Clostridium difficile (C. difficile) is a Gram-positive bacillus that is a common cause of diarrhea in the hospital environment, with a documented incidence of up to 10%. There are different methods to detect it, but a widely used test in our environment is the immunoassay for toxins A and B.
AimsThe aim of our study was to 1) estimate the positive predictive value of the immunoassay for the detection of the C. difficile toxins A and B, 2) to establish the incidence of C. difficile associated diarrhea in the hospital, and 3) to know the most common associated factors.
MethodsA diagnostic test accuracy study was conducted within the time frame of January 2010 to August 2013 at the Hospital Christus Muguerza® Alta Especialidad on patients with symptoms suggestive of C. difficile-associated diarrhea that had a positive immunoassay test and confirmation of C. difficile through colon biopsy and stool culture.
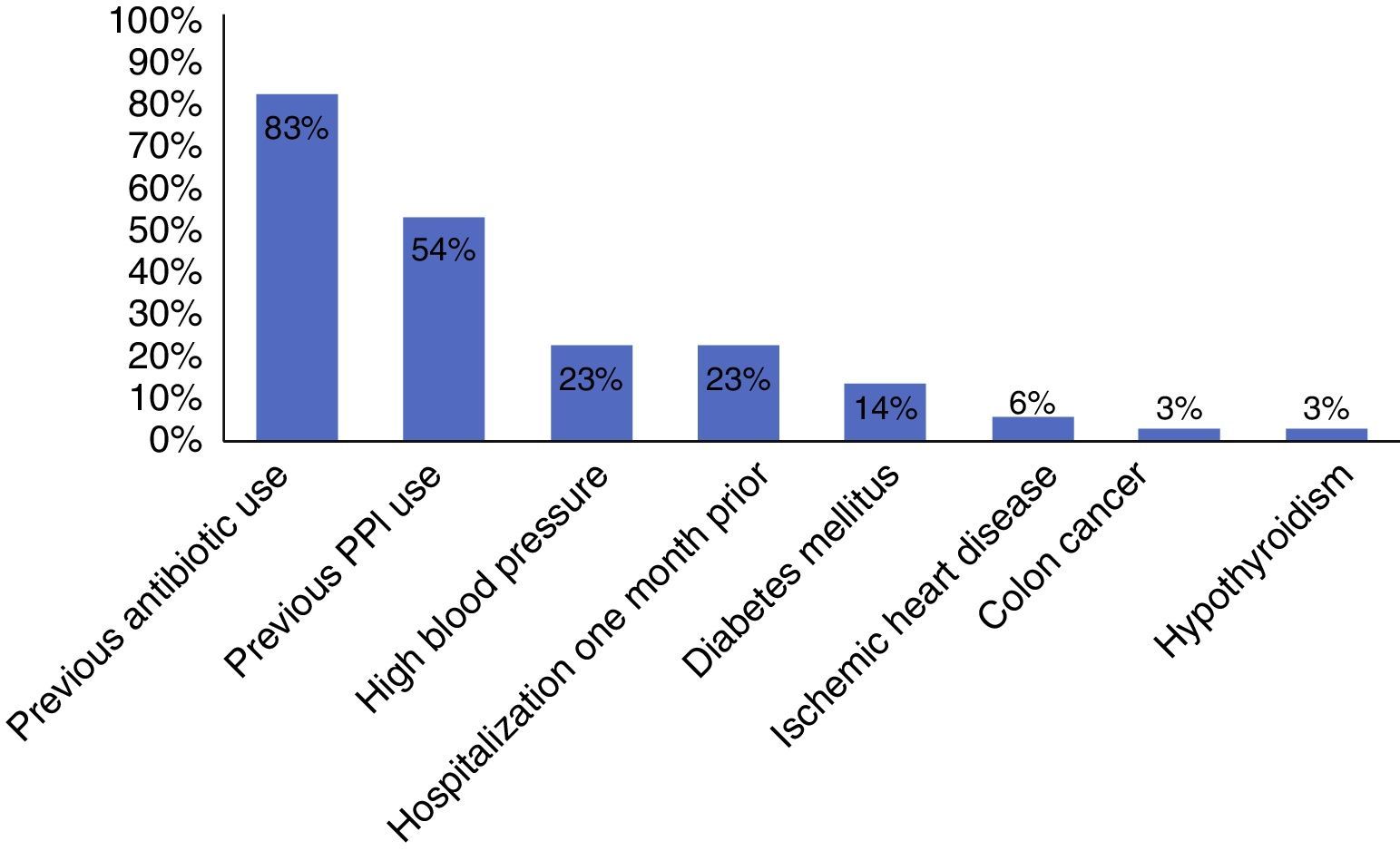
ResultsThe immunoassay for toxins A and B was performed in 360 patients. Fifty-five of the cases had positive results, 35 of which showed the presence of C. difficile. Incidence was 10.2% and the positive predictive value of the test for C. difficile toxins A and B was 0.64 (95% CI, 0.51-0.76). Previous antibiotic therapy (n=29) and proton pump inhibitor use (n=19) were the most common associated factors.
