The incidence of esophageal adenocarcinoma (EAC) has increased. Although there are screening and surveillance programs, especially for patients with Barrett’s esophagus (BE), they have limited effectiveness in detecting early disease. Post-endoscopy esophageal adenocarcinoma (PEEC), diagnosed after previous negative endoscopies, raises concerns about the accuracy of current endoscopic practices in high-risk patients.
AimsPrimary aim: to estimate the rate of PEEC and analyze its characteristics in patients at three hospital centers in Medellín, Colombia.
Specific aims• To compare characteristics between patients with PEEC and those diagnosed at the first endoscopy.
• Evaluate the prevalence of BE in the two cohorts and its relation to PEEC.
• Analyze the anatomic location of PEEC.
Materials and methodsAn observational cohort study was conducted that included 473 patients diagnosed with esophageal cancer between 2012 and 2023 at three centers in Medellín, Colombia, 31 of whom had PEEC. Their demographic, clinical, and survival data were evaluated using the STROBE guidelines for cohort studies (pages 22–26).
ResultsThe PEEC rate was 6.6%. Patients with PEEC presented with fewer alarm symptoms (35% vs 63%, p = 0.002), a higher prevalence of BE (42% vs 23%, p = 0.016), and were diagnosed at an earlier stage of disease. The previous endoscopies failed to detect lesions, especially in the proximal esophagus.
ConclusionsPEEC is a frequent entity, especially in patients with BE and proximal lesions. Optimizing endoscopy through advanced imaging techniques and strict surveillance protocols is required.
El adenocarcinoma esofágico (ACE) ha incrementado su incidencia, y aunque existen programas de cribado y vigilancia, especialmente para pacientes con esófago de Barrett (EB), su efectividad en la detección temprana es limitada. El cáncer de esófago postendoscopia (CEPE), diagnosticado tras endoscopias negativas previas, pone en duda la precisión de estas prácticas en pacientes de alto riesgo.
ObjetivosObjetivo principal: Estimar la tasa de CEPE y analizar sus características en pacientes de tres centros en Medellín, Colombia.
Objetivos específicos• Comparar características entre pacientes con CEPE y aquellos con diagnóstico en la primera endoscopia.
• Evaluar la prevalencia de EB en ambas cohortes y su relación con CEPE.
• Analizar la localización anatómica del CEPE
Materiales y métodosEstudio observacional de cohortes con pacientes diagnosticados con cáncer de esófago entre 2012 y 2023 en tres centros de Medellín. Se incluyeron 473 pacientes (31 con CEPE), evaluando datos clínicos, demográficos y de supervivencia. Para su elaboración se utilizó la lista de comprobación STROBE: cohortes (pág. 22–26).
ResultadosLa tasa de CEPE fue del 6,6%. Estos pacientes presentaron menos síntomas de alarma (35% vs. 63%, p = 0,002), mayor prevalencia de EB (42% vs. 23%, p = 0,016) y diagnóstico más temprano. Las endoscopias previas fallaron en detectar lesiones, especialmente en el esófago proximal.
ConclusionesEl CEPE es frecuente, sobre todo en pacientes con EB y lesiones proximales. Se requiere optimizar la endoscopia mediante técnicas de imagen avanzadas y protocolos estrictos de vigilancia.
