Bariatric surgery is the most effective treatment for obesity and its comorbidities but there are barriers that prevent its general acceptance. The growing obesity epidemic has resulted in the need for the creation of new, less invasive treatments, with a wide margin of safety and effectiveness for conditioning weight loss, at least greater than that resulting from treatment based on diet and exercise. Emerging therapies include devices that are endoscopically placed and removed, classified as: space-occupying devices, restrictive or anatomic-remodeling procedures, endoluminal bypass, and duodenal mucosal resurfacing. Percutaneous techniques and less invasive surgeries are also included.
In general, results have shown improvement in glucose metabolism in diabetic patients. With respect to weight loss, results do not surpass those of bariatric surgery, but are better than results with conservative treatment (diet and exercise) and have a low rate of adverse events. Clinical use of a new technique should be carried out within a multidisciplinary management program that includes nutritional, psychologic, physical activity, and medical support. It must be understood that novel therapies are not being created to substitute bariatric surgery, but rather to increase treatment options in the general population, with greater reach and impact. The aim of the present study was to provide an up-to-date literature review on emerging technologies for the treatment of obesity.
La cirugía bariátrica es el tratamiento más efectivo para tratar la obesidad y sus comorbilidades, sin embargo, hay barreras que evitan que la cirugía bariátrica sea aceptada de forma generalizada. Ante la creciente epidemia de obesidad, surge la necesidad de crear nuevos tratamientos menos invasivos, los cuales deben tener un amplio margen de seguridad y efectividad para condicionar pérdida de peso, al menos mayor que un tratamiento basado en dieta y ejercicio. Estos incluyen dispositivos que se colocan y retiran por endoscopia y se clasifican en: dispositivos ocupativos, procedimientos restrictivos o remodelantes de la anatomía, bypass endoluminal y remodelación duodenal. También se dispone de técnicas percutáneas y de cirugías menos invasivas.
En general, los resultados obtenidos muestran mejoría en el metabolismo de la glucosa de los pacientes diabéticos y en cuanto a la pérdida de peso, aunque no superan a la cirugía bariátrica, se observan resultados que tienen mayor alcance si se comparan con el tratamiento conservador (dieta y ejercicio). La tasa de eventos adversos es baja. El uso clínico de una nueva tecnología debe practicarse dentro de un programa de manejo multidisciplinario que incluya soporte nutricional, psicológico, deportivo, y médico. Es de suma importancia reconocer que estas terapias no son creadas para sustituir a la cirugía bariátrica, sino a aumentar las opciones de tratamientos para generar mayor alcance e impacto en la población general. Este trabajo tiene por objeto una revisión actualizada de la literatura sobre tecnologías emergentes para el tratamiento de la obesidad.
Bariatric surgeries and certain less invasive treatments have currently shown greater efficacy than diet, exercise, and even drug therapy, in the management of obesity.1 Among the most widely known and available procedures are Roux-en-Y gastric bypass (RYGB), vertical sleeve gastrectomy (VSG), duodenal “switch”, and single anastomosis bypass.1,2 Despite the fact that the efficacy and safety of bariatric surgeries have been widely described, less than 1% of patients with morbid obesity are estimated to have access to or accept undergoing any of those procedures to achieve weight loss.3 The reason for that is multifactorial and includes the high cost of the surgery, patient preference for conservative treatment, access to information, and especially, the fear of complications or death.1,3,4 Even though the mortality rate for bariatric surgery has decreased to a level of being comparable to that of a cholecystectomy, hysterectomy, or knee replacement, the overall frequency of complications or adverse events remains between 8 and 17%.3 Based on the above and the growing obesity epidemic, there is a need to create safe and less invasive treatments that achieve greater reach and acceptance in the general population.4 Those therapies should also be highly effective, at least more so than conservative weight loss treatment.5,6
