Insulin-like growth factor 1 is modulated by the insulin-like growth factor-binding proteins (IGFBPs) that are synthesized in the liver. The aim of the present study was to evaluate the concentrations of IGFBPs 1–7 in patients with chronic hepatitis C and study their association with fibrosis stage.
Patients and methodsA prospective, cross-sectional study was conducted that included patients with chronic hepatitis C. The stages of fibrosis were determined through FibroTest and FibroScan and the patients were compared with a control group. Serum levels of IGFBPs 1–7 were quantified through multiple suspension arrays. The Kruskal-Wallis test, Mann-Whitney U test, Spearman’s correlation, and ROC curves were used for the statistical analysis.
ResultsUpon comparing the patients and controls, the highest concentrations were found in IGFBPs 1, 2, 4, and 7 (p=0.02, 0.002, 0.008, and <0.001, respectively). IGFBP-3 levels had a tendency to be lower in the patients (p=0.066), whereas values were similar between patients and controls for IGFBP-5 and 6 (p=0.786 and p=0.244, respectively). Of the seven IGFBPs, IGFBP-3 concentrations were the highest. There were significant differences between fibrosis stages for IGFBP-5 and IGFBP-7.
ConclusionIGFBPs play a relevant role in the fibrotic process in liver damage. IGFBP-7, in particular, differentiates fibrosis stages, making it a potential serum biomarker.
El factor de crecimiento tipo insulinoide 1 es modulado por las proteínas de unión al factor de crecimiento de tipo insulinoide (IGFBPs), dichas proteínas son sintetizadas el hígado. El objetivo de este trabajo fue evaluar la concentración de IGFBPs 1–7 en pacientes con hepatitis C crónica y estudiar la asociación con el grado de fibrosis.
Pacientes y MétodosEstudio prospectivo, transversal. Se incluyeron pacientes con hepatitis C crónica, el grado de fibrosis se determinó por medio de Fibrotest y Fibroscan, los pacientes se compararon con un grupo control. Los niveles séricos de IGFBPs 1–7 fueron cuantificados por arreglo en suspensión múltiple. Para el análisis estadístico se utilizó Kruskal Wallis, U de Mann Whitney, correlación de Spearman y curvas ROC.
ResultadosAl comparar entre pacientes y controles, las concentraciones fueron más altas en las IGFBPs:1,2, 4 y 7(p=0.02, 0.002, 0.008y<0.001 respectivamente). IGFBP-3 con tendencia a ser menor en los pacientes (p=0.066). Mientras que la IGFBP-5y 6 tuvieron valores similares entre pacientes y controles (p=0.786 y p=0.244 respectivamente). De las siete IGFBPs, las concentraciones de IGFBP-3 fueron las más altas. De acuerdo con el grado de fibrosis, se encontraron diferencias significativas en IGFBP-5 e IGFBP-7.
ConclusiónLas IGFBPs tienen papel relevante en el proceso de daño fibrogénico hepático, en especial la IGFBP-7 participa de manera diferencial en los estadios de fibrosis, por lo que puede ser un potencial biomarcador sérico.
The insulin-like growth factor (IGF) family are proteins that have a high sequence similarity to insulin. The IGF system functions as an endocrine, paracrine, and autocrine regulating axis for cell proliferation, survival, and apoptosis in different types of cells.1 In general, the IGF system consists of 2 surface receptors (IGF-1R and IGF-2R), 2 ligands (IGF-1 and IGF-2), and a family of proteins that bind to IGF (the insulin-like growth factor-binding proteins or IGFBPs). IGFBPs play an important role as physiologic regulators of IGF interaction with their cell receptors in different organs, including the gastrointestinal tract and the liver.1 At present, different types of IGFBPs that are mainly produced by hepatocytes and secreted into serum have been described. Much has been written about those proteins having a high affinity (IGFBP-1 to IGFBP-6) or low affinity (IGFBP-7, among others) for binding to the IGFs.2 Normal serum levels of IGF-1 are approximately 40nmol/L, but 99 % of circulating IGF-1 is associated with the different IGFBPs, mainly with IGFBP-3.2
Specifically, IGFBP-1, as well as IGFBP-3, IGFBP-4, and IGFBP-6, are known to negatively regulate the capacity of IGF interaction with IGF-1R, which has been directly associated with cell growth, differentiation, and metabolism, along with their participation in the decrease of the effects of IGFs on cancers of the lung, breast, colon, and prostate.1 In addition to those functions, IGFBPs have been reported to have certain mechanisms independent of IGFs, participating in energy metabolism and carcinogenesis, according to the organ and their interaction with cell surface molecules.1