Over the past decades, there has been a significant increase in the incidence of esophageal cancer (EC), in particular, of esophageal adenocarcinoma (EAC), having increased 7-fold in the last 50 years. Despite advances in the management of Barrett’s esophagus (BE) and endoscopic screening programs, the 5-year survival rate continues to be under 20%.1 EC holds seventh place worldwide in incidence, sixth place in mortality, and is predominant in men, at 70% of cases.2
Given that tumor stage at diagnosis determines prognosis, guidelines recommend selective screening in patients with chronic gastroesophageal reflux and additional factors, such as age above 50 years, male sex, central obesity, smoking, and a family history of EC or BE.3,4 However, more than 90% of patients with EAC have no previous diagnosis of BE and most patients presenting with BE do not develop cancer, raising doubts as to the efficacy and cost-benefit of said programs.5–7
In addition, over half of the cases of EAC are diagnosed at advanced stages, illustrating the limitations of current surveillance strategies.8 The medical literature also reports cases of EC detected a short time after negative endoscopies, a situation similar to that seen in post-colonoscopy colorectal cancer.9–12 To standardize this phenomenon, the terms post-endoscopy esophageal adenocarcinoma (PEEC) and post-endoscopy esophageal neoplasia (PEEN) have been proposed. Undetected high-grade dysplasia and EAC are their primary causes.13–15
Different technical factors may contribute to these diagnostic errors, including insufficient BE segment inspection time, non-compliance with the Seattle biopsy protocol, incomplete mucosa sampling, or difficulty in identifying subtle lesions.14–16 Nevertheless, the information available on PEEC is limited and comes from small case series, lacking clarity on tumor stage at diagnosis.15,17,18 If early-stage esophageal cancers have a long natural history,17,19 then any cancer detected 3 years after endoscopy could have been missed before, as shown in previous studies.15,18,20,21
The present study aims to compare the clinical, endoscopic, and survival characteristics of patients with EC detected during their first endoscopy with those of patients whose EC was diagnosed after three consecutive normal endoscopies, prior to the cancer diagnosis, to provide evidence on the scope and characteristics of PEEC.
MethodsData sourcesAn observational, analytic, longitudinal, ambispective cohort study was conducted, utilizing the STROBE guidelines for cohort studies. The study was based on a group of patients with a histologic diagnosis of EC, who underwent a prospective follow-up and were treated by oncologic and gastrointestinal surgeons between July 2012 and June 2023. The data were obtained from the clinical records of 3 tertiary care institutions that are cancer referral centers: the Instituto de Cancerología-Clínica las Américas, the Clínica el Rosario, and the Centro Oncológico de Antioquia (COA) in Medellín, Colombia.
Study population and proceduresThe inclusion criterion was patients with histologically confirmed EC, diagnosed during the study period, at one of the 3 participating centers.
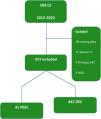
The exclusion criteria were 1) previous diagnosis and follow-up for BE with any grade of dysplasia or EC, 2) achalasia, 3) adenocarcinomas of the esophagogastric junction with a greater gastric component (Siewert 3), 4) patients with no follow-up at the participating centers, and 5) patients with lost values in more than 10% of the variables. Fig. 1 is a CONSORT diagram.
The clinical histories of all the patients with EAC at each center were reviewed, including those with negative endoscopies performed at other hospitals. Demographic data (age and sex), clinical data (tobacco and alcohol use as binary categories, a history of BE before the diagnosis of EAC, obesity [defined as a body mass index > 30 kg/m2]), indication for endoscopy (dysphagia, hematemesis, melena, vomiting, and constitutional syndrome, considered alarm symptoms), and treatment with proton pump inhibitors (PPIs), in the cases of diagnostic endoscopy, as well as the cases of false-negative endoscopy.
AimsThe study’s primary aim was to determine the PEEC rate.
The secondary aims were to compare the demographic, clinical, endoscopic, and survival characteristics between the patients with PEEC and those with detected esophageal cancer (DEC); to evaluate the prevalence of BE in the two cohorts; and to analyze the detection rate of PEEC in the different anatomic locations of the esophagus.
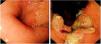
Endoscopic aspectsThe dates and primary diagnoses were collected from the records of both the negative and diagnostic endoscopies. The presence of esophageal ulceration and lesion location (upper, middle, or distal, including the cardia) were obtained from the endoscopy report. When more than one negative endoscopy was available, the most recent was selected for the analysis. Histologic subtype (squamous cell carcinoma, intestinal adenocarcinoma, or diffuse adenocarcinoma), differentiation grade (undifferentiated/poorly differentiated or moderately differentiated/well differentiated), and the presence of BE were obtained from the pathology report (Fig. 2).
Tumor stage was determined, according to the cTNM system, 8th edition, of the American Joint Committee on Cancer.22 Oncologic treatment was registered as binary results: neoadjuvant therapy, curative-intent surgery, adjuvant therapy, and palliative chemotherapy. Survival status was established, utilizing the date of diagnosis of EAC and the date of death. Survivors were right-censored on the date of their last medical visit (hospital or primary care).
