The patency capsule is an effective diagnostic method for preventing video capsule retention in the small bowel during capsule endoscopy. The most frequently associated complication when using the patency capsule is symptomatic retention. The aim of the present study was to evaluate the effectiveness and safety of patency capsules administered to patients at a tertiary care hospital center.
Materials and methodsA retrospective observational study was conducted that included all the patients with confirmed Crohn’s disease that were administered a patency capsule, within the time frame of January 2019 and December 2020. PC diagnostic yield, sensitivity, specificity, positive predictive value, and negative predictive value were evaluated, in relation to capsule endoscopy and double-balloon endoscopy findings. Complications associated with the patency capsule were also identified.
ResultsThirty patients were included, in whom the patency capsule had 83% sensitivity, 100% specificity, 100% positive predictive value, and 96% negative predictive value, with a diagnostic yield of 96.7%. There was one complication (3.3%) and it resolved spontaneously.
ConclusionsThe patency capsule is a safe and effective method for reducing video capsule retention during capsule endoscopy in patients with Crohn’s disease.
La cápsula Patency es un método diagnóstico efectivo para prevenir la retención de las cápsulas endoscópicas en el intestino delgado. La complicación más frecuente asociada al uso de la cápsula Patency es la retención sintomática de la misma. La intención de este estudio es evaluar la efectividad y la seguridad de las cápsulas Patency colocadas en un centro de tercer nivel.
Material y métodosEstudio observacional retrospectivo que incluyó a todos los pacientes con enfermedad de Crohn confirmada a los que se les colocó la cápsula Patency de enero de 2019 a diciembre de 2020. Se evaluó el rendimiento diagnóstico, la sensibilidad, la especificidad, el valor predictivo positivo y el valor predictivo negativo comparado contra la cápsula endoscópica o enteroscopia doble balón. También se identificaron las complicaciones asociadas a la colocación de la cápsula Patency.
ResultadosSe incluyeron 30 pacientes, en los cuales la cápsula Patency mostró una sensibilidad del 83%, una especificidad del 100%, un valor predictivo positivo del 100% y un valor predictivo negativo del 96%, respectivamente, con un rendimiento diagnóstico del 96.7%. Se presentó una complicación (3.3%), que se resolvió de forma espontánea.
ConclusionesLa cápsula Patency es un método seguro y efectivo para disminuir la retención de la cápsula endoscópica en pacientes con enfermedad de Crohn confirmada.
Capsule endoscopy (CE) is a noninvasive diagnostic method that enables visualization of the small bowel (SB). It is useful in different diseases of the SB, such as suspected bleeding, Crohn’s disease (CD), polyposis syndromes, celiac disease, and other inflammatory diseases, as well as in the search for tumors1,2.
Video capsule retention is the main complication of CE and is defined as the capsule remaining in the SB for more than 14 days post-ingestion or when symptoms of bowel obstruction present. Its extraction requires medical, endoscopic, or surgical management3. Capsule retention rate in CE depends on the indication for the procedure and can vary from below 1% in patients suspected of SB bleeding to 21% in the context of obstruction in patients undergoing CE, enteropathy due to nonsteroidal anti-inflammatory drugs, SB tumors, SB anastomosis, and enteropathy due to radiation4.
The patency capsule (PC) (Pillcam™ Given Imaging, Yoqneam, Israel) is the same size as the video capsule utilized in CE (length: 26 mm, diameter: 11 mm)5 and is designed to be used before CE, to detect the presence of SB strictures that could cause video capsule retention. The PC is made of a polyurethane membrane that contains lactose, barium, and a radiofrequency identification tag (3 × 13 mm) and is sealed at both ends with a wax plug. Post-ingestion, intestinal secretions degrade its content, enabling the release of the barium. The device then wrinkles, and the remaining empty membrane and radiofrequency tag pass through any stricture that is present6. The PC has been shown to be effective in preventing capsule retention during CE in SB strictures, with a negative predictive value of 100%7.
Despite the fact that PC ingestion is a safe test8, it is not exempt from complications. Symptomatic retention is the main complication, causing bowel obstruction, even though the degradation of the PC continues, enabling its passage through the stricture and symptom relief, in the majority of cases. Very infrequently, medical treatment with steroids has been required, and surgical treatment is exceptional9. Another complication is aspiration of the PC into the airway, but it is uncommon, with only a few isolated cases reported10,11. The aim of the present work was to analyze the effectiveness and safety of PCs used in patients at a tertiary care center.
Materials and methodsStudy designA retrospective, descriptive, cross-sectional study was conducted that included all the PCs administered to patients at the Endoscopy Service of the Hospital de Especialidades, Centro Médico Nacional Siglo XXI of the Instituto Mexicano del Seguro Social, within the time frame of January 2019 and December 2020. The effectiveness and safety of PC use was analyzed.
Patient selectionAll the study patients had confirmed CD and required CE to evaluate treatment response. Due to the high risk of video capsule retention, the decision was made to have the patients undergo a PC test before the CE. All the patients fasted for 8 h prior to PC ingestion. No preparation with laxatives or prokinetics was carried out. The patients were given an appointment 30 h post-PC ingestion. If the PC was expelled in a bowel movement during the 30 h period, it had to be collected or photographed, to evaluate its physical integrity. If that was not possible, a plain abdominal x-ray in two positions was taken, to verify the location of the PC in the gastrointestinal tract.
