Aorto-enteric fistula (AEF) is associated with a high mortality rate of approximately 77% and is fatal without treatment.1,2 AEF is an abnormal connection between the aorta and the gastrointestinal tract3 and is commonly caused by anastomotic leak and peptic ulcer formation.4
A 42-year-old man, with a history of hypertension and ischemic heart disease, reported difficulty swallowing and 10 kg weight loss over the past 4 months. Three months earlier, he underwent cardiac stenting following a myocardial infarction (MI). On esophagogastroduodenoscopy (EGD), an ulcerated, friable tumor was found at 36 cm from the incisors, extending to 41 cm, involving the gastroesophageal junction (GEJ). The tumor predominantly involved the gastric cardia and the lesser curvature along with GEJ. Biopsy showed moderately differentiated adenocarcinoma (Siewert type Ⅲ). Computed tomography (CT) of the abdomen and chest revealed lymph node involvement. A PET CT scan confirmed the diagnosis of adenocarcinoma of the GEJ, with 18F-fluorodeoxyglucose (FDG)-avid gastrohepatic nodes but no distant metastases, resulting in a T3N1M0 staging. Liver metastases, ascites, and malignant cells (fluid cytology) were not found on staging laparoscopy. Following neoadjuvant fluorouracil, leucovorin, oxaliplatin, docetaxel (FLOT 4) chemotherapy regimens, the tumor bulk was successfully resected with clear margins (histopathology) via minimally invasive (abdominal laparoscopy and video-assisted thoracoscopic surgery) 2-stage esophagectomy.
As per our center’s routine practice, no nasogastric tube was inserted. From postoperative day one, liquid feed was started. On post-operative day 6, CT with contrast showed no leaks and the patient was discharged.
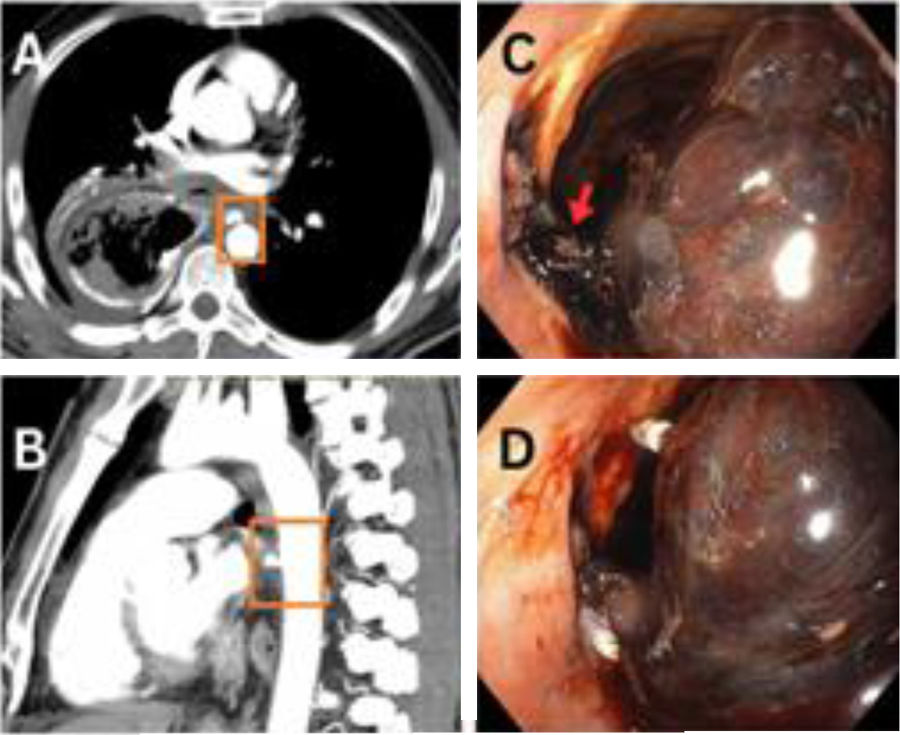
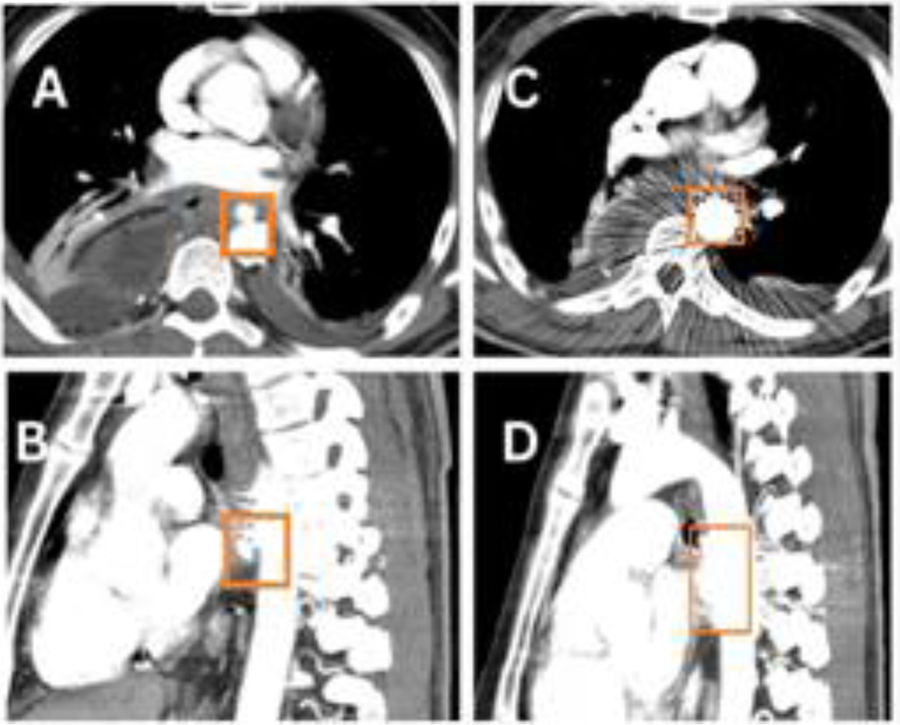
Unfortunately, 17 days after surgery, on Eid day (a national holiday), the patient arrived at the emergency room, having presented with 6–8 episodes of hematemesis. The patient was resuscitated with blood products, tranexamic acid, and vitamin K. His hemoglobin dropped from 7.7 to 5.6 over the next 20 h. A CT scan found an aneurysm at the T6 level on the anterior aspect of the aorta, measuring 10 × 7 mm, with a fistulous connection to the anastomotic site (Fig. 1A–C). There was no pleural leakage and no communication with the thoracic cavity. Endoscopy revealed a large adherent clot and fresh blood at the anastomosis site. Endoscopic attempts to close the fistula with endoclips (Fig. 1D) were unsuccessful. Urgent aortic stenting was performed, and follow-up CT angiography showed that the stent (length 2.9 cm) partially covered the fistulous communication (Fig. 2A and B). Re-stenting with a 4.6 cm stent completely covered the fistula (Fig. 2C and D). The patient was extubated and a nasojejunal tube was inserted. He was discharged on day 10, with long-term oral antibiotics. The patient’s post-operative rehabilitative state is good, and he is undergoing regular surveillance.