Emerging technologies are defined as procedures that differ from accepted practice and whose results have not been widely described. They offer us the opportunity to improve and broaden the therapeutic armamentarium and are essential for the evolution of medical practice. The impact of the advances in bariatric surgery, resulting from the work of pioneers in surgical innovation, is a clear example.2,5
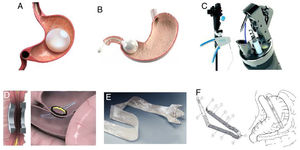
Endoluminal therapies, devices, and novel technologies are currently in different stages of development for the primary treatment of obesity and the control of metabolic problems, as well as for managing previous failed bariatric surgeries or surgical complications.2 In recent years, different endoluminal procedures, such as gastric (Orbera, Obalon, Spatz-3, ReShape) or intestinal (BAROnova, EndoBarrier) space-occupying therapies, technology for endoscopic anatomic remodeling (POSE, StomaphyX, Apollo OverStitch), surgical implants (vBloc and Bariclip), and percutaneous therapies (AspireAssist and gastric fundus embolization) have gained in popularity in the effort to reduce the gap between surgical and medical treatment (Fig. 1).1,5,6 Endoluminal interventions are an attractive alternative for a large group of patients that reject surgical treatment. However, there is little information in the medical literature on the emerging technologies and it is limited to small case series. Thus, there are no clinical practice guidelines on the use of new technologies for the treatment of obesity.2Table 1 shows the most widely studied therapies and devices, classifying them by placement route, device subtype, and primary organ to treat. In 2018, the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) reported 25,359 (4%) primary endoluminal procedures worldwide7 and the most commonly described were gastric balloons, endoscopic suture devices, and EndoBarrier. The actual number is thought to be higher, given that reliable data could be obtained from only 35% of the associations that belong to the IFSO. In addition, the data collected were compared with the annual sales reported by the manufacturers, and the sales figures were higher that the procedure figures reported.1
The most widely accepted novel technologies/procedures.
| Approach | Type | Organ(s) | Subtype | Device | FDA |
|---|---|---|---|---|---|
| Endoscopy | Space-occupying | Stomach | Balloon | Orbera | Yes |
| Endoscopy | Space-occupying | Stomach | Balloon | ReShape | Yes |
| Endoscopy | Space-occupying | Stomach | Balloon | Spatz-3 | No |
| Ingestible/Endoscopy | Space-occupying | Stomach | Balloon | Obalon | Yes |
| Endoscopy | Space-occupying | Stomach /duodenum | “Pyloric transporter” | BARONova | Yes |
| Endoscopy | Remodeling | Stomach | Endoscopic suture | POSE | No |
| Endoscopy | Remodeling | Stomach | Endoscopic suture | OverStitch | Yes |
| Endoscopy | Remodeling | Stomach | Endoscopic suture | EndoCinch | Yes |
| Endoscopy | Re-epithelialization | Duodenum | NA | DMR (Revita) | No |
| Endoscopy/Laparoscopy | Barrier | Gastro-duodenal-jejunal | NA | ValenTx | No |
| Endoscopy/Laparoscopy | Barrier | Duodenal-jejunal | NA | EndoBarrier | No |
| Endoscopy/Laparoscopy | Magnets | Jejunum/Ileum | NA | IAS | No |
| Endoscopy/Laparoscopy | Magnets | Jejunum/Ileum | NA | IMAS | No |
| Endoscopy/Laparoscopy | Magnets | Jejunum/Ileum | NA | Magnamosis | No |
| Percutaneous | Aspiration | Stomach | NA | AspireAssist | Yes |
| Percutaneous | Embolization | Gastric fundus irrigation | NA | NA | No |
| Laparoscopy | Pacemaker | Stomach | Gastric stimulation | Tantalus | No |
| Laparoscopy | Pacemaker | Stomach | Gastric stimulation | Abiliti | No |
| Laparoscopy | Pacemaker | Vagus nerve | Vagus nerve blocking | vBloc | Yes |
| Laparoscopy | Implantable | Stomach | NA | Bariclip | No |
FDA: Food and Drug Administration; NA: not applicable.
