Acute cholecystitis is one of the most frequent diseases faced by the general surgeon. In recent decades, different prognostic factors have been observed, and effective treatments described, to improve the results in patients with said pathology (lower morbidity and mortality, shorter hospital stay, and minimum conversion of laparoscopic to open procedures). In general, laparoscopic cholecystectomy is the standard treatment for acute cholecystitis, but it is not exempt from complications, especially in patients with numerous comorbidities or those that are critically ill. Percutaneous cholecystostomy emerged as a less invasive alternative for the treatment of acute cholecystitis in patients with organ failure or a prohibitive surgical risk. Even though it is an effective procedure, its usefulness and precise indications are subjects of debate. In addition, there is little evidence on cholecystostomy catheter management. We carried out a review of the literature covering the main aspects physicians involved in the management of acute cholecystitis should be familiar with.
La colecistitis aguda es una de las enfermedades más frecuentes ante la cual debe enfrentarse el cirujano general. En las últimas décadas se han observado distintos factores pronósticos y descrito modalidades de tratamiento efectivas con la finalidad de mejorar los resultados en pacientes con este padecimiento (baja morbilidad y mortalidad, menor estancia hospitalaria y mínima conversión de procedimientos laparoscópicos a abiertos). En general, la colecistectomía laparoscópica es el tratamiento estándar de la colecistitis aguda, sin embargo, dicho procedimiento quirúrgico no está exento de complicaciones, principalmente en pacientes con múltiples comorbilidades o en estado crítico. La colecistostomía percutánea surgió como una alternativa menos invasiva para el tratamiento de colecistitis aguda en pacientes con falla orgánica o riesgo quirúrgico prohibitivo. Aunque es un procedimiento efectivo, existe controversia sobre su utilidad e indicaciones precisas. Aunado a lo anterior, la evidencia sobre el manejo de la sonda de colecistostomía es escasa. Realizamos una revisión abordando las principales cuestiones que los médicos involucrados con el manejo de esta patología deben conocer.
Gallbladder pathology is one of the most frequent diseases faced by the general surgeon. Currently, laparoscopic cholecystectomy (LC) is considered the gold standard as treatment for acute cholecystitis (AC). An effort has been made to reduce the morbidity and mortality associated with the procedure in high-risk surgical patients through less invasive interventions, such as percutaneous cholecystostomy (PC).
AC is an inflammatory disease of the gallbladder whose pathophysiology consists of the obstruction of drainage pathways of the gallbladder or dysmotility of its walls, which conditions an increase in intraluminal pressure, wall edema, altered vascularity with ischemia or necrosis, bacterial proliferation, or gallbladder perforation. The aim of PC in AC is to enable bile duct drainage to resolve the acute symptoms and prevent the development of local and systemic complications. PC is generally reserved for patients with AC that are high-risk surgical patients or that present with organ failure. PC offers useful advantages in that context: general anesthesia is not required, it can be performed at the patient’s bedside, it is a rapid procedure, its success rate is above 95%, it has a low complication rate, and it can be used as bridging therapy before elective surgery.
Gallbladder drainage is a treatment option that is included in the main international guidelines for AC management, but there is still some controversy as to its results in critically ill patients, its precise indications, and its superiority when compared with definitive surgical treatment.
There is little evidence on the surveillance and care of patients that undergo PC. The majority of studies focus on the selection of patients that will benefit from the procedure, but few describe subsequent patient management. We analyze herein the evidence on the usefulness of PC in AC and suggest an algorithm for PC catheter management.
Criteria for classifying acute cholecystitis severityThe 2018 Tokyo Guidelines (TG18) on AC classify disease severity as mild (grade I), moderate (grade II), and severe (grade III).1 Said classification takes into account patient comorbidities, symptom duration, physical findings upon hospital admission, signs of systemic inflammatory response, the presence of marked local inflammation in imaging studies, and the development of organ dysfunction. The TG classification is useful because disease presentation severity correlates with mortality, morbidity, the length of hospital stay, and the rate of conversion from laparoscopic surgery to open surgery (Tables 1 and 2).