Barrett’s esophagus and esophageal adenocarcinomaFor the present study, patients who were not in a previous follow-up due to BE with some grade of dysplasia were taken into account. The cases of EAC diagnosed in the interval between the day of BE diagnosis and 60 days later were considered prevalent EAC. The interval of 30 days after BE diagnosis was incorporated into the definition for considering additional procedures necessary for adequately stratifying the neoplasia identified at the time of BE diagnosis. EAC cases diagnosed between 30 and 365 days after the index endoscopy that diagnosed BE were considered PEEC (i.e., EAC that could have been prevalent and was not detected at the diagnosis of BE).13 Cases of EAC diagnosed more than 365 days after the diagnosis of BE were considered incidental (i.e., EAC that most likely was not present at the time of BE diagnosis). The analysis of those patients with BE in follow-up and the finding of cancer will be the subject of another study.
Statistical analysisMean, standard deviation, median, and interval were calculated for the continuous variables and frequency and percentage for the categorical variables. The data were analyzed using parametric methods for normally distributed continuous data (t test) and nonparametric methods for not normally distributed continuous data (Mann-Whitney U test). The chi-square test and Fisher’s exact test were used for the categorical data.
In addition, undiagnosed cancers were compared in the first year and between 1 and 3 years before the diagnostic endoscopy. One and 5-year survival probabilities were calculated for PEEC and non-PEEC, through the Kaplan-Meier method. The log-rank test was utilized to evaluate the differences in overall survival. All the analyses were two-tailed, and a p-value < 0.05 was considered statistically significant. All the statistical calculations were performed using the SPSS (Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp) program.
Ethical considerationsThe study protocol was designed in strict conformity with the principles established in the Declaration of Helsinki and the Guideline for Good Clinical Practice, ensuring the respect of the participants’ rights and wellbeing. The study was reviewed and approved by the Research Ethics Committee of the Clínica del Rosario, guaranteeing that all practices met the national and international ethical standards.
Given the study’s observational design and the fact that it is based on the review of data previously compiled during routine clinical procedures, exemption of individual informed consent was requested and granted, for including the participants in the study. This exemption was justified by the complete lack of direct intervention on the patients and the exclusive use of historic information, minimizing the risks for the participants (1993 Health Ministry Resolution 8430).
Nevertheless, informed consent had been previously obtained in all cases for the endoscopic procedures carried out as part of clinical care. Likewise, strict measures were implemented for secure and ethical data management, including information anonymization and limiting access to sensitive data to authorized personnel only, thus ensuring that the personal data protection norms were met.
ResultsThe initial cohort of linked data included 509 patients diagnosed with EAC between July 2012 and June 2023, of which only 473 met the inclusion criteria (Fig. 1). Table 1 summarizes the demographic and clinical characteristics of the cohort.
Demographic, clinical, and endoscopic characteristics of the patients.
| DEC (%) | PEEC (%) | p | Total (%) | ||
|---|---|---|---|---|---|
| 442 (93.4) | 31 (6.6) | 473 | |||
| Sex | Male | 326 (69) | 22 (71) | 0.733 | 348 (74) |
| Female | 116 (31) | 9 (29) | 125 (26) | ||
| Age | <50 | 39 (9) | 3 (10) | 0.993 | 42 (9) |
| 51 – 60 | 93 (21) | 6 (19) | 99 (21) | ||
| 61 – 70 | 133 (30) | 10 (32) | 143 (30) | ||
| 71 – 80 | 124 (28) | 9 (29) | 133 (28) | ||
| > 80 | 53 (12) | 3 (10) | 56 (12) | ||
| Alcohol use | Yes | 241 (58) | 15 (48) | 0.423 | 256 (54) |