The development of endoscopic techniques is a new trend in the treatment of obesity, and unlike bariatric surgery, they are less invasive, produce less morbidity in percentage and severity, and the majority of complications are reversible. Those therapies are classified as: space-occupying devices, restrictive or anatomic “remodeling” procedures, endoluminal bypass, and duodenal mucosal “resurfacing”.6,8
Space-occupying devicesIntragastric balloons, made of silicone and filled with liquid or air, partially occupy the space destined for food (Fig. 1A). They can be individual balloons or consist of 2 interconnected spheres. The balloon remains in the stomach from 6 months to one year, depending on the recommendations of each manufacturer.6 Orbera Intragastric Balloon System (previously known as BioEnterics, Orbera, Apollo Endosurgery, Austin, TX, USA) is a device made of silicone that is resistant to the acidic environment of the stomach. It is filled under direct endoscopic vision with physiologic solution at volumes ranging from 500 to 750 ml, with the addition of methylene blue for opportune leakage detection. Different studies have reported very good results. For example, there was 58% ± 19 excess weight loss (EWL) in 122 patients at the time of extraction (6 months), 39% ± 14 at one year, 25% ± 8 at 2 years, and 17% ± 8 at 5 years.8 In a meta-analysis that included 3,698 patients, the mean weight loss at 6 months was 14.7 kg (32.1% EWL) and the mean decrease in body mass index (BMI) was 5.7 kg/m², associated with significant improvement in blood pressure, fasting glucose, and lipid profile. HbA1c% improved in 87.2% of the patients with type 2 diabetes mellitus (DM2), as well. In relation to severe adverse events, two patients died (both of whom had undergone previous gastric surgery).8,9
The ReShape device (ReShape Medical, San Clemente, CA, USA) is another type of intragastric balloon that consists of 2 interconnected balloons. It is designed to prevent device migration if one of the balloons becomes deflated. The data on ReShape showed a total weight loss of 15.4 ± 8% at the time of balloon removal.10
The Spatz3 balloon is designed to be inflated or deflated, meaning that it can be calibrated according to patient progression. It has the big advantage of not needing to be removed for up to 12 months. Several studies have described the efficacy of different intragastric balloons, with a mean EWL of 25.4% at 6 months from device removal. Seventy-five percent of the adverse events in clinical trials on Orbera and ReShape were hospital readmissions due to nausea and vomiting, as well as abdominal pain, conditioning the early extraction of the device.8 Other adverse events reported with the use of ReShape were esophageal injury or perforation, bleeding gastric ulcer, and aspiration pneumonitis. Regarding Orbera, gastric outlet obstruction with diffuse gastritis, gastric perforation, esophageal tears, laryngeal spasms, and infected balloons were reported. Premature intragastric balloon removal occurred in 18% of the cases with the Orbera balloon and 15% with the ReShape balloon.8 The Spatz3 balloon is less studied because it has not been approved by the Food and Drug Administration (FDA), but it has the advantage of an adjustable balloon that is potentially better tolerated during placement, given that it can be started out with lower volumes of liquid, and also enables longer treatment duration.11 Finally, the ingestible air-filled balloon (Obalon Balloon System [OBS; Obalon, Inc., Carlsbad, CA, USA]) was recently analyzed prospectively in 1,343 patients (BMI > 25 kg/m²), finding a total weight loss of 10 ± 6.1% for patients with a BMI between 30 and 40 kg/m². Severe adverse events occurred in 0.15% of the cases.12 No adequately designed studies showed one balloon to be better than another. Support therapy by a multidisciplinary team is essential for achieving the best results. Even though intragastric balloons have been shown to be effective for conditioning weight loss, patients have been reported to present with weight regain once the balloon is removed.6,8,9
Another space-occupying device is the Transpyloric Shuttle (TPS, BARONova Inc, San Carlos, CA, USA). It consists of 2 spheres, the smallest of which is placed posterior to the pylorus in the duodenal bulb. Traction and intermittent occlusion of the pylorus is provided by the larger sphere, which is placed anterior to the pylorus (Fig. 1B). The general idea is to cause slow gastric emptying, with consequently greater satiety and less caloric intake. That device showed a total weight loss of 14% ± 5.8 at 6 months from placement in a group of 20 patients, producing adequate quality of life.13 In a recent double-blind randomized study on 270 patients, the device produced an EWL at 12 months of 30.9% in the patients with the device vs. 9.8% in those with no device. Important adverse events were reported in 2.5% of the patients, 10.3% of whom required premature device removal.14 Based on that study, the device was approved by the FDA.