Liver fibrosis, which results from a chronic hepatocellular lesion, is a dynamic process characterized by an increased accumulation of extracellular matrix (ECM) proteins, which are produced by the hepatic stellate cells (HSCs). In addition, fibrosis is considered the final stage in the majority of liver diseases.3 A significant increase in IGFBP-1 levels has been observed in nonalcoholic fatty liver disease (NAFLD), mainly in the advanced stages of fibrosis.4 At the same time, serum IGFBP-3 concentrations have been found to be low, correlating with liver dysfunction severity and poor outcome in hepatocellular carcinoma.5 On the other hand, experimentally, IGFBP-7 expression has been found to induce HSC activation and hepatocyte apoptosis.6 There is little evidence on serum concentrations of IGFBP-2, IGFBP-4, IGFBP-5, and IGFBP-6 and their association with liver diseases. However, those proteins have been reported to inhibit angiogenesis (IGFBP-4),7 regulate the role of TNF-α and tumor growth (IGFBP-5),8 and promote prostate cancer cell migration (IGFBP-6).9
Liver fibrosis, even in advanced stages, has been reported to be reversible, stimulating considerable research aimed at identifying new molecules for the development of anti-fibrotic therapies.3 IGFBPs are produced in the liver, but there is little evidence of their participation in the process of liver damage in humans. Therefore, their study can contribute new knowledge about the pathophysiology of liver fibrosis, enabling the discovery of therapeutic or complementary targets for diagnosing liver fibrosis.
The aim of the present study was to determine the serum concentrations of the different IGFBPs in patients with chronic hepatitis C (cHC), according to the stage of fibrosis. Our results showed higher serum concentrations of IGFBP-1, IGFBP-2, IGFBP-4, and IGFBP-7 in patients with cHC, compared with the control group. In addition, some of those proteins were found to directly correlate with the stage of fibrosis. Thus, we believe that IGFBPs are regulated differentially and can importantly participate in the development of liver fibrosis in patients with cHC.
Materials and methodsA cross-sectional study was conducted that included patients seen at the Hospital General de México “Eduardo Liceaga”, the Hospital Universitario de la Universidad Autónoma de Nuevo León, and the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, within the time frame of January 2011 to December 2015. The patients were treatment-naïve, underwent fibrosis stage evaluation with FibroTest and Fibroscan,10 and were divided by stage into F0, F1, F2, F3, and F4. However, given the number of cases in stages F1 and F2, those patients were placed in a single group (F1-F2). The patients that had at-risk alcohol consumption (AUDIT>8) and no concordance between the methods employed for diagnosing fibrosis were excluded. Likewise, no patients with comorbidities (e.g., diabetes, high blood pressure) were included. The data comparison was carried out using samples from healthy subjects (blood donors), considered the control group, that were at no risk for alcohol consumption (AUDIT<8) and had negative serologic test results for the hepatitis A, B, and C viruses.
Anthropometric and laboratory variablesSex, age, and the anthropometric variables of height (measured in centimeters, with a stadiometer), weight (measured in kilograms with a manual scale), and body mass index (BMI) (kg/m2; weight/height2 formula) were obtained for each study subject. The biochemical analysis included total bilirubin, direct bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels. In addition, a thorough clinical history was carried out, specifically searching for the presence of clinical data of liver damage.
Insulin-like growth factor-binding protein determinationFor IGFBP determination, blood samples were drawn from all the participants (10mL) and processed to obtain serum that was then stored at −80°C until its use. The concentrations of said proteins were determined through multiple suspension array technology (HIGFBMAG-53K07 kit, Merck Millipore®), based on the use of antibodies that enable the simultaneous analysis of a variable number of proteins with no cross-reactivity, reducing intra-assay (CV<10 %) and inter-assay (CV<15 %) error. The data were acquired utilizing Luminex 200 MAGPX® SYSTEMS (Series No. 10294005) equipment, following the supplier’s specifications (Merck, Millipore). In addition, the sensitivity of the minimum and maximum detection values for each protein was established, using the Luminex XPONENT software.