| No | 191 (42) | 16 (52) | 207 (46) | ||
| Tobacco use | Yes | 256 (55) | 17 (55) | 0.737 | 273 (58) |
| No | 186 (45) | 14 (45) | 200 (42) | ||
| History of Barrett's esophagus | Yes | 101 (23) | 13 (42) | 0.016 | 114 (24) |
| No | 341 (77) | 18 (58) | 359 (76) | ||
| PPI use | Yes | 232 (52) | 18 (58) | 0.547 | 250 (54) |
| No | 210 (48) | 13 (42) | 223 (46) | ||
| Obesity | Yes | 72 (15) | 4 (13) | 0.707 | 76 (16) |
| No | 370 (85) | 27 (87) | 397 (84) | ||
| Alarm symptoms | Yes | 278 (63) | 11 (35) | 0.002 | 289 (61) |
| No | 164 (37) | 20 (65) | 184 (39) | ||
| Pathology | Adenocarcinoma | 292 (66) | 19 (61) | 0.588 | 311 (66) |
| Squamous cell | 150 (33) | 12 (39) | 162 (33) | ||
| Location | Upper-middle | 82 (19) | 11 (35) | 0.021 | 93 (20) |
| Lower-cardia | 360 (71) | 20 (65) | 380 (80) | ||
| T | 0−1 | 22 (5) | 5(16) | 0.016 | 27 (6) |
| 2 | 66 (15) | 8 (26) | 74 (16) | ||
| 3 | 292 (66) | 15 (48) | 307 (65) | ||
| 4 | 62 (14) | 3 (10) | 65 (13) | ||
| TNM | Stage 0−1 | 24 (5) | 6 (19) | 0.013 | 30 (6) |
| Stage 2 | 81 (18) | 7 (23) | 88 (19) | ||
| Stage 3 | 241 (55) | 14 (45) | 255 (54) | ||
| Stage 4 | 96 (22) | 4 (13) | 100 (21) | ||
DEC: detected esophageal cancer; PEEC: post-endoscopy esophageal adenocarcinoma; PPI: proton pump inhibitor.
The mean age (± SD) of the patients at EAC diagnosis was 69.3 ± 10.4 years, and 7 out of every 10 patients were men. Three-quarters of the cancers (74%) were located in the lower part of the esophagus. At diagnosis, 24% of the cohort had BE. Of the patients with known TNM stage at diagnosis, 75% presented with cancers in stage III or IV, whereas only 6% were diagnosed with stage 0 (carcinoma in situ) or stage I cancers.
Demographic and clinical aspectsPEEC was not associated with sex, age group, a history of alcohol or tobacco use, obesity, PPI use, or cancer histology (Table 1). However, the rate of PEEC was significantly associated with the absence of alarm symptoms (p = 0.002), the presence of BE (p = 0.016), early T stage, and of course, a tumor in the pre-malignant stage (p = 0.01).
Post-endoscopy cancer percentage and rateOf the 473 patients considered for the study, 31 (6.6%) had undergone at least one endoscopy in the 3 years before diagnosis of their cancer (undiagnosed cancer rate, 6.1%), 166 (35%) underwent an endoscopy between 3 and 12 months before the diagnosis, and 307 (64%) had an endoscopy between 1 and 3 years before the EAC diagnosis. There were no significant differences between the three hospital centers in that regard. The median time interval between a diagnosis negative for EAC and diagnosis of PEEC was 18.6 months (range: 3.2–34.1) and was below 2 years in 84% of the patients. The median number of negative endoscopies in the PEEC group was 1 (range: 1–2).
Most frequent endoscopic findings in PEECThe most frequent finding in the endoscopy before the cancer diagnosis was esophagitis (12/31, 39%): 4 were cases of Los Angeles classification grade A peptic esophagitis, 2 cases were grade B, 3 were grade C, 2 were grade D, and there was one case of esophageal candidiasis, followed by apparent peptic esophageal stricture (4/31, 13%). No biopsy was taken nor was adequate endoscopic follow-up carried out in any of the cases of stricture or peptic esophagitis. The most probable explanation for PEEC was an unseen or missed lesion (19/31, 61%), followed by inadequate follow-up (7/31, 23%), and an error in biopsy sampling (5/31, 16%).
Table 2 distinguishes the endoscopies performed during the period (1 or 3 years) before the EAC diagnosis, in the PEEC group. Patients with EAC diagnostic endoscopies carried out after the last negative endoscopy had more studies performed due to BE (42%, 95% CI: 24.56% to 59.31% versus 23%, 95% CI: 18.94% to 26.77%, p = 0.026), they had fewer alarm symptoms (65% versus 85%), a higher number of proximal locations (32%, 95% CI: 18.64% to 52.33% versus 19%, 95% CI: 14.93% to 22.18%, p = 0.034), and a tendency to present with cancer at an early stage (0 to I) (19%, 95% CI: 18.64% to 52.33% versus 5%, 95% CI: 14.93% to 22.18%).