Endoscopic gastric remodeling and duodenal mucosal resurfacingThe stomach is an organ that is easily accessed for endoscopic remodeling to condition weight loss. Success is dependent on overcoming certain challenges, the main ones of which are imitating or improving the functioning acquired through surgical treatments, assimilating the manner in which the tissue responds to foreign bodies, and understanding the functioning of the neuro-enteric pathway during the interaction between food ingestion, hunger, and satiety. The first remodeling efforts failed because of technical limitations, as well as deficient knowledge about the role of the stomach in weight loss. Current methods, especially endoscopic sleeve gastroplasty, utilize technology that is continuously being perfected.6,9 The results of liquid food tests have shown a decrease in caloric intake capacity after endoscopic gastric remodeling because the velocity of gastric emptying is reduced 2 months after the procedure but returns to normal in 6 months. On the other hand, changes in the secretion of hormones, such as ghrelin and PYY, have been reported but it is not clear if the hormonal changes are due to the anatomic modifications or the later weight loss.9
Those procedures reduce the volume of the stomach, employing sutures, staples, or tissue anchoring. USGI Medical (San Clemente, CA, USA) has provided the technology for endoscopic tissue anchoring in the performance of full thickness plicatures. That device is utilized to perform the so-called Primary Obesity Surgery Endoluminal (POSE) procedure, which creates parallel plicatures in the gastric fundus. The procedure was evaluated in the ESSENTIAL clinical trial,9 which had a randomized and controlled design and was conducted at 11 sites in the United States. A group of patients that underwent POSE were compared with a group that underwent a sham treatment. At follow-up month 12, the POSE group (221 patients, 91% of whom had follow-up) had a total weight loss of 4.95 ± 7.04%, which was 3.6-times higher than that of the control group. A great disadvantage was that 77.8% of the patients experienced procedure-related pain, nausea, and vomiting. However, those adverse events resolved in one week in the majority of patients and did not condition sequelae. Only 1.8% of the participants had device-related adverse events (gastric erosion, pain, and oral trauma). No deaths were reported.15
Another device, the OverStitch (Apollo Endosurgery, Austin, TX, USA), is a tool utilized endoscopically that enables full-thickness suturing (Fig. 1C). Its acceptance is one of the fastest growing worldwide and was recently approved by the FDA. Its uses include the creation of an endoscopic sleeve gastroplasty, as well as tissue apposition in the gastrointestinal tract (e.g., fistula management).8 The data derived from the 6 most recent higher quality studies included 1,600 patients. The percentage of EWL and total weight loss at 6 and 12 months after the procedure were 15.6% and 17.5%, and 59.7% and 64.1%, respectively. Only one study reported a 24-month follow-up and 18.6% total weight loss. Likewise, improvement in DM2, dyslipidemia, and systemic arterial hypertension was reported.16 The percentage of adverse events was 3.2%. When comparing the 6-month follow-up of endoscopic sleeve gastroplasty with laparoscopic sleeve gastrectomy, total weight loss was 23.6% vs. 17.1% (p < 0.01). In addition, the rates of adverse events and gastroesophageal reflux were greater in laparoscopic sleeve gastrectomy, compared with the endoscopic procedure: 16.9% vs. 5.2% (p < 0.05) and 14.1% vs. 1.9% (p < 0.05), respectively.16
The suturing system, EndoCinch (Davol, Warwick, Rhode Island, USA), now known as the RESTORe suturing system (Davol, Murray Hill, NJ, USA), creates a superficial suture that only includes the mucosa. The TRIM clinical trial reported safety and efficacy with endoscopic transoral gastric volume reduction for achieving weight loss. Of the 18 patients with a mean age of 40 years and a mean BMI of 38 kg/m2 initially enrolled in the trial, only 14 of them completed the 12-month follow-up. On average, the weight of the participants decreased by -11 ± 10 kg and the mean EWL was 27.7 ± 21.9%. No severe adverse events were reported. Complementarily, patients with high blood pressure had improved hypertension figures. Nevertheless, the control endoscopy at the year of follow-up showed partial or total plicature release in the majority of cases.17 Endoscopic transoral gastric volume reduction has been shown to be safe and well-tolerated and is efficacious for achieving modest weight loss and decreasing blood pressure. Despite its positive clinical effects, plicature is not lasting and the effects vary widely among patients.17
Endoscopic duodenal mucosal resurfacing (DMR) consists of hydrothermal ablation of the duodenal mucosa utilizing Revita DMR (Fractyl, Lexington, MA, USA). The procedure is carried out by advancing a balloon catheter into the duodenum (distal to the ampulla of Vater), followed by inflating the 2.0 cm-long balloon in the catheter with hot water for the ablation, all under direct vision. That therapy does not result in significant weight loss, but it improves glycemic control. The theory is that the re-epithelialization of the “unhealthy” duodenal mucosa after ablation will result in “healthy” mucosa.8,9,18 In the first study on humans, 39 diabetic patients were divided into 2 groups: long segment ablation (segment length ≥ 9 cm, n = 28) and short segment ablation (segment length < 6 cm, n = 11). There was an absolute reduction in HgbA1c of 1.2 ± 0.3% at 6 months in the entire cohort (p < 0.001). Those results are particularly interesting because they occurred in the context of minimum weight loss (3% of total body weight at 6 months).8,17 The results of that first study were recently updated, reporting that the effect obtained remained at one year after the intervention.19
Gastrointestinal diversions with devicesThe exclusion of the biliopancreatic segment of the small bowel appears to result in glucose metabolism improvement after gastric bypass, regardless of weight loss. In duodenal-jejunal bypass that only modifies the anatomy of the small bowel, reduced glycosylated hemoglobin has been observed, despite minimal weight loss, as well.