Statistical analysisThe qualitative variables were described through absolute and relative frequencies (%) and the continuous variables through mean±standard deviation. The chi-square test was employed for the qualitative variables and a non-parametric analysis (Kruskal-Wallis test and Mann-Whitney U test) for the quantitative variables. Correlation was analyzed using the Spearman’s rank correlation coefficient. ROC curves were constructed to analyze sensitivity and specificity and statistical significance was set at a p<0.05. The SPSS version 22 (IBM) program was employed for the statistical analysis.
Ethical considerationsThe present study was approved by the ethics committees of the Hospital General de México "Dr. Eduardo Liceaga" (DI/16/107/03/082) and the School of Medicine of the UNAM (FM/DI/15/2015). All participants provided their informed consent and the study protocol followed the ethics guidelines of the 1975 Declaration of Helsinki.
ResultsOne hundred and twenty patients were included in the study population, with a predominance of women in the cHC group and a predominance of men in the control group. No differences were observed in the BMI values between the two groups, whereas the total bilirubin, direct bilirubin, AST, and ALT levels were significantly higher in the patients with cHC (Table 1).
Demographic data of the study subjects.
| cHC (n=120) | CS (n=103) | p | |
|---|---|---|---|
| Sex, n (%) | |||
| Men | 25 (29) | 138 (89) | < 0.001 |
| Women | 95 (71) | 27 (11) | |
| Age (years) | 51±10 | 37±9 | < 0.001 |
| BMI (kg/m²) | 27±4 | 28±4 | 0.464 |
| Total bilirubin (mg/dL) | 1.37±0.22 | 0.78±0.03 | < 0.001 |
| Direct bilirubin (mg/dL) | 1.21±0.16 | 0.68±0.03 | < 0.001 |
| AST (IU/L) | 84±7 | 30±1 | < 0.001 |
| ALT (IU/L) | 90±6 | 28±2 | < 0.001 |
Data are expressed as mean±standard deviation. Statistical analysis: chi-square and Mann-Whitney U tests.
ALT: alanine aminotransferase; AST: aspartate aminotransferase, BMI: body mass index; cHC: Chronic hepatitis C; CS: control subjects.
IGFBP-1 concentration (ng/mL) was higher in the patients, compared with the controls (1.35±0.26 vs. 0.65±0.12) (p=0.02), as were the IGFBP-2 values (16.26±3.81 vs. 3.91±0.35) (p=0.002). IGFBP-3 had the highest concentrations of all the IGFBPs, with a trend toward lower values in the patients (778±36) than in the healthy subjects (878±40) (p=0.066). Serum concentrations of IGFBP-4 were also higher in the patients, compared with the controls (59±14 vs. 21±1.9) (p=0.008). No significant differences in IGFBP-5 concentrations were found between the patients and controls (251±26 vs. 241±21) (p=0.786), nor were there significant differences in IGFBP-6 values between the patients and controls (131±6.6 vs. 122±4.2, p=0.244). Regarding IGFBP-7, concentrations were significantly higher in the patients (57±4.4), compared with the controls (33±3.1) (p<0.001).
Fibrosis stage and insulin-like growth factor-binding protein analysisThe 120 patients included in the study were divided according to the stage of fibrosis, as follows: F0 (n=35), F1-F2 (n=11–14), F3 (n=21), and F4 (n=39). There were significant differences when IGFBP concentration and fibrosis stage were compared. For IGFBP-1, IGFBP-2, IGFBP-4, and IGFBP-6, there were differences mainly between the stages of fibrosis and the control group level (Table 2), whereas the differences for IGFBP-5 and IGFBP-7 concentrations were between the stages of fibrosis (Table 2).
IGFBP concentrations according to stage of fibrosis.
| IGFBP(ng/mL) | F0(n=35) | F1-F2(n=11–14) | F3(n=21) | F4(n=39) | CS(n=103) | p |
|---|---|---|---|---|---|---|
| 1 | 0.9±0.5 | 1.5±0.5 | 1±0.5 | 1.4±0.5 | 0.65±0.12 | F4vsCS** |
| 2 | 8.8±8.4 | 10±5 | 26±9 | 18±7 | 3.9±3.5 | F0vsCS*F1-F2vsCS*F3vsCS**F4vsCS** |
| 3 | 695±202 | 620±350 | 844±304 | 756±391 | 878±406 | NS |
| 4 | 25±17 | 88±76 | 37±30 | 77±29 | 21±19 | F1-F2vsCS*F3vsCS*F4vsCS** |
| 5 | 97±71 | 237±186 | 107±36 | 324±292 | 241±118 | F4vsCS*F0vsF4**F1-F2vsF3** |
| 6 | 136±53 | 112±68 | 168±81 | 126±59 | 122±42 | F3vsCS** |
| 7 | 20±10 | 42±30 | 91±23 | 60±42 | 33±31 | F3vsCS**F4vsCS**F0vsF3**F0vsF4*F1-F2vsF3*F3vsF4* |
Data expressed as mean±standard deviation. Statistical analysis: Mann-Whitney U test.