Characterization of the relevant findings on the endoscopies performed 1 and 3 years before the cancer diagnosis.
| DEC (%) | PEEC and previous endoscopies | p | ||||
|---|---|---|---|---|---|---|
| 442 (93.4) | 3−12 meses | 1−3 años | Total | |||
| n = 12 (%) | n = 19 (%) | n = 31 (%) | ||||
| History of Barrett's esophagus | Yes | 101 (23) | 8 (67) | 5 (26) | 13 (42) | 0.026 |
| No | 341 (77) | 4 (33) | 14 (74) | 18 (58) | ||
| Alarm symptoms | Yes | 278 (63) | 7 (58) | 3 (16) | 10 (32) | 0.027 |
| No | 164 (37) | 5 (42) | 16 (84) | 21 (68) | ||
| Location | Upper-middle | 82 (19) | 7 (58) | 4 (21) | 11 (32) | 0.034 |
| Lower-cardia | 360 (71) | 5 (42) | 15 (79) | 20 (68) | ||
| TNM | Stage 0-I | 24 (5) | 0 (0) | 6 (32) | 6 (19) | 0.019 |
| Stage II | 81 (18) | 1 (8) | 6 (32) | 7 (23) | ||
| Stage III-IV | 337 (77) | 10 (92) | 8 (36) | 18 (58) | ||
The prevalence of BE was 24% in the entire cohort. The number of patients with BE in the PEEC cohort was significantly higher than in the patients in the DEC group (42%, 95% CI: 24.56% to 59.31%, versus 23%, 95% CI: 18.94% to 26.77%, p = 0.026). The diagnosis of PEEC associated with BE was made in the first year after negative endoscopy in 26% of patients, in contrast with the 13% of patients without BE (p = 0.040).
Utilizing the classification of PEEC and its relation to BE proposed by the American Gastroenterological Association (AGA), 88% of our patients were considered prevalent EAC, 7% PEEC, and 5% incident EAC.
Frequency of previous endoscopiesOf the 473 patients who underwent endoscopies in the 3 years prior to EAC diagnosis, 361 (76%) had just one procedure during that period. The number of patients with a single previous endoscopy was lower in the patients who had the endoscopy between 3 and 12 months before the cancer diagnosis (56%), compared with those who had the procedure between 1 and 3 years before the diagnosis (81%). Of the patients who underwent an endoscopy between 3 and 12 months before the EAC diagnosis, 32 (17.0%) had undergone 3 or more endoscopies in the previous 3 years.
TNM tumor stageTNM stage at diagnosis showed a significant association with the number of endoscopies performed in the 3 years before the cancer diagnosis (p = 0.014) (Table 3). Eighty-one percent of the patients with stage IV cancer had undergone only one endoscopy in the 3 years before diagnosis, compared with 50% of the patients with stage 0/1 cancer. Only 23% of the stage 0/1 cancers were diagnosed after 3 or more endoscopies in the 3 years before diagnosis.
Number of endoscopies performed before diagnosis, stratified by tumor status at diagnosis.
| Endoscopies | TNM at diagnosis | Total | |||
|---|---|---|---|---|---|
| 0−1 | 2 | 3 | 4 | ||
| n = 30 (%) | n = 88 (%) | n = 255 (%) | n = 100 (%) | n = 473 (%) | |
| 1 | 15 (50) | 66 (75) | 199 (78) | 81 (81) | 361 (76) |
| 2 | 8 (27) | 15 (17) | 31 (12) | 14 (14) | 68 (14) |
| 3 | 2 (7) | 3 (3) | 18 (7) | 4 (4) | 27 (6) |
| 4 or more | 5 (16) | 4 (5) | 7 (3) | 1 (1) | 17 (4) |
The median follow-up was 5.2 years (interquartile range [IQR], 3.5–8.6 years). There were no significant differences between the two groups in the survival analysis, summarized in Fig. 3.