Endoscopic gastro-duodeno-jejunal bypass sleeve (GDJBS, ValenTx Endoluminal Bypass, ValenTx Inc) is a 120 cm “fluoropolymer liner or sleeve” anchored to the gastroesophageal junction, extending from the stomach to the jejunum. It was originally placed through hybrid endoscopy/laparoscopy, with a mean procedure duration of 75-90 min. Studied in 22 patients (BMI 42 kg/m2, range 35.4–50.8 kg/m2), a mean EWL of 39.7% (range 27-64%) at 3 months was found. Five patients required early removal of the device 3 weeks after implantation due to dysphagia, and they were not included in the weight loss analysis. A second pilot test on 12 patients (BMI 42 kg/m2) resulted in an EWL of 35.9% at 12 months.20 That device, which does not have FDA approval, is currently still being studied, and a new one is being developed.21
The duodenal-jejunal diversion device, “EndoBarrier” (GI Dynamics, Lexington, MA, USA), is a nickel-titanium implant attached to a 60 cm polymer liner. It prevents food from coming into contact with the small bowel mucosa but allows pancreaticobiliary secretions to move along the device into the jejunum (Fig. 1E). It is endoscopically placed, anchored at the level of the duodenal bulb through fluoroscopic guidance, with the patient under general anesthesia.9 Its effectiveness and safety have been studied in multiple trials outside the United States, reporting overall EWL of 35.4% at 12 months of follow-up. However, randomized controlled trials showed a difference of 9.5% in EWL, compared with controls.8,9 Likewise, A1C hemoglobin level values are known to decrease by a mean 1.5 units. Device migration in 4.9% of the cases, gastrointestinal bleeding in 3.9%, obstruction in 3.4%, liver abscess in 0.13%, cholangitis in 0.13%, acute cholecystitis in 0.13%, and esophageal perforation in 0.13% were among the adverse events observed. Premature removal of the device was necessary in 24% of the cases.9 A multicenter study in the United States that recruited 325 cases was stopped due to liver abscess in 3.5% of the patients, who were then treated with parenteral antibiotics combined with percutaneous drainage. Early device removal was required in 11.7% of the cases.8,9 Recent studies with greater implant duration confirmed its effectiveness at 12 and 24 months in relation to weight loss and glycemic control. However, as expected, glucose levels worsened upon device removal.22
The use of magnetic force for creating a junction and intestinal anastomosis has been employed since the 1980s. The most promising platforms include the Incisionless Anastomotic System (IAS), the Incisionless Magnetic Anastomotic System (IMAS) (GI Windows, W. Bridgewater, MA, USA), and the Magnamosis (Magnamosis Inc., San Francisco, CA, USA).23 The most widely studied is the IMAS, which uses magnets to create a two-way enteral bypass that enables the flow of nutrients through the native anatomy and a jejunal-ileal anastomosis (Fig. 1D). It is created by the simultaneous release of self-assembling magnets in the proximal jejunum and the ileum, through colonoscopy and fluoroscopic guidance. The appropriate positioning of the magnets is examined laparoscopically, and the length of the intestinal segments is measured. Once the magnets are implanted, the tissue around the magnets necrotizes, and the subsequent remodeling of the surrounding tissue makes the anastomosis possible (simulating an entero-enteral fistula).24 In the first pilot study on humans, 14 patients (BMI of 30 to 50 kg/m²) were enrolled but the device was successfully placed in only 10. Sixty percent of the patients completed the 12-month follow-up (mean BMI of 41 kg/m²). Mean procedure duration was 115 min. At the end of follow-up, the mean total weight loss was 14.6% and EWL was 40.2%. Initial HbA1c in the diabetic participants was 7.8 ± 2.4% and it decreased to 5.9 ± 0.5%. All the patients presented with diarrhea after the procedure but had short-term remission. There was recurrence of diarrhea (40%) that appeared to be related to the composition of the diet and was resolved by reducing simple carbohydrate consumption and the prescription of loperamide.25