CSs: control subjects; IGFBP: insulin-like growth factor-binding protein; NS: not significant.
The Spearman’s correlation was then carried out to determine the correlation between the IGFBPs and the stages of fibrosis. The results showed a correlation between the concentration of IGFBP-1, IGFBP-2, IGFBP-4, and IGFBP-7 and the stage of fibrosis. For IGFBP-1, the association with the stages of fibrosis was mild, showing an r of 0.185 (p=0.015). For IGFBP-2, the association with the stages of fibrosis was moderate, with an r of 0.537 (p<0.001). With respect to IGFBP-4 and fibrosis stages, there was an increase in concentrations in F1-F2, F3, and F4, compared with the controls. When IGFBP-4 was correlated with fibrosis stage, an r of 0.445 was obtained (p<0.001), which was a moderate correlation. IGFBP-7 concentration was 2-times higher in the patients than in the controls. In the fibrosis stage evaluation, there was a gradual increase in the concentration of that protein, with significant differences between F0 vs. F3 (p<0.001), F0 vs. F4 (p<0.001), F1-F2 vs. F3 (p=0.002), and F3 vs. F4 (p=0.005). When IGFBP-7 was correlated with the stages of fibrosis, a statistically significant r of 0.364 was obtained (p=0.001), which was a moderate association.
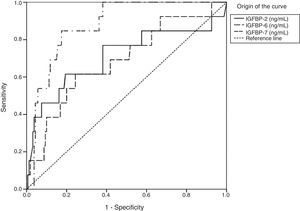
Insulin-like growth factor-binding protein sensitivity and specificity in the last stage of fibrosisTo evaluate IGFBP sensitivity and specificity for diagnosing the stages of fibrosis, especially in stages F3 and F4, ROC curves were constructed and areas under the curve were calculated (Figs. 1 and 2). For stage F3, the statistically significant results were in IGFBP-2, IGFBP-6, and IGFBP-7 (Fig.1 and Table 3).
Statistical data of the area under the curve of IGFBP-2, IGFBP-6, and IGFBP-7 in stage F3.
| Variables | Area | *SE | p | 95 % CI | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| IGFBP-2 | 0.708 | 0.093 | 0.013 | 0.525 | 0.891 |
| IGFBP-6 | 0.687 | 0.080 | 0.025 | 0.530 | 0.845 |
| IGFBP-7 | 0.877 | 0.037 | <0.001 | 0.804 | 0.949 |
CI: confidence interval; IGFBP: insulin-like growth factor-binding protein; SE: standard error.
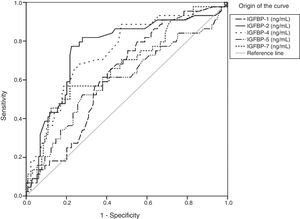
Regarding stage F4, the areas under the curve showed significant results for IGFBP-1, IGFBP-2, IGFBP-4, IGFBP-5, and IGFBP-7 concentrations (Fig. 2 and Table 4).
Statistical values of the area under the curve of IGFBP-1, IGFBP-2, IGFBP-4, IGFBP-5, and IGFBP-7 in F4.
| Variables | Area | SE | p | 95 % CI | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| IGFBP-1 | 0.627 | 0.045 | 0.012 | 0.538 | 0.717 |
| IGFBP-2 | 0.760 | 0.045 | 0.000 | 0.672 | 0.847 |
| IGFBP-4 | 0.744 | 0.043 | 0.000 | 0.659 | 0.828 |
| IGFBP-5 | 0.600 | 0.053 | 0.049 | 0.496 | 0.703 |
| IGFBP-7 | 0.674 | 0.049 | 0.001 | 0.579 | 0.770 |
CI: confidence interval; IGFBP: insulin-like growth factor-binding protein; SE: standard error.