Classification of acute cholecystitis severity according to the 2018 Tokyo guidelines.1
| Grade I (mild) | Grade II (moderate) | Grade III (severe) |
|---|---|---|
| Any of the following conditions:
| Any of the following organ dysfunctions:
|
Early LC (within 72h) should ideally be performed in cases of grade I and grade II AC, if the Charlson comorbidity index (CCI) and functional capacity according to the classification system of the American Society of Anesthesiologists (ASA) suggest that the patient can tolerate surgery. To the contrary, initial medical treatment should be established (antibiotics and general support) and surgical treatment considered once conditions improve. In grade II AC, the recommendation of performing percutaneous drainage is added when the patient cannot tolerate surgical treatment.1
The 2013 Tokyo Guidelines (TG13) classify AC as grade III when there is evidence of organ dysfunction, defining it when there is cardiovascular, neurologic, respiratory, renal, hepatic, or hematologic insufficiency. According to the TG13, emergency LC is contraindicated if there is dysfunction in those organ systems. However, in 2017, Yokoe et al.2 provided information on the treatment of AC in a Japanese and Taiwanese population (n=5,329), in which LC was successfully performed on a majority of patients with grade III AC. The same cohort was later re-analyzed, placing an emphasis on those patients with organ dysfunction that could safely undergo surgical treatment. The patients with a CCI>6 points presented with a higher mortality rate. The main usefulness of that study was its multivariate logistic regression analysis, which showed that jaundice, neurologic dysfunction, and respiratory dysfunction were independent predictive factors for mortality at 30 days in patients with grade III AC. Nevertheless, there was no evidence of increased mortality in patients with grade III AC that presented with other types of organ failure and that underwent LC.3 Thus, the TG18 define neurologic dysfunction, respiratory dysfunction, or the presence of jaundice (total bilirubin levels ≥ 2mg/dl) as predictive factors of mortality in grade III AC. On the other hand, renal dysfunction and cardiovascular dysfunction are considered favorable types of organ failure, given that they can often be reversed after the establishment of initial medical treatment, and consequently, not contraindicate surgical treatment.1
Indications for percutaneous cholecystostomy placementIn general terms, the most frequent indication for placing a PC is severe AC.4–9 Less common indications are those whose objective is to divert the biliary tract by means of transcholecystic access, which is considered relatively second-line in the majority of cases due to the preference for carrying out endoscopic or transhepatic diversions. Said scenarios are summarized in Table 3.10–13
Indications for percutaneous cholecystostomy placement.
| Severe acute cholecystitis according to the 2018 Tokyo Guidelines |
| Acute cholecystitis and ASA classification >3 or Charlson comorbidity index >6 |
| Second-line access to the biliary tract:Malignant biliary tract lesion bridgingBile duct stricture dilationBile duct fistula output diversionBiliary tract decompression in cholangitisOthers |
Unlike that established in the TG13,14 the TG18 underline the fact that emergency gallbladder drainage can be carried out in grade II AC when laparoscopy is not available and there is an inadequate response to initial medical treatment, mainly in patients that present with “poor” general conditions (CCI>6 points or ASA classification >3). That suggestion is supported by a 2017 multicenter retrospective study15 that included 1,764 patients over a 2-year period but only paired 330 patients into two groups for comparison. Group 1 consisted of 330 patients that underwent transhepatic percutaneous drainage and transhepatic percutaneous aspiration and group 2 consisted of 330 patients that underwent endoscopic transpapillary gallbladder drainage. The results were analyzed on post-procedure days 3 and 7. At day 3, the therapeutic success rate was 62.5% (cholecystostomy) and 69.8% (endoscopic treatment). At day 7, the therapeutic success rate was 87.6% (cholecystostomy) and 89.2% (endoscopic treatment). No significant differences were found in the early or late success rates. Complication frequency was 4.8% in the cholecystostomy group and 8.2% in the endoscopic drainage group, and there were no statistical differences in the statistical analysis. The complications reported in the cholecystostomy group were 8 patients with catheter migration, one patient with bleeding, and 3 patients with biliary leakage. That study showed percutaneous drainage to be effective, having the same efficacy as endoscopic management. Even though the patients were not stratified according to AC severity, the authors stated in the discussion section that bias most likely occurred in the study due to the inclusion of a high frequency of patients with severe (grade III) and moderate (grade II) AC.