Percutaneous proceduresThe AspireAssist system (approved by the FDA) is a novel endoscopic therapy that entails the placing of a percutaneous gastrostomy tube, a port for skin, and an accessory device. The system enables the “washout” of the stomach with saline solution, followed by partial aspiration of ingested food. Treatment should be accompanied by lifestyle changes directed at reducing caloric intake and increasing physical activity. At one year of follow-up in a single center pilot study, AspireAssist produced weight loss that was 3-times higher than lifestyle changes alone.6 The results from a multicenter, randomized, controlled study showed that the participants in the AspireAssist group, at 52 weeks, lost 31.5% ± 26.7 of excess body weight (12.1% ± 9.6 of total body weight), whereas those in the Lifestyle Counseling group lost only 9.8% ± 15.5 of excess body weight (3.5% ± 6.0 of total body weight).9 The conclusion was that the AspireAssist system resulted in considerable weight loss and was more efficacious than intense lifestyle modification. The system is designed for long-term treatment and requires periodic monitoring. It has the advantage that it can be removed if treatment were to be suspended, causing no anatomic changes that would preclude the performance of future bariatric surgery.8
Even though it does not directly involve the manipulation of a digestive organ, bariatric arterial embolization is a new percutaneous transcatheter procedure performed mainly by the interventional radiologist. It is designed to induce weight loss through the arterial embolization of the blood supply to the gastric fundus. The fundus of the stomach is one of the principal anatomic sites for the production of the orexigenic hormone, ghrelin. That hormone is involved in the mechanisms that cause weight loss after bariatric surgery. Thus, the premise is that bariatric embolization creates ischemia in the ghrelin-producing cells in the gastric fundus. Two representative clinical trials were approved by the FDA: GET LEAN and BEAT Obesity.6 In the GET LEAN study, 4 patients (mean age 41 years, mean BMI 42 kg/m², one diabetic) underwent embolization through the left radial or femoral approach, with 300-500 μm particles (BeadBlock, BTG International, West Conshohocken, PA, USA). No severe events were reported. A minor complication was superficial gastric ulcers that resolved in 30 days. The mean body weight change at 6 months was 9.2 kg (range: -2.7 to -9.5 kg), corresponding to a 17% EWL.26,27 The BEAT Obesity study was approved for 20 patients with obesity (16 women, mean age 44 years, mean BMI 45 kg/m²), reporting EWL of 8.2% at one month, 11.5% at 3 months, 12.8% at 6 months, and 11.5% at 12 months. Asymptomatic superficial gastric ulcers were diagnosed at 2 weeks after embolization in 8 patients and had disappeared at 3 months. All the patients complained of initial symptoms of nausea, vomiting, and epigastric pain, conditioning hospitalization only for observation and symptom treatment. One participant presented with subclinical pancreatitis that resolved spontaneously within a few days.28
“Less” invasive surgeriesIntermittent vagus nerve blocking (vagus nerve blocking [vBloc] device) was developed as a less invasive alternative to standard bariatric surgery. Its conception and development were based on reports about the influence of vagotomy on weight loss. Its mechanism of action is thought to be involved in appetite reduction.6 The laparoscopically implanted Maestro Rechargeable System device sends intermittent, high frequency, low-energy electrical pulses to the intra-abdominal vagal trunks for a predetermined number of hours per day. Previous studies showed significant weight loss and improvement of associated comorbidities, such as DM2, with a low rate of serious complications. The ReCharge Trial is a clinical trial that included patients with class 2 and 3 obesity for a 5-year weight loss, adverse event, and quality of life evaluation utilizing vBloc. At present, information on 123 patients from the follow-up at 2 years is available. The participants had a mean EWL of 21% (8% total body weight loss) and diabetic patients had a -0.3% reduction in HbA1c. There were 4.3% total adverse events that included heartburn, dyspepsia, and pain at the implantation site, none of which were considered severe.29
Autonomous selective activation is the aim (unlike vBloc) of implantable pacemakers. The “gastric pacemaker” stimulates the vagus nerve through a cable implanted in the gastric wall (the lesser curvature) by laparoscopy. The devices that are currently under study are the Diamond (TANTALUS) System (MetaCure Inc.) and the Abiliti System (IntraPace, Inc.).30
