Celiac disease (CD) is one of the most common autoimmune enteropathies of the small bowel. It affects persons with a genetic predisposition and is derived from exposure to gluten, a protein present in wheat, barley, and rye. CD causes changes in the duodenal mucosa, characterized by intestinal villa atrophy, crypt hyperplasia, and lymphocytic infiltration, often producing malabsorption.1
A 41-year-old woman had a past medical history of hypothyroidism and infertility (G2-A2), history of constipation and vomiting of 10-year progression, defecation pattern of <3 bowel movements per week with Bristol 1 and 2 stool consistency, and difficulty in passing the stools. She stated having no anal blockage sensation, incomplete evacuation, or digital manipulation. Stools had no mucus or blood, and bowel movements improved with fiber intake. She also stated having postprandial vomiting that contained bile and food, but no borborygmi, abdominal pain, or bloating. She said she experienced early satiety and postprandial fullness once or twice a week, triggered by fat and carbohydrate intake. She had no weight loss. Neurologic and abdominal physical examinations were unremarkable. Perianal inspection was postponed due to patient modesty.
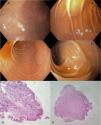
Initial endoscopy reported Los Angeles grade A esophagitis, a 10 mm Forest III ulcer on the anterior surface of the greater curvature in the gastric antrum and flattening of the villi in the second and third parts of the duodenum. Gastric ulcer biopsies showed reparative epithelial changes, with no atypia, and were negative for Helicobacter pylori. There was mild villous atrophy, Giardia test was negative, and 50% of the CD3/CD8 intraepithelial lymphocytes (IELs) in the bulb and second and third parts of the duodenum were classified as MARSH 3A. Tissue transglutaminase IgA antibodies (tTg IgA) and serum IgA were both negative. Standard dose esomeprazole for 12 weeks was the clinical management (Fig. 1).
Esophagogastroduodenoscopy images. A) Duodenal bulb (D1), with endoscopic changes suggestive of villous atrophy during the immersion maneuver. B and C) Villous flattening in D2. D) Moderate flattening of the villi in D3. E) Histopathology reporting mild villous atrophy and the presence of CD3/CD8 intraepithelial lymphocytes of 50% (Marsh 3A).
Source: Patient’s clinical history.
Given the findings in the duodenum and the suspicion of CD versus seronegative enteropathy, testing for endomysial antibodies (EMA), deamidated gliadin peptide-IgA antibodies (DGP-IgA), and deamidated gliadin peptide-IgG antibodies (DGP-IgG) was ordered. CD was diagnosed by means of the positive DGP-IgA test finding and gluten restriction was started. After three months of treatment, the patient said her vomiting, constipation, and dyspeptic symptoms had improved (Table 1).
Panel of results for celiac disease.
| Determinations | Result | Normality range |
|---|---|---|
| Leukocytes | 8.82 × 103 /μl | 4.4−10 |
| Neutrophils | 5.45 × 103 /μl | 2−8 |
| Lymphocytes | 2.77 × 103 /μl | 1−4.4 |
| Hemoglobin | 14.2 g/dl | 13.5−17.3 |
| MCV | 91.9 fl | 76−96 |
| MCH | 30.2 pg | 28−33 |
| Glucose | 86 mg/dl | 50−100 mg/dl |
| TSH | 1.71 IU/mL | 0.27−4.2 |
| Free T3 | 5.91 pg/mL | – |
| Free T4 | 1.09 ng/dl | 0.8−2 |
| Sodium | 138 mEq/l | 135−145 mEq/l |
| Potassium | 3.2 mEq/l | 3.5−4.5 mEq/l |
| Calcium | 8.9 mg/dl | 8.2−10.5 mg/dl |
| Magnesium | 1.8 mg/dl | 1.7−2.2 mg/dl |
| Total bilirubin | 0.94 mg/dl | 0.1−1.2 |
| Direct bilirubin | 0.78 mg/dl | 0.0−0.14 |
| Indirect bilirubin | 0.16 mg/dl | |
| AST | 17.9 IU/l | 0−40 |
| ALT | 27.7 IU/l | 0−45 |
| Alkaline phosphatase | 146.3 IU/l | 0−400 |
| GGT | 49.4 IU/l | 11−50 |
| ESR | 10 mm/h | 10−20 |
| PCR | 1.2 mg/l | 0.5−3.58 |
| Immunoglobulin A (IgA) | 2.87 g/l | 0.7−4.0 g/l |
| Anti-tissue transglutaminase IgA antibodies (tTg IgA test) | 1.10 U/mL | 0−25 U/mL |
| Anti-tissue transglutaminase IgG antibodies (anti-tTg IgG) | 1.80 U/mL | Negative: < 10 U/mL |
| Anti-endomysial antibody (anti-EMA) | Negative | |
| Deamidated gliadin peptide-IgA (DGP-IgA) | 25.80 RU/mL | Positive: ≥ 25 RU/mL |
| Deamidated gliadin peptide-IgG (DGP-IgG) | < 2 RU/mL | Positive: ≥ 25 RU/mL |
| Fecal antigen for H. pylori | Negative | |
| Fecal calprotectin | 15 μg/g | < 50 μg/g |
ALT: alanine aminotransferase; AST; aspartate aminotransferase; ESR: erythrocyte sedimentation rate; GGT: gamma-glutamyl transferase; MCH: mean corpuscular hemoglobin; MCV: mean corpuscular volume PCR: polymerase chain reaction; TSH: thyroid stimulating hormone.