DiscussionIn the present population cohort study, 6.6% of the patients diagnosed with esophageal cancer had undergone one endoscopy between 3 and 36 months before the cancer diagnosis. This results in an estimated overall misdiagnosis rate of 6.1% in the study cohort (the interval cancer rate = number of interval cancers/number of interval cancers + total number of detected cancers x 100). This figure falls within the lower range of estimates from previous studies, which have reported rates from 4.0% to 14.3%.15,18,19,21,23,24 In addition, there was an association between patients having undergone a previous endoscopy and their tumor stage at diagnosis; a negative endoscopy was more frequent in patients with disease stage 0/1. Patients with BE, alarm symptoms, and esophageal cancer in the upper third of the esophagus, also had a higher probability of having undergone an endoscopy before the cancer diagnosis.
Studies suggest that either some esophageal cancers are not detected at endoscopy or that the natural evolution of a significant number of cancers progresses from an early lesion that is endoscopically invisible to an advanced cancer, in a relatively short period of time. Considering the latter scenario, an alternate interpretation would be that only stage 2–4 cancers diagnosed within 3−36 months following a negative endoscopy are potentially missed, corresponding to a more conservative estimated omission rate of 5.6%. Nevertheless, we believe that the overall figure may be more exact. Even though there are very few studies on the natural history of untreated early esophageal cancer, two small cohort studies suggest that some patients with early cancer survive more than 5 years,25,26 and there are increasingly more confirmations of the slow progression of endoscopically visible dysplasia in patients with BE.27,28
Previous negative endoscopies were more frequent in patients with early cancers, and some of those patients may have been under periodic surveillance. This is supported by our finding that BE was more frequent in those patients (42% vs 58%, p = 0.026).
Several reasons have been proposed for a cancer not being diagnosed during the initial endoscopy.29,30 They include the fact that the endoscopist may not identify a possible lesion, or if identified, its importance is not recognized, and so a biopsy sample is not taken or is taken with an insufficient number of samples.17 The endoscopic appearance of an incipient cancer can be very subtle, and the only sign may be slight changes in the color or contour of the mucosa.4 Thus, these lesions are difficult to detect, reinforcing the need to perform high-resolution white light endoscopy; adding improved imaging techniques (narrow-band imaging [NBI], blue laser imaging [BLI], iScan) may be considered an alternative but their preferred use over conventional white light endoscopy is not recommended in the guidelines, not even in high-risk populations.
The use of proton pump inhibitors before endoscopy may also increase the probability of lesions not being detected,31 by favoring mucosal healing. Ideally, endoscopies should be performed before the prescription of antacid medications. It has been suggested that the middle and upper esophagus may be poorly inspected, given that the endoscope may be rapidly withdrawn during the final phases of the procedure, which reduces the possibility of visualizing subtle lesions.29 In agreement with other studies,30,32 we found a higher number of patients with missed lesions in the upper third of the esophagus, who had undergone an endoscopy one year before EAC diagnosis, than in the patients with lesions in the lower third of the esophagus or the gastroesophageal junction.
More than one-third of the negative endoscopies were performed due to alarm signs, such as dysphagia, and those symptoms were more frequent in patients with DEC (37% versus 63%, p = 0.027). This concurs with the fact that alarm symptoms tend to be associated with advanced disease and with a lower rate of previous endoscopies in patients with stages T3 and T4.
The relation between BE and PEEC deserves special attention. If the number of cases of PEEC were calculated without including the 114 patients with BE, the PEEC rate in our study would have dropped from 6.6% to 5.3%. The percentage of PEEC cases with a history of BE at one year was 63%, compared with 37% of EAC cases without BE, representing an alarmingly high percentage of undetected EAC that should be addressed. Our results highlight the need for stricter compliance with the diagnostic protocols for BE recommended in guidelines, greater attention paid to making diagnoses through advanced imaging and to biopsy protocol, and a more pro-active attitude in the treatment of gastroesophageal reflux disease (GERD). Different case series, when analyzing PEEC, exclude patients with BE18,21,32–34 or with BE and no dysplasia,23,35,36 whereas Yalamarthi et al.37 call attention to the error-inducing role of histologic interpretation (27%) at diagnosis.