The “gastric clip” (BariClip) is a nonadjustable device that is placed vertically parallel to the lesser curvature. The clip restricts oral intake without changing the gastric anatomy and does not require stapling, cause malabsorption, or require any maintenance or monitoring, and it is reversible (Fig. 1F). There is only one study on humans, with a 39-month follow-up. It monitored weight loss and adverse events in 117 patients. Mean weight was 112 kg (range: 85 to 138 kg) and mean BMI was 44 kg/m2 (range: 35 to 56 kg/m2). Eighty-nine percent of the patients were women and their mean age was 31 years (range: 19 to 38). EWL of 31.7%, 45.1%, 51.4%, 58.9%, and 66.7% was reported at 3, 6, 12, 18, and 24 months, respectively. The device was placed by laparoscopy, with a mean surgery duration of 69 min and a mean hospital stay of 1.3 days. Nine patients developed postoperative nausea that resolved in a few days and 7 patients presented with gastroesophageal reflux that was controlled with a proton pump inhibitor within 2 to 3 weeks. At the beginning of the study, 9 patients presented with device slippage, causing gastric outlet obstruction, for which changes in the surgical technique were carried out. One patient was found to have asymptomatic erosion located in the antrum during routine endoscopy at month 24 of follow-up, requiring conversion to laparoscopy.31
EndoVac (aspiration therapy for complication management)The development of leakage or fistula is associated with greater morbidity and mortality, and therefore is of much concern to the bariatric surgeon. Even though management of those complications is multimodal, endoscopy plays a very important role. Well-known alternatives are endoprostheses, glues, clips, dilations, septotomies, and the placement of internal drainage catheters. Centers specializing in the treatment of obesity have recently acquired experience in the use of vacuum aspiration systems similar to the negative pressure systems used in abdominal surgeries to accelerate cicatrization and wound closure.32–34 An endoscopic vacuum-assisted closure sponge, or EndoVac therapy, has been used for managing leaks associated with intra-abdominal septic foci. There are 2 treatment variants: the intracavitary, in which the aspiration system sponge is placed through the esophageal defect into an extraluminal cavity; and the intraluminal, in which the aspiration sponge is placed in the esophageal lumen.32 In a study on 5 patients, the sponge was attached to a drainage tube for continuous suction and endoscopically placed into the wound cavity, changing the sponge at regular intervals. The 5 patients had fistula closure after a median of 9 sponge changes (median 28 days).32,34 Loske et al. reported a case series of 14 patients with esophageal perforations, treated with EndoVac. Esophageal defect closure was achieved in 13 patients, with a mean therapy duration of 12 days and 4 changes.34 That therapy could be very useful in the presently available armamentarium, but further studies are needed.
ConclusionsEmerging treatments for the management of obesity have arisen from the need to gain greater acceptance among patients and other specialists. Those therapies are less invasive and probably present with fewer risks. Drastic risk reduction could result in greater acceptance of their use, even if they do not provide benefits as significant and lasting as those of bariatric surgery. Clinical use of a new technology should be practiced within a multidisciplinary management program, i.e., with nutritional, psychologic, physical activity, and medical support. It is of the utmost important to recognize that emerging therapies are not meant to “compete” with the universally accepted surgeries, but rather to increase the possibility of treatment and achieve greater reach. Ideas, devices, therapies, surgeries, and more ways to treat overweight and obesity are continuously being developed but patient safety must be the most important cornerstone, regardless of weight loss. Little is known about the long-term effects of many of the devices described herein, nor the characteristics of the endoscopists/surgeons needed to perform the new techniques, which can potentially put the patient at risk. Finally, the ethical aspect must always be present, given that promoting devices that evoke a false sense of safety in the patients or whose results are obtained from specific, small, controlled populations can convert the proposed solution into an even bigger problem. Just because a less invasive device aids in weight loss, even if its use is approved, does not mean that it will substitute basic obesity management, which is to seek a change of habits in each patient, on an individual basis.
Ethical considerationsGiven that the present article was an up-to-date review of the literature on emerging technologies for the treatment of obesity, patient informed consent was not required. No data identifying any patient were published.
Financial disclosureNo financial support was received in relation to the present review.
Conflict of interestThe authors declare that there was no conflict of interest.
Please cite this article as: Zerrweck C, Espinosa O. Nuevas tecnologías y avances en terapias para la pérdida de peso. Revista de Gastroenterología de México. 2020;85:452–460.