosas-Camargo V, Gamboa-Domínguez A. Sistema de reparación de errores de emparejamiento en carcinoma colorrectal. Frecuencia, fenotipo y seguimiento, Revista de Gastroenterología de México. 2022;87:432–438.