The study’s strengths include its multicenter analysis and its assessment of patient characteristics (both clinical and endoscopic), as well as tumor features, such as stage at diagnosis and its association with the performance of previous endoscopies. Endoscopies performed within 3 months prior to the cancer diagnosis were excluded to minimize the likelihood of including patients who were under follow-up after an initial concerning finding.
The retrospective design, a relatively small patient cohort, and medium-term follow-up are some limitations of our study that could potentially bias the results. Additionally, there was no standardization in the performance of the endoscopies or in the interpretation of the histopathologic reports.
Inadequate evaluation of premalignant or focal lesions, inadequate quality of the endoscopy and biopsy, and poor decision-making regarding surveillance or follow-up plans have been identified as the most common explanations of PEEC.30 The unadjusted PEEC rate was 6.6% in our study, which is within the goal of <10% proposed in a position statement on quality norms in endoscopy in the United Kingdom.38Table 4 presents a more comprehensive overview of the problem by compiling different case series reviewed and published on PEEC, highlighting their heterogeneity.
Characteristics of the different publications that make reference to post-endoscopy esophageal adenocarcinoma.
| Author, year | Country | Type | Design | Population | Conditioning factors | Period | EGD | Location | CA | PEEC | M | Age | Months | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Goetz et al.,21 2022 | Australia | SC | RC | General | – | 2011- 2016 | 17,131 | EC, GC | 126 | NR | NR | 6 --36 | 2,4 | |
| Januszewicz et al.,15 2022 | Poland | POP | RC | General | – | 2012−2018 | 5,877,674 | EC, GC, DC | 33,241 | 1993 | 60 | 68 | 6–36 | 6,0 |
| Vajravelu et al.,17 2022 | USA | POP | RC | Follow-up | Barrett’s esophagus without dysplasia | 2004−2019 | NR | EAC | 366 | 50 | 74 | NR | 1–12 | 14 |
| Dhaliwal et al.,36 2021 | USA | POP | RC | Follow-up | Barrett’s esophagus without dysplasia | 1991−2019 | NR | EAC | 22 | 2 | NR | NR | <12 | 13 |
| Gavric et al.,20 2020 | Slovenia | SC | RC | General | Excludes Barrett’s esophagus | 2007−2015 | 29,617 | EC, GC | 422 | 29 | 64 | 71 | <36 | 7,3 |
| Tai et al.,29 2020 | United Kingdom | SC | CC | General | Excludes ulcers | 2012−2017 | 60,214 | EC, GC | 672 | 48 | 62 | 72 | <36 | 7,7 |
| Van Putten et al.,35 2018 | Northern | POP | CR | Follow-up | Barrett’s esophagus without dysplasia | 1993−2010 | NR | EAC | 210 | 26 | 76 | 67 | 3–12 | 12,7 |
| Ireland | ||||||||||||||
| Rodríguez de Santiago et al.,22 2019 | Spain | MC | CR | General | Excludes Barrett’s esophagus | 2008−2015 | 123,395 | EC | 391 | 25 | 84 | 67 | <36 | 6,4 |
| Wang et al.,40 2016 | USA | POP | CC | General | – | 2000−2007 | NR | EC, GC | 751 | 52 | 52 | 77 | 6–36 | 6,9 |
| Cheung et al.,33 2016 | United Kingdom | POP | CC | General | Excludes Barrett’s esophagus | 2002−2012 | NR | EC, GC | 9487 | 633 | 59 | 70 | 12–36 | 6,7 |
| Chadwick et al.,24 2014 | United Kingdom | POP | CC | General | – | 2011−2012 | NR | EC | 6943 | 537 | 70 | 71 | 3–36 | 7,8 |
| Raftopoulos et al.,32 2010 | Australia | SC | RC | General | Excludes Barrett’s esophagus | 1990−2004 | 28,064 | EC, GC, DC | 822 | 55 | 80 | 67 | <12 | 24 |
| Bloomfield et al.,34 2005 | USA | SC | CC | General | Excludes Barrett’s esophagus | 1997−2001 | NR | EC | 110 | 10 | 90 | 57 | <24 | 9,1 |
| Yalamarthi et al.,37 2004 | United Kingdom | SC | CC | General | – | 1994−2001 | NR | EC, GC | 305 | 30 | NR | NR | <36 | 7,2 |
| Current series, 2024 | Colombia | MC | RC | General | Previous EC – Excludes Barrett’s esophagus | 2012−2023 | NR | EC | 442 | 31 | 74 | 69 | 3−36 | 6.6 |
CA: cancer; CC: cases and controls; DC: duodenal cancer; EAC: esophageal adenocarcinoma; EC: esophageal cancer; GC: gastric cancer; MC: multi-center; NR: not reported; POP: population; RC: retrospective cohort; SC: single center.
