Celiac disease (CD) is an autoimmune enteropathy triggered by gluten ingestion in genetically susceptible individuals. Prevalence in Mexico is estimated at 0.9%, and its diagnosis is complicated by the variability of clinical manifestations.1,2
An 18-month-old female infant, the first child of nonconsanguineous healthy parents from Tabasco, with no family history of CD or autoimmune diseases, was delivered at full-term by cesarean section, weighing 3.5 kg and measuring 52 cm in length. She had her first bowel movement 24 h after birth. The infant was both breastfed and received anti-constipation formula from birth to 6 months of age, and then was breastfed until 12 months. Wheat was introduced to her diet after one year of age, a decision made by her mother. She presented with normal psychomotor development.
At the age of 15 months, the patient presented with constipation (no bowel movements for up to 2 weeks), vomiting, abdominal pain, and bloating. She received prokinetics, laxatives, and enemas, with no improvement. Physical examination revealed skin pallor, a painful and distended abdomen with a perimeter of 48 cm, liver 3-5-5 cm under the costal margin, and fecal impaction in the entire colon. Digital rectal exam found adequate sphincter tone and hard feces. The patient’s weight was 10.6 kg, her height was 79 cm, and her brachial perimeter was 13 cm. A barium enema showed abundant feces in the descending colon, and no transition zone. Polyethylene glycol at 1.5 g/kg/day was started.
At the follow-up at 22 months of age, the patient continued to be constipated and dependent on up to 2.5 g/kg/day of polyethylene glycol. Her stools were classified as type 2 on the Bristol stool scale, and treatment adherence was poor, with intermittent suspensions. She presented with increased abdominal pain and bloating, gastro-biliary vomiting, and a generalized rash that remitted in 24-48 h and was not associated with food intake. Physical examination showed abdominal distension, liver 4-6-6 cm under the costal margin, dermatosis extending to the axillas, and the inguinal region characterized by erythema and eczema. The patient’s weight was 12.9 kg, her height was 85 cm, and her brachial perimeter was 15 cm. She was well-nourished at the follow-up, but her weight was not reliable due to hepatomegaly.
Laboratory test results reported elevated aminotransferases (ALT 119 U/l, AST 70 U/l) and alkaline phosphatase (350 U/l). Hemoglobin, platelets, bilirubin, albumin, gamma glutamyl transpeptidase, and immunoglobulins were normal. Ova and parasite exam, stool culture, and Giardia lamblia antigen test in stool were negative. The tissue transglutaminase IgA/IgG test (tTG-IgA) and IgA endomysial antibody test (EMA) were negative.
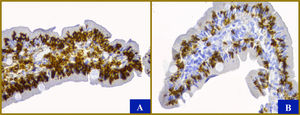
Endoscopy was performed, taking three and four biopsies from the duodenal bulb and distal duodenum, respectively, that reported preserved duodenal architecture. Immunohistochemical reactions with CD45 and CD8 antibodies were positive in the intraepithelial lymphocytes, which are changes consistent with CD (Fig. 1). Allergy was ruled out, by the absence of clustered or intraepithelial degranulated eosinophils in the lamina propria, and autoimmune enteropathy was ruled out, by the absence of Paneth cell damage. Colonoscopy revealed preserved intestinal architecture.
Histologic characteristics. Between 8 and 12 villi are shown, with a villus to crypt ratio of 2-3:2. A slightly enlarged mononuclear infiltrate with up to 6 eosinophils per high power field were found in the lamina propria (x40). Immunohistochemistry with CD45 (A) and CD8 (B) antibodies show more than 100 mainly cytotoxic lymphocytes (in dark brown) for every 100 enterocytes, despite the fact that the villi are the usual size (original magnification x40). There is no evidence of hyperplasia of the crypts or micro-organisms. The enterocytes, goblet cells, and Paneth cells are normal. The color of the figure can only be appreciated in the electronic version.
After the diagnosis of CD and having started a gluten-free diet, the patient was lost to follow-up.
The present case illustrates the fact that negative serologic tests do not rule out CD and that a high level of suspicion is the basis for making the diagnosis.
Less than 10% of cases present with constipation.3 In a study on 313 children with constipation and 990 healthy children, seroprevalence of CD of 2.5% and 0.6%, respectively, was reported.4
The European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) guidelines recommend total IgA and tTG-IgA testing as initial screening in children with suspected CD, and in subjects with age-specified normal IgA, tTG-IgA should be the initial serologic test, regardless of age. In patients with low total IgA levels, an IgG-based test (DGP, EMA, or tTG) should be performed as a second step.5
The Mexican clinical guidelines for the diagnosis and treatment of CD suggest the quantification of IgA and/or IgG antibodies against the deamidated gliadin peptide (DGP IgA and IgG) for children with a negative tTG-IgA test (especially those under 2 years of age), with suggestive symptoms. That was not performed on our patient, due to limited availability in our environment.6
The prevalence of tTG-IgA positivity varies from 0-88% and EMA positivity ranges from 8.6-79%, depending on the testing kit manufacturer.7 In a meta-analysis, the accuracy of diagnostic kits was calculated at a sensitivity of 94% (CI 89.9-96.5) and a specificity of 94.4%.8 The QUANTA LiteTM kit, with a sensitivity of 92.7% and a specificity of 91.6%, was utilized in the present case.
In the scenario described herein, histologic confirmation through duodenal biopsies was indispensable for making the diagnosis and determining mucosal damage.9
In accordance with the ESPGHAN, HLA DQ2 and/or DQ8 detection is not required in patients that are tTG-IgA-positive, if they are diagnosed with CD through biopsy or have high serum tTG-IgA (≥ x10) and positive EMA-IgA. In addition, positivity to those haplotypes does not confirm the diagnosis, added to its high cost and limited availability in our medical environment.5
Elevated aminotransferase levels appear to be more common in younger patients and have been reported to vary from 24-40%. In a retrospective cohort of 388 children (10.1 ± 4.4 years of age), a prevalence of 15.1% was reported but liver enzyme values were determined in only 185 (47.7%) patients at diagnosis. Although there are no evidence-based guidelines that address the need to test for liver dysfunction, several reviews recommend screening for liver disease in all newly diagnosed patients with CD. Abnormal transaminases normalize in the majority of cases within the first year of a gluten-free diet.10
CD must be consciously looked for in the appropriate clinical context, combining clinical history, serologic testing, and duodenal biopsy.
Ethical considerationsThe authors declare that the present article contains no personal information that could identify the patient, because it is a review of a clinical case record, no authorization by an ethics committee was needed.
Financial disclosureNo financial support was received in relation to this article.
Conflict of interestThe authors declare that there is no conflict of interest.
Please cite this article as: Espriu-Ramírez MX, Rivera-Suazo Y, Valencia-Mayoral PF. Enfermedad celiaca seronegativa: para encontrarla, hay que buscarla. Reporte de caso pediátrico. Revista de Gastroenterología de México 2021;86:317–319.