Result evaluationThree criteria were considered PC predictive values of possible retention: 1) radiologic evidence of the PC located in the SB, 30 h after its ingestion, 2) expulsion of the disintegrated PC, and/or 3) the presence of obstructive symptoms during the passage of the PC through the SB (pain, bloating, and/or diarrhea). If there were no PC predictive criteria for retention, CE with a SB3 video capsule (Given Imaging, Yoqneam, Israel) was administered within 48 h. If there were PC predictive criteria for retention, they were confirmed through anterograde or retrograde double-balloon enteroscopy (DBE), depending on the location of the probable stricture site.
Statistical analysisThe quantitative variables were calculated as frequencies and percentages and the qualitative variables as mean and standard deviation or median and interquartile range (25th, 75th percentiles) according to their distribution. A 2 × 2 table was utilized to analyze sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic yield. PC diagnostic yield was determined in relation to the findings identified through CE or DBE. SPSS version 25 (IBM, Chicago IL, USA) software was used for the statistical analysis.
Ethical considerationsAll study patients remained anonymous and each of them signed written statements of informed consented. The present study meets the current bioethics research regulations and was authorized by the Research and Ethics Committee of the Hospital (R-2018-3601-188).
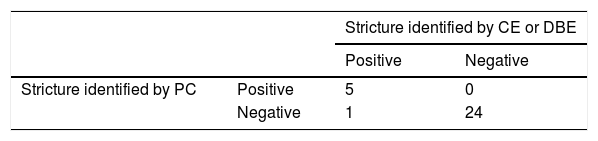
ResultsThe study included 30 PCs administered to the same number of patients. Nineteen of the patients were women (63.3%) and mean patient age was 55 years (±13.81). The PC identified predictive data of SB stricture in 5 patients. In 3 of those cases, a plain abdominal x-ray showed PC retention in the SB, 30 h post-ingestion, and in one case, a deformed PC was expelled in a bowel movement, within the first 30 h. In the remaining case, the patient went to the emergency service 6 h post-ingestion, with signs of bowel obstruction that were then confirmed through a plain abdominal x-ray. Twelve hours after symptom onset, the patient expelled a deformed capsule in a bowel movement, spontaneously resolving the situation12. Stricture was corroborated in the 5 cases through DBE; one was identified in the jejunum and the others were found in the terminal ileum, at least one meter from the ileocecal valve. None of the patients had symptoms prior to the bowel obstruction. During the DBE, strictures were dilated in 3 patients: to 15 mm in one patient and to 18 mm in two patients. CE was carried out in the 25 patients that met no PC retention criteria, one to two days after PC ingestion. Only one case presented with video capsule retention (Table 1). The stricture that caused the retention was in the jejunum and the video capsule was expelled in a bowel movement 27 days post-ingestion. The patient was asymptomatic during the time the capsule was retained. Comparing the PC predictive criteria for retention with the findings identified by CE and DBE, sensitivity was 83%, specificity was 100%, PPV was 100%, NPV was 96%, and PC diagnostic yield was 96.7%. There was one complication (3.3%) in the cases, and it resolved spontaneously.
DiscussionIn a study conducted in Japan, Yoshimura et al.13 administered 99 PCs in patients with CD. There were no PC-related data suggestive of stricture in 84 patients (84.8%) that underwent CE. Fifteen patients had PC-related data suggestive of SB stricture; 10 of them underwent enteroscopy and 2 had enteroclysis; stricture was confirmed in 11 of the cases. PC had 91.7% sensitivity, 98.8% specificity, 98.9% PPV, and 91.7% NPV, results very similar to those of our study, in which specificity and PPV were close to 100%.
In another study, Silva et al.14 included 54 patients diagnosed with CD. PC retention was identified through plain abdominal x-ray in 20% of the cases, at 30 h post-ingestion. Abdominal tomography scans carried out later identified retention in only 9%. In the rest of the cases, the PC was located in the colon. We did not perform abdominal tomography to corroborate whether the PC that was retained in the SB, identified through abdominal x-ray 30 h post-ingestion, was in fact in the SB, and not in the colon. However, in the 4 cases in which PC retention was identified through abdominal x-ray, SB stricture was confirmed through enteroscopy.
Kopylov et al.15 carried out the largest multicenter study evaluating the safety of PCs administered over a 10-year period and included 1,615 patients. They found symptomatic retention in only 20 cases (1.2%). One of those patients (5%) required surgery, whereas the rest resolved spontaneously or after treatment with corticosteroids. In the case series we present herein, symptomatic retention was slightly higher (3.3%), which could be due to the smaller study population, and the fact that one case resolved spontaneously.
The PC should not be utilized in all cases prior to CE. Its use should be reserved for cases in which there is a high risk for retention, such as patients with confirmed CD, symptoms of obstruction, chronic use of nonsteroidal anti-inflammatory drugs, or a history of abdominal and pelvic radiation16. Due to the low risk of capsule retention in CE in patients with other diseases, such as SB bleeding or suspected CD, the diagnostic yield of PC would be lower than in high-risk populations17.
Among the limitations of the present study were the number of patients and the fact that it was conducted at a single tertiary care center. Another limitation was not having carried out abdominal tomography scans to corroborate the location in the SB of the retained PC. Nevertheless, the present study on PC is the largest case series reported in Latin America, and it can be concluded that PC is a safe and effective method for reducing video capsule retention during CE, in patients with confirmed CD.
Financial disclosureAll the patency capsules utilized in the study were donated by Medtronic and Endomédica.
Conflict of interestGerardo Blanco-Velasco is a speaker for Medtronic. The rest of the authors declare that they have no conflict of interest.
Please cite this article as: Blanco-Velasco G, Ramos-García J, Solórzano-Pineda OM, Martínez-Camacho C, Murcio-Pérez E, Hernández-Mondragón OV. Seguridad y eficacia de la cápsula Patency. Rev Gastroenterol Méx. 2023;88:132–135.