Treatment recommendations in cases of grade III AC were some of the biggest changes in the TG18, with respect to the TG13. Previously, emergency gallbladder drainage (endoscopic or transhepatic) was the only recommendation,13 indicating a nonexplicit tendency toward avoiding emergency surgical treatment, to not increase morbidity and mortality. Even though the TG18 reiterate that recommendation, the option of performing early LC, after establishing antibiotic treatment and normalizing organ function, was added.16 Therefore, upon diagnosing AC, especially grade III, calculating the CCI and ASA functional capacity and identifying whether the patient has favorable predictive factors of organ failure (renal or cardiovascular injury) are essential. Those patients could present with a rapid recovery after beginning medical treatment, becoming candidates for definitive treatment with LC.
The World Society for Emergency Surgery, recommends carrying out percutaneous drainage of the gallbladder in “high-risk” patients, but it does not provide a precise definition of said patients, which can result in different decisions being made by different professionals involved in the treatment of acute cholecystitis.17
Despite the generalized acceptance of PC placement in patients with severe AC,18,19 and the reported evidence on a relatively low mortality rate (17%),20 the best treatment option is still a subject of debate, given that some groups report an acceptable outcome with emergency LC in critically ill patients.21–25
Risk of conversion from laparoscopic cholecystectomy to open surgeryDifferent factors have also been described for predicting the risk of conversion from LC to open cholecystectomy.26,27 Some authors propose that patients at high risk for conversion should be considered candidates for initial PC placement, and once the inflammatory process is resolved, undergo late LC (Table 4).28,29
Risk factors for conversion to open cholecystectomy.
| Lipman et al. (2007)26 | Male sex (OR 4.06, 95% CI: 2.42–6.82)Leukocytosis 3.01 (OR 3.01, 95% CI: 1.77–5.13),Hypoalbuminemia (OR 2.90, 95% CI: 1.70–4.96),Pericholecystic fluid (OR 2.36, 95% CI: 1.25–4.47)Diabetes mellitus (OR 1.87, 95% CI: 1.03–3.42)Elevated total bilirubin (OR 1.85, 95% CI: 1.01–3.39) |
| Kim et al. (2014)27 | Advanced age (OR 1.05, 95% CI: 1.008–1.10),Male sex (OR 5.0, 95% CI: 1.31–19.05),Pericholecystic fluid (OR17.22, 95% CI: 4.36–67.94). |
| Masri et al. (2018)79 | Female sex (OR 0.09, 95% CI: 0.03–0.25)Advanced age (OR 2.15, 95% CI: 1.62–2.85)History of abdominal surgery (OR 4.66, 95% CI: 1.78–12.17)History of pulmonary disease, e.g., COPD (OR 6.03, 95% CI: 1.21–29.97)Serum hemoglobin < 9mg/dl (OR 36.57, 95% CI: 3.16–423.72) |
| Morales Maza et al. (2019)a | Male sex (OR 1.64, 95% CI: 0.48–5.58)Gallbladder wall diameter (OR 1.35, 95% CI: 1.03–1.76)Advanced age (OR 1.02, 95% CI: 1.00–1.04)Pericholecystic fluid (OR 2.84, 95% CI: 1.04–7.69) |
The usefulness of initial conservative treatment, first placing a PC and then performing interval LC in patients with AC symptoms of more than 72-h progression, has also been studied. Karakayali et al.30 analyzed a group of patients with AC of more than 72-h duration and symptom progression of 48h after initial medical management. The patients (n=92) were non-randomly assigned to either undergo PC and then interval LC or undergo emergency LC. The group that underwent interval LC had a lower conversion rate (40% vs. 19%; p=0.029), less intraoperative blood loss (33% vs. 9%; p=0.006), shorter hospital stay (5.3 days vs. 3.0; p=0.001), and lower frequency of complications in general (35% vs. 9%; p=0.003).
El-Gendi et al.31 randomized a group of patients (n=150) with AC for more than 72h of progression to undergo emergency LC vs. PC and interval LC 6 weeks later. Their analysis showed that the PC and interval cholecystectomy group had a lower conversion frequency (24% vs. 2.27%; p<0.001), less intraoperative blood loss (mean 41.73ml vs. 26.33ml; p=0.008), shorter surgery duration (87.8min vs. 38.09min; p<0.001), a lower subtotal cholecystectomy rate (17.3% vs. 0.0%; p<0.001), shorter hospital stay (51.71h vs. 10.76h; p<0.001), and fewer postoperative complications (26.7% vs. 2.7% p<0.001), respectively.