(A) Axial and (B) sagittal views of the initial CT scan, showing fistulous communications between the aorta and gastric conduit. (C) Endoscopic image showing a hematoma (red arrow) and some active bleeding from the fistula site. (D) Endoscopic image showing the attempted endoscopic clipping (white clips).
(A) Axial and (B) sagittal views of the CT aortogram after the first stent placement in the aorta, showing partial covering of the fistulous communication. (C) Axial and (D) sagittal views of the CT aortogram after the second stent placement in the aorta, showing complete covering of the fistulous communication.
AEF management focuses on preventing hemorrhage from aortic rupture and sepsis due to esophageal perforation.2 Primary fistulas develop without any prior surgical interventions or injuries to the aorta, whereas secondary fistulas arise following aortic repair surgery.5 In our case, the patient had a primary aortoenteric fistula.
For aortic aneurysms with AEFs, endovascular repair (EVAR) is frequently employed. Studies have shown that the mortality rate for open surgical methods was 33.9%, whereas for EVAR it was only 7%.6,7 In our case, EVAR stenting was deployed twice.
AEF is a surgical emergency and requires immediate intervention, as the mortality rate is nearly 100% without any treatment.8 Timely optimal resuscitation and a CT angiography are crucial to confirm the diagnosis. EVAR stenting is a safe and effective treatment for this highly morbid and fatal condition.
The present case report highlights timely management of an AEF using minimally invasive procedures. It also shows that minimal resources, when combined with a multidisciplinary approach, yield rewarding results.
CRediT authorship contribution statement- •
Conceived and designed the analysis: Tanees Asim and Zubair Shabbir Khanzada.
- •
Collected the data: Tanees Asim and Muhammad Waqas.
- •
Contributed data or analysis tools: Tanees Asim, Mahnoor Asim, and Zubair Shabbir Khanzada.
- •
Drafted the article or revised it: Tanees Asim and Zubair Shabbir Khanzada.
- •
Gave final approval of the version to be submitted: Zubair Shabbir Khanzada.
Written informed consent was obtained from the patient, and institutional review board (IRB) approval was obtained from the institution (SKMCH).
Financial disclosureNo financial support was received in relation to this article.
The authors declare that there is no conflict of interest.