ConclusionsC. difficile incidence in our environment is similar to that found in the literature reviewed, but the positive predictive value of the test for toxin A and B detection was low.
Clostridium difficile (C. difficile) es un bacilo grampositivo, causa común de diarrea en el medio hospitalario; siendo la incidencia documentada hasta un 10%. Hay distintos medios para su detección sin embargo en nuestro medio, una prueba muy utilizada es el inmunoanálisis de toxinas A y B.
Objetivos1) Estimar el valor predictivo positivo de la prueba de inmunoanálisis para detección de toxinas A y B de C. difficile; 2) establecer la incidencia de diarrea por C. difficile en el hospital, y 3) conocer los factores asociados más comunes.
MétodosEstudio de prueba diagnóstica. Se incluyó a los pacientes con cuadro indicativo de diarrea por C. difficile de enero del 2010 a agosto del 2013, en el Hospital Christus Muguerza Alta Especialidad y con prueba de inmunoanálisis positiva, y que se comprobó por biopsia de colon y cultivo de muestra fecal para determinación de C. difficile.
ResultadosSe analizó a 360 pacientes a los que se le solicitaron toxinas A y B, de los cuales 55 casos resultaron positivos; en 35 se demostró la presencia de C. difficile. La incidencia fue del 10.2% y el valor predictivo positivo (VPP) de la prueba de toxinas A y B de C. difficile fue de 0.64 (intervalo de confianza del 95%, 0.51-0.76). Se encontró que el uso de antibioticoterapia previa (n=29) y de inhibidores de bomba de protones (n=19) fue el factor asociado más común.
ConclusionesLa incidencia de C. difficile en nuestro medio es similar a la literatura revisada; sin embargo, el VPP de la prueba de detección de toxinas A y B fue bajo.
Clostridium difficile (C. difficile) is the most common infectious cause of diarrhea in hospitalized patients and is associated with substantial mortality and elevated economic cost.1 In the United States, 1 out of every 100 hospitalized patients develops diarrhea due to C. difficile, whereas community incidence is 1 out of every 100,000.2
C. difficile is a Gram-positive anaerobic toxigenic spore-forming bacillus that has a wide range of manifestations. The result of colonization ranges from being an asymptomatic carrier to the development of pseudomembranous colitis.3
Inoculation occurs in 5-15% of healthy persons and transmission is usually through the same healthcare workers that become contaminated by other infected patients. In the United States, the reported incidence is 1-2% in hospitalized patients.4 Some of the main causes that predispose to infection are: antibiotic use, among which clindamycin, cephalosporins, amoxicillin, and fluoroquinolones are the most related; age above 65 years; prolonged hospitalization; and the use of proton pump inhibitors.5–8 Mortality associated with C. difficile-caused diarrhea is high, reaching 65% in the untreated elderly.9,10
The definitive diagnosis is made when C. difficile cytotoxins are demonstrated in stool samples. The reference method is the toxin B cytotoxicity test in cell cultures (gold standard) that is capable of detecting a small quantity of toxin (10 pg) in stool with elevated sensitivity (94-100%) and specificity (99%).11 The main disadvantages are that cell cultures are not available in all laboratories, together with the incubation period of 48-72hours. Given its speed (2-6h) and lower cost, the most widely used method is the immunoassay for Toxins A and B, which has 71-94% sensitivity and 92-98% specificity. The latex agglutination test has insufficient sensitivity at approximately 48 to 59%.12,13 Another utilized method is that of the nucleic acid amplification tests, such as the polymerase chain reaction (PCR) test. It has 100% sensitivity and 96% specificity, but it is a complex and expensive technique and detects asymptomatic carriers.11
The aims of the present study were to determine the positive predictive value of the immunoassay used in our hospital for the detection of the C. difficile A and B toxins, to establish the incidence of C. difficile-associated diarrhea, and to know the most common associated factors.
