A 73-year-old man had a past history of hypercholesterolemia, high blood pressure, hypothyroidism, and squamous cell carcinoma of the larynx treated with surgery, radiotherapy, and chemotherapy. He had no personal or family history of neurofibromatosis (NF), Cowden syndrome, or multiple endocrine neoplasia type 2B (MEN 2B). His habitual treatment was acetylsalicylic acid, levothyroxine, valsartan, and pitavastatin.
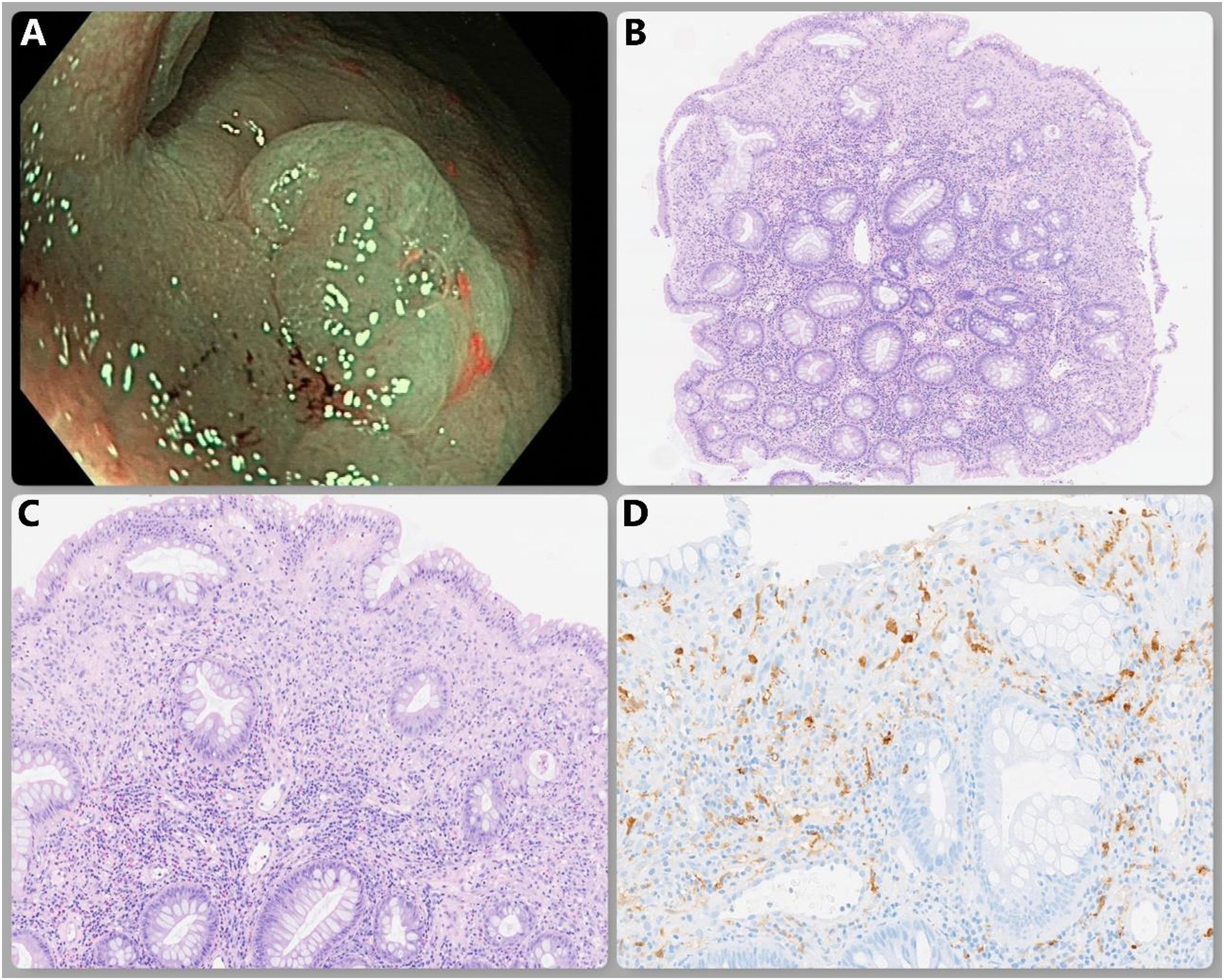
The patient was asymptomatic and underwent a complete and adequately clean colonoscopy that was ordered as a follow-up of adenomas. Diverticula were seen in the left colon, along with numerous 3 to 10mm polyps, all of which were resected with a cold snare. The histologic results were hyperplastic polyps, except for one 5mm flat-elevated polyp with no erosions or ulcerations on its surface (Fig. 1A), resected in the transverse colon. An associated mucosal Schwann cell hamartoma (MSCH) was identified. This diagnosis was made after the histology and immunohistochemistry (IHC) studies were performed, ruling out other possibilities.