Patients with acalculous AC are another group worth mentioning. They are frequently critically ill patients, with multi-organ failure, and are candidates for PC as initial treatment of choice for resolving the acute symptoms. However, patients with signs of gallbladder perforation, gangrene, or symptom progression after PC placement should undergo emergency cholecystectomy.32
Contraindications for percutaneous cholecystostomy placementThere are no absolute contraindications for PC. The relative contraindications are few, and they include coagulopathy that conditions the risk for severe bleeding (platelets < 50,000×109/l or INR>1.5), allergy to iodized contrast medium (employed to confirm adequate catheter placement through fluoroscopy (albeit it could be done through ultrasound),33 and overlapping of intestinal segments at the puncture site (extremely rare because the liver impedes segment overlap).34
Paracentesis is suggested in patients with ascites, before PC placement. It should be emphasized that the frequency of PC complications in patients with ascites is low and not significantly different, when compared with patients that do not have ascites. A gallbladder tightly packed with gallstones can also impede the secure placement of the PC catheter.35
Is there a decrease in mortality with percutaneous cholecystostomy placement in “high-risk” patients?Detailing the existing evidence on the decrease in mortality in high-risk patients with AC that undergo PC, a 2014 study analyzed the database of the California Health System. Its primary aim was to determine mortality and its temporality in 3 groups of patients with AC: those that had PC placement, those that underwent LC, and those that underwent neither of the two strategies due to the severity of their condition.36 A total of 43,341 patients were studied, resulting in a 61.7% mortality rate in the patients treated with PC, 42% in the patients that had no invasive treatment, and 23% in the patients that underwent LC. There was no difference in survival in patients with severe sepsis and septic shock during the comparison of the PC group and the non-intervention group (p=0.256), and there was greater survival in the patients that underwent LC (p<0.001). Even though mortality was greater in the patients that underwent PC, they had a higher CCI (5 versus 3.8), compared with the patients that underwent LC. Thus, those study limitations must be considered when interpreting the results and drawing conclusions. In another study published in 2014, reduced morbidity and mortality in patients managed with PC was not able to be demonstrated,37 but it should be mentioned that the protocol of PC management was heterogeneous and varied between centers, which could have partially contributed to the variability of results.
The CHOCOLATE protocol was published in 2012 with the intention of providing a more conclusive answer to the PC debate. It was a multicenter, randomized, controlled study on intervention superiority. It attempted to compare the result between performing LC versus PC placement in patients with AC that had an APACHE score >7 points. The number of patients to recruit was 284 over a 2-year period, at 30 high-volume centers. The primary aim was to analyze the complications within the first 3 months after the intervention and the mortality rate at one year (Netherlands Trial Register [NTR]; NTR2666).38 The results of the protocol were not published, given that it was suspended in 2016, after the preliminary analysis showed greater morbidity and mortality in the patients treated with PC.
Those studies place the real usefulness of PC in patients with severe AC in doubt. However, there is much controversy surrounding it, given that randomized studies are needed so that selection bias can be eliminated. The majority of currently available evidence is retrospective and includes a large number of patients that are “so severe” they have a high mortality rate, regardless of the intervention offered.
It is important to underline that patients that are not high-risk, have no comorbidities, and are candidates for LC, do not benefit at all from PC placement. Some studies suggest that outcome can even worsen with the use of said strategy.39,40 In our experience, we do not consider it advisable to perform laparoscopic cholecystostomy as a rescue method after a failed LC, because it is more recommendable to perform a subtotal cholecystectomy in that scenario, if there were technical difficulties during the procedure. In the studies that have compared PC versus open cholecystectomy in critically ill patients with acalculous cholecystitis, less morbidity, fewer days of hospital stay, and lower associated costs have been observed in patients that have undergone PC placement.41
Description of the technique for ultrasound-guided placement of percutaneous cholecystostomyOnce the decision has been made to perform PC, the approach to be used for placing the drain, whether transhepatic or transperitoneal, must first be defined. Depending on each patient and each surgeon, one or the other can be chosen, each with its advantages and disadvantages.