Artificial intelligence (AI) has emerged as a powerful tool in gastroenterology, especially in the early detection of neoplastic and pre-neoplastic lesions. A multicenter study conducted in China shows the potential of AI for improving the detection of superficial squamous cell carcinomas of the esophagus and precancerous lesions in areas where conventional methods, such as white light endoscopy and NBI, may have limitations. Their trial showed that AI reduced the undetected lesion rate, with a failure rate per lesion of 1.7% in the AI-assisted group, compared with 6.7% in the routine group. Although the difference was not statistically significant, the trend suggests that AI may have a potential clinical benefit, particularly in terms of diagnostic sensitivity. In addition, AI could aid in reducing interpretation variability among endoscopists, a well-known challenge in the detection of subtle lesions. However, considering the long-term evaluation of cost-benefit and effectiveness in real-world clinical settings is crucial. The integration of AI into daily practice should be carefully weighed against additional costs and the need to train personnel.39
Additional studies are needed to determine whether endoscopic detection improved through advanced imaging techniques (NBI, BLI, iScan), a longer inspection time, a higher number of biopsy samples, and the performance of repeat endoscopy within defined time limits would result in the surveillance of BE having a real impact on reducing the incidence and prevalence of EAC. In addition, cost-effectiveness analyses of the proposal should be supported by the implications of the findings.
Based on our findings and the present medical literature, we suggest implementing the following strategies that could contribute to reducing the rate of PEEC: 1) Strict compliance with the biopsy protocol. It is essential to rigorously adhere to the established protocols, such as the Seattle biopsy protocol, for patients with BE, guaranteeing adequate sampling of the mucosa for the early detection of dysplasia or neoplasia, 2) Use of advanced imaging techniques and photo-documentation. The incorporation of improved imaging techniques, such as NBI, BLI, iScan, or chromoendoscopy, increases sensitivity in the detection of subtle lesions, especially in the proximal esophagus, where said lesion may go unnoticed; photo-documentation with at least 4 images of the esophagus (upper, middle, cardia, and retrovision) is also recommendable, 3) Increased inspection time and sedation use. It is essential to spend adequate time on the endoscopic inspection, suggesting a minimum of 7 min for the endoscopy, or one minute for each centimeter of BE, to minimize the risk of missing neoplastic lesions; sedation can be a valuable tool for ensuring patient collaboration and reducing discomfort during the procedure, 4) Proactive follow-up in high-risk patients. Patients with tumors of the head and neck, BE, or a family history of EAC should have regular endoscopic surveillance, with a proactive focus on the early detection of malignant changes, and 5) Negative endoscopy reevaluation. In cases of previously negative endoscopies, especially in patients who are asymptomatic, at high risk, and with equivocal findings, early reevaluation utilizing advanced techniques should be considered, as well as possibly repeating the endoscopy to ensure that no important lesions have been missed.
ConclusionOur study results showed that 6.6% of esophageal cancers were diagnosed after a previously negative endoscopy, a diagnostic omission that was significantly more common in patients with BE, proximal tumors, asymptomatic disease, and early disease stages. These results underline the need for optimizing endoscopic practice, focusing on a meticulous inspection of the proximal esophagus and strict adherence to biopsy protocols in groups identified as high-risk, to reduce the rate of PEEC.
Financial disclosureThis study was financed using the authors own resources.
Declaration of Generative AI and AI-assisted technologies in the writing processArtificial intelligence was not utilized at any stage of this scientific study, including the design, data collection, analysis, or interpretation of results. All the procedures and analyses were carried out manually by the research team, guaranteeing that the conclusions are based solely on traditional methods of scientific research and medical judgement.
The authors declare they have no conflict of interest.