In our study, 71 % of the patients were women and 29 % were men, coinciding with the characteristics of cHC populations in Mexico.11 With respect to BMI, the mean in the two groups was within the overweight range (25–29.9kg/m2), according to World Health Organization figures. It is important to keep in mind that obesity is a health problem in Mexico. Even though no clinical or biochemical (AST and ALT) evidence of liver damage was found, a more detailed analysis should be carried out in future studies to rule out possible hepatic steatosis in the two groups of the study. Nevertheless, our data revealed significant differences in the group comparisons, showing that the differences in the IGFBP concentrations and the fibrosis stages due to hepatitis C virus (HCV) were the result of chronic liver disease.
Fibrosis and cirrhosis of the liver (final stage) alter the production and metabolism of IGF system proteins, suppressing the correct functions of the organism.1,2 The authors of different studies consider that IGFBP-1 is an insulin-sensitive protein that participates in the development of metabolic diseases in patients with or without liver disease, such as insulin resistance or metabolic syndrome.12 In addition, IGFBP-1 has been identified as a possible biomarker for alcoholic liver disease (ALD) because its increased expression has been described.13 In cases of NAFLD, a decrease in serum IGFBP-1 concentrations has been reported due to the interaction with insulin.12 However, Hagström et al. reported high values in patients with advanced fibrosis.4 High concentrations of IGFBP-1 have also been related to hepatocellular carcinoma, albeit its role is still controversial.14
In our results, IGFBP-1 concentrations were higher in the cHC patients. According to the stage of fibrosis, differences were only found in relation to the severe stage of fibrosis and the controls. Likewise, the association of that protein with the stages of fibrosis was mild. Nevertheless, studies on patients with cirrhosis of the liver due to different causes (hepatitis C, hepatitis B, NAFLD, ALD, and autoimmune hepatitis) have shown that IGFBP-1 is increased in stages of advanced fibrosis (F3, F4).4 IGFBP-2 has been associated as a biomarker for metabolic disease, diabetes, and insulin resistance.15 In our study, IGFBP-2 concentrations were 5-times higher in the patients, with a gradual increase according to fibrosis stage but with no significant difference between the stages. However, it is important to consider that said protein was moderately associated with fibrosis stages and had a high sensitivity and specificity for diagnosing the final stages of fibrosis, suggesting its participation in the development of fibrosis caused by HCV.
Interestingly, IGFBP-3 presented with the highest serum levels, when compared with the other IGFBPs, but with a tendency to be lower in the patients than the controls. IGFBP-3 has been the most widely studied protein due to its high affinity for IGF-1. It has been described as a biomarker for liver dysfunction according to the Child-Pugh scale, with lower values in patients with Child-Pugh class C.16 In patients with cirrhosis due to different causes, a decrease in IGFBP-3 concentrations and an increase in IGF-1 have been found.17 Low IGFBP-3 levels have also been associated with a high risk for hepatocellular carcinoma and a poor prognosis.5 Aleem et al. concluded that said protein is a better predictor than IGF-1 for the development of hepatocellular carcinoma in patients with cirrhosis due to HCV.18 Miller et al. carried out a serum proteome in patients with NAFLD, and found a decrease in IGFBP-3 in the comparison with control subjects. IGFBP-3 was also able to distinguish between the different stages of fibrosis.19 In 2017, Chishima et al. studied the GH, IGF-1, IGFBP-3 axis in patients with NAFLD and cHC and the relation to the histologic severity of NAFLD. IGFBP-3 levels were lower in patients with cirrhosis caused by NAFLD, but did not decrease according to fibrosis stage in patients with cHC.17 Our results concurred with those of that study.
On the other hand, IGFBP-4 has been associated with the progression of lung cancer, finding its high expression in lung tissue, showing a decrease in survival.20 We found that IGFBP-4 concentration was 2-times higher in the patients, compared with the controls, but with no significant differences according to fibrosis stage. Its association with the stage of fibrosis was moderate. Likewise, it had a high sensitivity and specificity for F4. Experimental studies have shown a regulation of the increase of IGFBP-4, together with IGFBP-1 and IGF-1, by AMPc, IL-6, IL-1b, and TNF-a.21 Regarding IGFBP-5, studies on animal models of progressive intrahepatic cholestasis suggest it has a possible role in the pathogenesis of chronic cholangiopathy. Those same authors reported that IGFBP-5 increased the expression of pro-fibrotic markers in human hepatic stellate cells (LX-2), concluding that it plays a role in liver fibrosis progression.22 In our study, IGFBP-5 concentrations were similar between the patients and controls. However, when we classified the concentrations by fibrosis stage, there were differences between F0 vs. F4 and F1-F2 vs. F3, findings that concur with those of Colak et al., who showed that IGFBP-5 played an important role in many of the pathophysiologic stages of liver fibrosis.23 We also found that IGFBP-5, like IGFBP-4, had moderate sensitivity and specificity that could be used to diagnose F4. Improving the transdifferentiation of HSCs into myofibroblasts, improving the survival of those cells through the anti-apoptotic effects on activated HSCs, and increasing the expression of the profibrotic genes, such as collagen 1a1, TIMP-1, and MMP-1, are among the functions of IGFBP-5,21 whereas IGFBP-6 is capable of inducing chemotaxis of T-cells and monocytes, but not of B-cells. IGFBP-6 also increases oxidative stress and is a late factor of neutrophil activation.24 However, there are few studies on IGFBP-6 in liver diseases. In our study, IGFBP-6 concentrations were the same in the patients and controls, but upon analyzing fibrosis stage, there were differences in the patients in F3, compared with the controls.