Microscopic examination (Fig. 1B, C) revealed colonic mucosa composed of elongated glands, with serrated morphology in the upper and middle thirds, covered by a columnar epithelium with abundant intracytoplasmic mucin and rounded nuclei located in the intermediate or basal layer, without atypia. Focally, there was stromal expansion due to neural lineage proliferation (S-100+) (Fig. 1D) made up of spindle cells that had fibrillary eosinophilic cytoplasm and nuclei with irregular oval-shaped contours. Some of the nuclei were hyperchromatic and had a certain pleomorphism, but they lacked mitotic activity and marked atypia.
After 18 months of follow-up, the patient remains asymptomatic and has not presented with any complications.
MSCH is a benign entity of mesenchymal origin that was first described in 2009 by Gibson and Hornick.1 It consists of neural proliferation without the presence of ganglion cells. It has been identified in different gastrointestinal organs, such as the stomach, gallbladder, and duodenum, but it is most frequently located in the colon.2–4
Its endoscopic appearance tends to be that of a 1 to 6mm polyp.2,3 When these lesions are evaluated with optical magnification, they have a surface with a Kudo I and II pattern and a non-neoplastic profile,5,6 but they can also appear as small intramucosal nodules, protruding subepithelial lesions, or even as normal mucosa (an unexpected result of random biopsies).3,6,7
MSCHs are extremely rare, with fewer than 100 cases presently reported in the medical literature.3 They appear to be more frequent in middle-aged women and usually coexist with other colon polyps, such as adenomas or hyperplastic polyps.4,8
Cases of MSCHs have been reported in patients with ulcerative colitis, primary sclerosing cholangitis, multiple sclerosis, and acute myeloid leukemia, but its etiology is still unknown.2,4 They have been postulated to be secondary to an inflammatory process in areas of previously exposed or erosive mucosa.2
Found in a majority of asymptomatic patients, MSCH is frequently an endoscopic incidental finding.8 Nevertheless, it has also been described in patients with diarrhea, melena, abdominal pain, and anemia, as well as in a case of intestinal intussusception.1,2,8
The differential diagnosis includes neuroma, perineuroma, schwannoma, mucosal benign epithelioid nerve sheath tumor (MBENST), granular cell tumor (GCT), gastrointestinal stromal tumor (GIST), and colonic leiomyoma.5,7,9
No Schwann cells are identified in neuromas or perineuromas (the former present with axon fibers and the latter do not). The presence of Schwann cells and abundant axons leads to suspected neurofibroma that would have positive IHC ganglion cell markers (CD34). If the axons are scarce or absent, MBENST, an intestinal schwannoma, or MSCH would be suspected. Schwannomas are polypoid masses that are not limited to the lamina propria.
IHC is key to making the differential diagnosis: MBENST is positive for CD34 (it also has an infiltrative, epithelioid morphology), GIST is positive for CD117, and leiomyoma is positive for smooth-muscle actin (SMA).10 Perineuromas are positive for epithelial membrane antigen (EMA) or claudin-1.2 GCT is more frequent in the esophagus but can be present in the right colon and PAS+and S-100+ cytoplasmic granules would be identified.9
MSCH is differentiated from all of the above because it is positive for S-100 and negative for claudin-1, CD34, CD117, and SMA.9
Unlike neurofibromas (associated with NF1), neuromas (associated with MEN 2B), and some ganglioneuromas (Cowden syndrome, juvenile polyposis syndrome, MEN 2B, and NF), MSCH is never associated with hereditary syndromes,9 which emphasizes the importance of a correct differential diagnosis, given the implication in terms of family member genetic counseling.7,10
At present, no cases of neoplastic degenerations in MSCH have been reported. Considered a benign lesion, the benefit of a follow-up colonoscopy after polyp resection is doubtful, and if it were to be performed, the most adequate time interval is not known. Given the rareness of the disease, there are no clinical practice guidelines for the management of MSCH.4 Therefore, it is necessary to report each individual case that is diagnosed, to broaden the knowledge about this entity.
Ethical considerationsThe authors declare that no experiments on humans or animals were conducted in relation to this research. The work center protocol on the publication of patient data was followed. The authors declare that neither the images nor the text includes data that could identify the patient, guaranteeing that complete patient anonymity was observed. Nevertheless, informed consent was obtained for the publication of this case.
Financial disclosureNo financial support was received in relation to this article.