In patients at greater risk for bleeding, the transperitoneal approach is traditionally described, to prevent liver puncture. On the other hand, the transhepatic approach is preferred in patients whose gallbladder is very distended (> 10cm at the long axis or > 5cm at the axial axis), to reduce the risk of perforation and biliary leakage, as well as bile peritonitis.
Another important aspect to take into account is patient habitus and capacity to move because it can impede one approach or the other due to poor visualization of the gallbladder or to difficulty in reaching the gallbladder because of a complex tract. Once all the abovementioned aspects have been analyzed, the most appropriate approach can be chosen.
In our experience, PC is usually a procedure performed at the patient’s bedside through the trocar technique, albeit the modified Seldinger technique is traditionally described. Our technical success rate is similar to that reported in the literature (data to be published).
The step-by-step technique employed for ultrasound-guided PC placement is described below (Fig. 1).
- 1
Perform the appropriate asepsis and antisepsis in the region of the right hypochondrium.
- 2
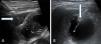
Scan the gallbladder through ultrasound to corroborate the diagnosis of AC.
- 3
Infiltrate the skin and subcutaneous cell tissue with 2% lidocaine.
- 4
Corroborate the presence of the material necessary for placing the catheter through the selected technique.
- 5
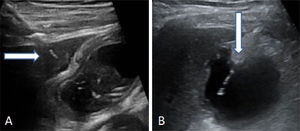
Locate the gallbladder through ultrasound scanning. Perform the transperitoneal or transhepatic puncture. Then introduce and advance the multipurpose catheter through the biliary tract (8.5 Fr) over the metal guidewire until it is positioned in the gallbladder (Fig. 2).
- 6
Extract the metal guidewire, leaving the catheter positioned inside the gallbladder.
- 7
Fix the catheter to the skin with a 3.0 prolene stitch.
- 8
Extract the biliary fluid to be sent to the microbiology laboratory for culture.
- 9
Place a collection bag at the distal end of the catheter, leaving the diversion.
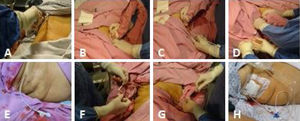
(A) Asepsis of the right hypochondrium. (B) Scanning of the gallbladder. (C) Ultrasound-guided introduction of the catheter together with the metal guidewire in the direction of the gallbladder. (D) The metal guidewire is extracted taking care to leave the catheter in place, facing the gallbladder lumen, and once again corroborating through ultrasound its adequate position inside the gallbladder. (E) The in-place cholecystostomy catheter. (F) The catheter is fixed with a simple stitch to the skin utilizing prolene 3-0. (G) Biliary fluid is aspirated and sent for culture. (H) The diversion cholecystostomy catheter with the collection bag.
PC placement is generally thought of as a safe and effective procedure. More than 90% of the patients are expected to present with improvement in pain and the systemic inflammatory response within the first 48h of PC placement.42 However, complications have been described in approximately 3% of cases. The most frequent complications are hemobilia, pneumothorax, biliary leakage, bile peritonitis, choledocholithiasis, and abscesses.43,44 Catheter exteriorization is always a risk, which could make placing it again difficult, once the gallbladder is decompressed.45,46
The risk of recurrence of AC after PC removal varies from 6 to 40%.47–49 AC recurrence has been reported, even in patients with a functional and permeable catheter, and generally occurs within the first 2 months of its placement.50 Longer hospital stay in patients with PC has been reported in some case series.51 That longer stay is most likely associated with the greater severity in the patients that are offered PC, rather than being a direct consequence of the procedure.52 Opportune PC placement, signifying within the first 24h from symptom onset, is effective in reducing hospital stay. That was documented in a 2015 study, in which only patients with late PC placement had longer hospital stay.53
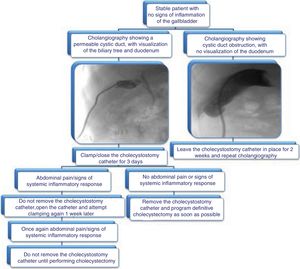
When to remove the cholecystostomy catheterIn general, the PC should be removed once the patient is stable and the acute picture has subsided. Nevertheless, the ideal removal time has not been precisely defined.54 Cholangiography performed through the PC has been carried out to evaluate cystic duct permeability. Lower AC recurrence rates have been reported in patients with a permeable cystic duct,55 but the data are contradictory. AC recurrence can present in some cases, even if cholangiography documents biliary tract permeability.56