Materials and methodsA single-center, observational, descriptive, retrolective, diagnostic test accuracy study was conducted. The incidence of C. difficile-associated diarrhea within the hospital and the predictive value of the immunoassay for the detection of C. difficile toxins A and B were evaluated and compared with the anatomopathologic result and culture. In addition, the most common associated risk factors in the patients were documented. The information was obtained from the case record registry of the Biostatistics department.
All the patients seen at the Hospital Christus Muguerza® Alta Especialidad above the age of 18 years that had clinical suspicion for C. difficile-associated diarrhea upon admission or during their hospital stay and in whom the immunoassay for the A and B toxins was ordered were included in the study. In the patients that had a positive test, their case records stated that the presence of C. difficile was confirmed through colon biopsy and colonoscopy culture within the time frame of January 2010 to August 2013.
Patients under the age of 18 years, that had positive titers for the C. difficile toxins A and B test in the previous 8 weeks, or whose case record information was insufficient, were excluded from the study.
The qualitative enzyme immunoassay Premier®Toxins A&B was the test used at the Hospital Christus Muguerza® Alta Especialidad for the detection of C. difficile A and B toxins. This immunoassay utilizes stool samples placed in microwells that are sealed with monoclonal and polyclonal antibodies specific for the toxins, which if present in the samples, form antibody-antigen complexes, converting the substrate/chromogen into a colored product that is compared with a negative and positive control that is a definite yellow. It detects levels > 1.4 ng/ml of toxin A and > 2.4 ng/ml of toxin B.
Statistical analysisThe quantitative variables are expressed as means ± standard deviation and the categorical variables are expressed as percentages. The incidence of C. difficile-associated diarrhea was calculated and the positive predictive value of the test was determined with a 2 x 2 table with 95% confidence intervals (CI) using the Wald formula. The Excel database sheet and Medcalc software were employed for the result compilation.
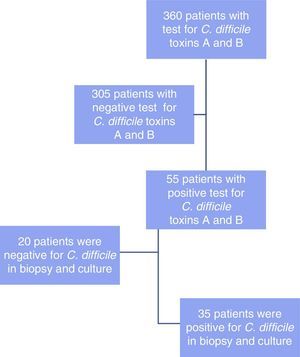
ResultsA total of 360 patients were analyzed (fig. 1) in whom the immunoassay for detecting the C. difficile toxins A and B was carried out. Of those patients, 55 cases had positive test results. The following variables were analyzed: age, sex, associated risk factors, days of hospital stay, stay in the intensive care unit (ICU), anatomopathologic result, and treatment.
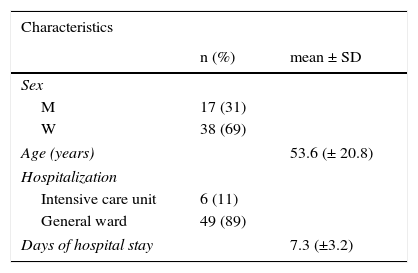
The mean age of the patients with positive immunoassay was 53.6 ± 20.8 years (Table 1) and the most frequent age was that of 50 to 65 years. In relation to sex distribution, 38 of the patients were women (69%) and 17 were men (31%).
Epidemiologic characteristics of the population.
| Characteristics | ||
|---|---|---|
| n (%) | mean ± SD | |
| Sex | ||
| M | 17 (31) | |
| W | 38 (69) | |
| Age (years) | 53.6 (± 20.8) | |
| Hospitalization | ||
| Intensive care unit | 6 (11) | |
| General ward | 49 (89) | |
| Days of hospital stay | 7.3 (±3.2) | |
M: Men; n: number of the population, SD: Standard deviation; W: Women.
Of the positive sample total, 6 patients (11%) were in the ICU, one of whom died while there, and 49 patients were in the general wards (89%).
The patients diagnosed with C. difficile-associated diarrhea had a mean hospital stay of 7.3 ± 3.2 days.
Colonoscopy was performed on the 55 patients, all of whom had suggestive data that was correlated with C. difficile through biopsy and culture in 35 of those patients (64%), whereas no correlation was found in 20 of the patients (36%).