Finally, different studies have shown that IGFBP-7 (IGFBPrP1), a protein with a low affinity for IGFs, contributes to liver fibrogenesis.25 That protein has been widely studied in experimental fibrosis models26 and its participation has been shown in the activation and transdifferentiation of HSCs and the increased production of EMC in vitro.26 Apart from the importance of IGFBPrP1 in the development of liver fibrosis, the use of an anti-IGFBPrP1 antibody has been associated with preventing the development of fibrosis, due to the suppression of HSC activation by the TGF-β1/Smad3 and ERK/MAPK signaling pathways.25,26 Our results showed a significant increase between the patients and a gradual increase according to fibrosis stage, being higher in F3. We also found that IGFBP-7 had a high sensitivity and specificity for diagnosing F3 and F4, which supports its possible participation as a serum marker in liver fibrosis. In biopsies of fibrotic and cirrhotic tissue, IGFBP-7 was shown to be a molecule that was involved in the progression of liver fibrogenesis.6 In in vitro studies, IGFBP-7 was found to induce liver fibrosis by HSC activation and hepatocyte apoptosis, through a Smad 2/3-dependent mechanism.27 It also acted as an initiator of liver fibrosis by inducing inflammation and ECM protein deposit through the ERK1/2 signaling pathway.28 In addition, it promoted fibrosis, increasing TGF-β1 expression, which in turn, increased Egr1, MAP2K2 (MEK2), and MAPK3 (ERK1) gene expression, while decreasing PTEN and Hhip mRNA expression.25 Li et al. found mutual regulation between IGFBP-7 and TGF-β1 in HSCs, which most likely accelerates the progression of liver fibrosis.29 IGFBP-7 inhibition attenuated fibrosis by re-establishing the MMP2/TIMP2 and MMP9/TIMP1 balance, concomitantly with the inhibition of HSC activation and profibrogenic mechanisms.30
The present study is the first to show evidence of IGFBP-1 to IGFBP-7 regulation and the correlation of those IGFBPs with the stages of fibrosis in patients with cHC, leaving the evaluation of the specific role of each of those proteins, except IGFBP-7, and their relation to the pathophysiology of the disease for future studies. However, the results of our study, have great relevance in the field of the development of diagnostic methods and/or possible therapeutic targets that would enable the fibrotic process to be reversed in chronic liver diseases.
ConclusionSerum IGFBP-1, IGFBP-2, IGFBP-4, and IGFBP-7 expression is differentially regulated in chronic hepatitis C. Based on our results, we strongly suggest that IGFBP-7 participates in the modulation and reuptake of ECM proteins and regulates the progression of chronic liver disease and the development of liver fibrosis. Therefore, we believe that IGFBPs can be candidates for serum biomarkers of liver fibrosis.
Financial disclosureThe present study was partially financed by the Consejo Nacional de Ciencia y Tecnología (CONACyT), number SALUD-2016-272579, and the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT- UNAM), number TA200515.
Conflict of interestThe authors declare that there is no conflict of interest.
The authors wish to express their gratitude with respect to the DGAPA-UNAM postgraduate scholarship program.
Please cite this article as: Rosique-Oramas D, Martínez-Castillo M, Raya A, Medina-Ávila Z, Aragón F, Limón-Castillo J, et al. Producción de las proteínas de unión al factor de crecimiento insulinoide durante el desarrollo de la fibrosis hepática por hepatitis C crónica. Revista de Gastroenterología de México. 2020;85:390–398.