A retrospective cohort study published in 2017 evaluated two groups. Group 1 (n=43) underwent routine cholangiography before cholecystostomy removal and group 2 underwent only on-demand cholangiography (n=41). The patients that had on-demand cholangiography had a better outcome, given that they had earlier drain removal, a higher early cholecystectomy rate, and less use of resources.57
At the time of performing LC as definitive treatment, special care must be taken, given that even though inflammation of the gallbladder has decreased or disappeared, patient comorbidities generally persist, meaning that surgery can be more complicated than first contemplated.58,59 A frequency of conversion to open surgery in that scenario has been reported between 11 and 32%.60–63 Interval cholecystectomy is generally performed with no technical problems in young patients with no comorbidities.64,65
Definitive treatmentCholecystostomy placement is ideally a bridging therapy before the definitive treatment that is performed once the patient presents with improvement in his/her general condition.66,67 More than 95% of patients have cholecystectomy as definitive treatment,68 but cholecystostomy has been reported in case series to remain indefinitely in 30 to 50% of patients.69–71 Some authors recommend PC placement as definitive and permanent treatment in elderly patients and those with a high surgical risk.72
In a descriptive study conducted within the time frame of 2000 to 2011 on 36 patients that had PC placement because they were considered “high-risk” patients, the authors reported 100% symptom resolution 3 days after PC placement in all the patients. PC was the definitive treatment in 63% of the patients, with definitive LC performed in only 6 patients (16%).73 Other authors are in favor of performing LC during the same hospitalization, because delayed LC is associated with patient loss to follow-up.74 Other study groups agree that the frequency of catheter-related complications is high and suggest removing the PC as soon as the acute inflammatory response has been controlled, even within the first 10 days of catheter placement.75,76
In the experience of our center, the cholecystostomy is removed once the patient is programmed for cholecystectomy within the following 2 weeks, for the single purpose of improving patient quality of life before surgery. The catheter is only removed if the patient is asymptomatic, has no signs of systemic inflammatory response, and has signs of a permeable cystic duct. It is also removed in patients that will undergo definitive cholecystectomy. If those criteria are not met, our position is to leave the cholecystostomy catheter in place indefinitely.
We propose the algorithm shown in Fig. 3 for cholecystostomy catheter removal.
It is important to emphasize that a greater frequency of surgical site infection has been reported in the patients that undergo cholecystostomy removal and then LC. Said infection has been described in up to 16% of cases.77
ConclusionsPercutaneous decompression of the gallbladder is a widely known treatment, but there is still no standardized strategy for cholecystostomy catheter management, making the uniform study of those patients difficult with the currently available literature. Despite the paucity of evidence, some considerations can still be established:
- •
High-risk surgical patients diagnosed with AC in whom emergency LC is contraindicated appear to be the best candidates for PC placement.
- •
There is no evidence supporting PC placement in stable patients with no contraindication for undergoing LC.
- •
PC catheter removal should only be carried out after cystic duct permeability has been documented through cholangiography, if the catheter causes the patient discomfort, and if the patient is a candidate for definitive surgical treatment within a “reasonable” and opportune period of time to reduce the possibility of presenting with recurrent acute cholecystitis.
There is little evidence on the management of percutaneous cholecystostomy catheters and randomized studies to evaluate the best strategy to follow in that group of patients are presently required.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this article.
Confidentiality of data. The authors declare that they have treated all patient data with confidentiality and anonymity, following the protocols of their work center.
Right to privacy and informed consent. The authors declare that no patient data appear in this article that compromise their identity and therefore it was not necessary to obtain their informed consent.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the physicians of the department of general surgery of the INCMNSZ.
Please cite this article as: Morales-Maza J, Rodríguez-Quintero JH, Santes O, et al. Colecistostomía percutánea como tratamiento de colecistitis aguda: ¿qué ha pasado en los últimos 5 años? Revisión de la literatura. Revista de Gastroenterología de México. 2019;84:482–491.