Of the 20 patients with negative results, nonspecific chronic and acute inflammation was documented in only 10 of them, Crohn's disease in 3, ulcerative colitis in 3, normal histopathologic studies in 3, and infectious colitis due to Cryptosporidium in one patient.
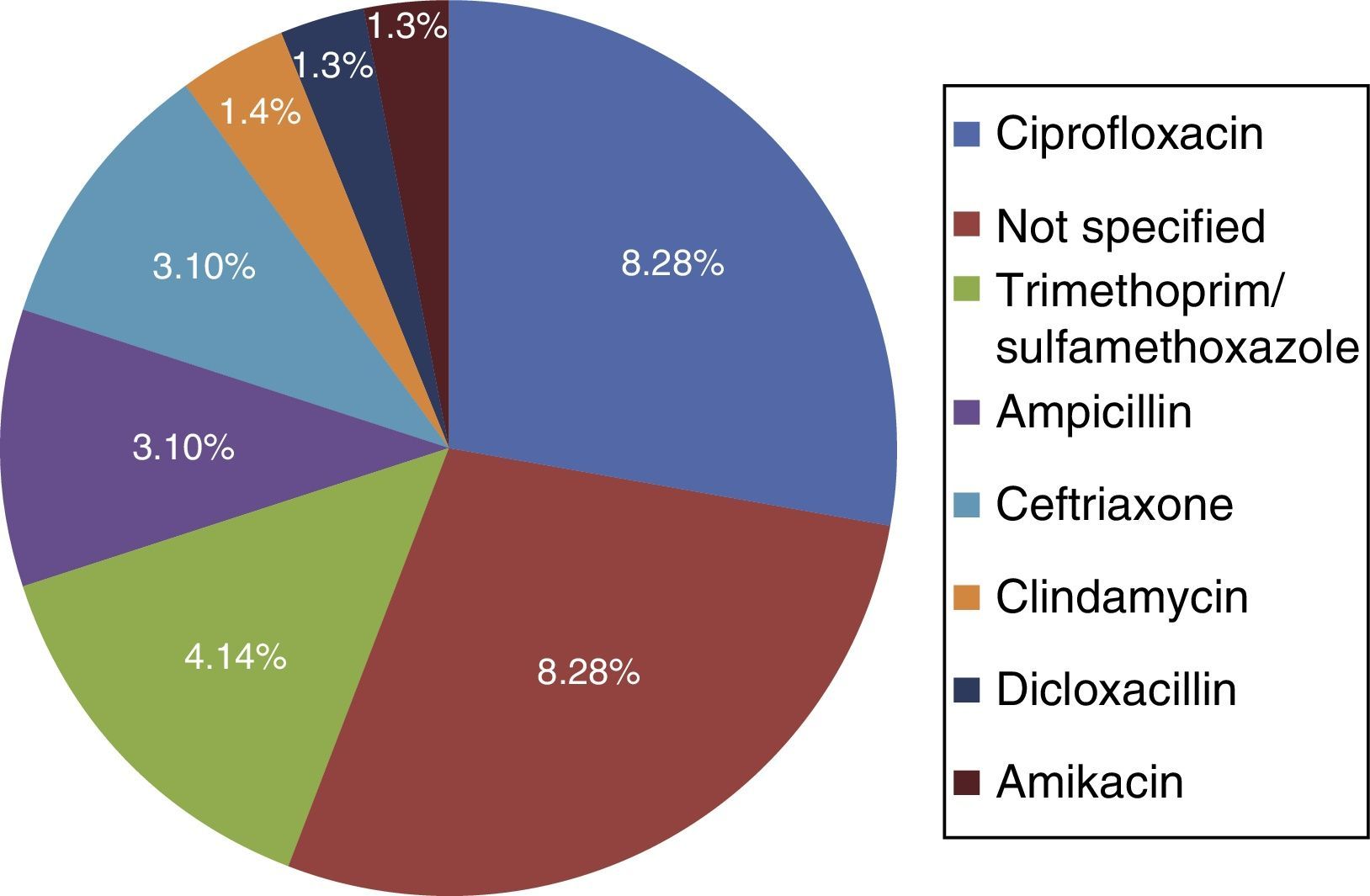
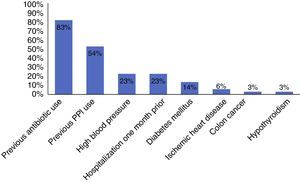
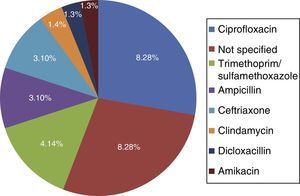
Among the risk factors associated with infection due to C. difficile (fig. 2) were the use of proton pump inhibitors and having taken an antibiotic, of which ciprofloxacin was the most related (fig. 3).