CD affects 1% of adults and the dietary restriction of gluten is the only efficacious treatment. Risk is higher in persons with autoimmune diseases, such as type 1 diabetes or autoimmune thryroiditis.2 Prevalence of 0.5−0.7% is reported in Mexico, whereas it has reached 1.3% in Latin America, with greater frequency in Argentina and Brazil. According to its presentation, CD is classified into symptomatic, asymptomatic, and potential disease.
Symptomatic CD is characterized by the presence of classic, extraintestinal, or non-classic gastrointestinal symptoms. The classic manifestations are diarrhea, steatorrhea, and weight loss. Non-classic CD is characterized by the absence of malabsorption symptoms with the predominance of other manifestations, such as abdominal pain and bloating, borborygmi, constipation, vomiting, and anemia, among others. A high level of suspicion is essential, given that less than 50% of adults present with classic gastrointestinal symptoms. On the other hand, the asymptomatic variant is characterized by the absence of symptoms, with positive serology and altered duodenal mucosa, whereas potential CD presents in persons at risk of developing the disease, who have normal histology and positive serology.3
The testing for tTg IgA antibodies plus serum IgA is indispensable for making the diagnosis, with 78% sensitivity and 98% specificity. Due to the variable diagnostic accuracy, anti-IgA-endomysial antibody (EMA) and anti-DGP-IgA antibody tests are useful for diagnosing patients highly suspected of presenting with CD who have negative tTg IgA + serum IgA tests. The anti-IgA-EMA test has 94% sensitivity and 100% specificity, and the anti-DGP-IgA antibody test has 74% sensitivity and 95% specificity. In cases of selective IgA deficiency, determining IgG antibodies supports the diagnosis.4
There is scant information on DGP-IgA/IgG antibodies in the adult population. Vootukuru et al. evaluated the diagnostic utility of positive DGP-IgG with negative tTg-IgA in a cohort of 26 pediatric patients. The positive finding on its own had a low diagnostic yield (OR 3.9%, 95% CI 0.7–18.9 %) and histologic support was recommended for making the diagnosis.5 On the other hand, Saadah et al. compared tTg IgA testing with DGP IgA and IgG in 26 pediatric patients with CD, and found a significant correlation between the two antibodies (r = 0.69, p < 0.001), considering tTg IgA testing a useful alternative for diagnosis and follow-up.6 Zingone et al., in an attempt to find diagnostic strategies without the need for biopsies, evaluated the performance of tTg-IgA + DGP-IgG antibodies in an adult population. The incorporation of DGP-IgG together with tTg-IgA testing in a one-step focus may be a valid confirmation strategy for the definitive diagnosis of CD without biopsies.7
Clinical manifestations of unexplained vomiting and constipation in a patient with a history of hypothyroidism and infertility, in whom Marsh 3A villous atrophy and negative tTg IgA serology without IgA deficiency were identified, led us to suspect non-classical symptomatic CD. IgA-EMA and DGP-IgA antibody testing provides an early diagnosis.8 Araujo et al. reported that late diagnosis due to initial negative serology led to fatal outcomes, such as lymphoma or intestinal adenocarcinoma, and the widening of the serologic panel enables early diagnosis and treatment.9
Ethical considerationsInformed consent was obtained from the patient for the treatment received and the publication of the present clinical case. This works meets the current bioethics regulations, but because it is a case report and not a study with research subjects, ethics committee approval was not required. Likewise, the authors declare that the article contains no information that could identify the patient, guaranteeing confidentiality and patient privacy.
CRediT authorship contribution statementPDME and MACC: Research and writing of the original draft and DACR: Performed the endoscopic procedure, carried out the photos, validation, editing, and supervision of the manuscript.
Financial disclosureNo specific grants were received from public sector agencies, the business sector, or non-profit organizations in relation to this article.
The authors declare that there is no conflict of interest.