Of the 55 patients with a positive test for C. difficile toxins A and B, 28 of them (51%) were treated with metronidazole, 13 (24%) with vancomycin, and 14 (25%) required combination therapy with metronidazole and vancomycin.
The incidence of C. difficile-associated diarrhea detected at the Hospital Christus Muguerza® Alta Especialidad through the Toxin A and B immunoassay was 10.2% during a 3-year period and the positive predictive value of the immunoassay utilized, in relation to the anatomopathologic diagnosis, was 0.64 (95% CI, 0.51-0.76).
DiscussionC. difficile infection is definitely a common etiologic agent of intrahospital diarrhea in our environment and its incidence is similar to that reported in the literature.1
For many years, toxin A and B detection in stools through cellular cytotoxicity was considered the gold standard for the diagnosis of C. difficile infection. Recent studies have shown that toxin culture has a greater diagnostic potential and therefore should be the new gold standard. However, both tests are slow, laborious, and require trained personnel. For these reasons, the enzyme immunoassays for toxin detection are the most widely used for the diagnosis of C. difficile infection.14
Novak et al.15 compared the different tests employed for the diagnosis of C. difficile infection and found a positive predictive value of 0.68 for the toxin A and B test, which was similar to that found in our study (0.64). In other analyses, such as those by Eastwood et al.16 and Planche et al.17 that evaluated the sensitivity and specificity of the tests for the detection of the C. difficile toxins A and B in stool samples, values of 83 and 95%, respectively, a positive predictive value of 70%, and a negative predictive value of 96% were reported.
Other potential diagnostic options that have been described in the literature are the detection of glutamate dehydrogenase glutamate, PCR, and the nucleic acid amplification test (NAAT).14
Glutamate dehydrogenase detection can be used as a screening test because it has close to 100% sensitivity, but it has the disadvantage of having low specificity.18 PCR and NAAT have excellent sensitivity and specificity, almost 90%, which is why they have become the diagnostic method of choice in some centers. However, both tests are costly and do not detect toxin production and thus cannot distinguish C. difficile infection from asymptomatic carriage.19,20
Current American and European guidelines have recommended a two-step algorithm for the laboratory diagnosis of C. difficile infection that uses a highly sensitive test, such as that which detects glutamate dehydrogenase, followed by a highly specific one, such as immunoassay toxin A and B detection.18 Some have even proposed a third step of ordering toxin culture for those patients negative for toxins, but that have strongly suspicious symptoms.21
The main documented associated risk factors were the previous use of antibiotics and proton pump inhibitors, as reported in the study by Camacho et al.7 Among the antibiotics, quinolone use was the most related to infection with respect to antimicrobials, according to the literature reviewed.5,10
In regard to therapeutic management, metronidazole was the main drug used, in addition to vancomycin or the combination of the two, concurring with the management reported in the literature.4,5 Due to the emergence of hypervirulent strains, disease severity, increased sporulation, and greater antimicrobial resistance, the therapeutic options for recurrent or treatment refractory C. difficile infection are limited. Rifaximin, fidaxomicin, nitazoxanide, and tigecycline have been used as alternatives in those cases. Fecal microbiota transplantation is also another therapeutic measure for recurrence.22
ConclusionsC. difficile incidence in our environment was similar to that in the literature reviewed, but the positive predictive value for the test was low. Proton pump inhibitor use and previous antibiotic therapy were the most highly associated risk factors.
The determination of C. difficile toxins A and B should continue to be an important diagnostic test for the opportune detection of C. difficile-associated infection in our environment because it has a low cost, is fast, available, and easy to perform. This is especially true for those centers that have no other methods for confirming symptoms suggestive of this entity.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for this study.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Pérez-Topete SE, Miranda-Aquino T, Hernández-Portales JA. Valor predictivo positivo de la prueba de inmunoanálisis para detección de toxina A y B de Clostridium difficile en un hospital privado. Revista de Gastroenterología de México. 2016;81:190–194.