The sofosbuvir-velpatasvir (SOF/VEL) combination is a direct-acting antiviral therapy that is authorized and available in Mexico, making the performance of a real-world multicenter study that evaluates the sustained virologic response at 12 weeks post-treatment a relevant undertaking.
MethodsA retrospective review of the case records of 241 patients seen at 20 hospitals in Mexico was conducted to assess hepatitis C treatment with the SOF/VEL combination (n = 231) and the sofosbuvir/velpatasvir/ribavirin (SOF/VEL/RBV) combination (n = 10). The primary efficacy endpoint was the percentage of patients that achieved SVR at 12 weeks after the end of treatment.
ResultsOverall SVR was 98.8% (95% CI 97.35–100%). Only three patients did not achieve SVR, two of whom had cirrhosis and a history of previous treatment with peg-IFN. Of the subgroups analyzed, all the patients with HIV coinfection, three patients with genotype 3, and the patients treated with the SOF/VEL/RBV combination achieved SVR. The subgroups with the lower success rates were patients that were treatment-experienced (96.8%) and patients with F1 fibrosis (95.5%). The most frequent adverse events were fatigue, headache, and insomnia. No serious adverse events were reported.
ConclusionTreatments with SOF/VEL and SOF/VEL/RBV were highly safe and effective, results coinciding with those of other international real-world studies.
La combinación de sofosbuvir-velpatasvir (SOF/VEL) es una terapia antiviral de acción directa que está autorizada y disponible en México. Esto hace que la evaluación de la respuesta virológica sostenida (RVS) 12 semanas después del tratamiento, por medio de la realización de una revisión multicentro en el mundo real, sea una tarea relevante.
MétodosSe efectuó una revisión retrospectiva de los registros de 241 casos de pacientes atendidos en 20 hospitales en México para evaluar el tratamiento contra la hepatitis C con la combinación SOF/VEL (n = 231) y sofosbuvir/velpatasvir/ribavirin (SOF/VEL/RBV) (n = 10). El objetivo de eficacia primario fue el porcentaje de pacientes que lograron la RVS 12 semanas posterior a la finalización del tratamiento.
ResultadosEn general, la RVS fue de 98.8% (IC 95% 97.35 a 100%). Solo tres pacientes no lograron la RVS, de los cuales dos padecían cirrosis y una tenía historia previa de tratamiento con interferón pegilado (peg-IFN). De los subgrupos analizados, todos los casos con infección de virus de la inmunodeficiencia humana (VIH), tres con genotipo 3 y aquellos tratados con la combinación SOF/VEL/RBV, lograron RVS. Los subgrupos con tasas menores de éxito fueron los pacientes que tenían experiencia con tratamiento (96.8%) y pacientes con fibrosis F1 (95.5%). Los eventos adversos más frecuentes fueron fatiga, cefalea e insomnio. No se reportaron eventos adversos graves.
ConclusiónLos tratamientos con SOF/VEL y SOF/VEL/RBV fueron altamente seguros y efectivos y los resultados coinciden con los de otros estudios internacionales realizados en el mundo real.
Sofosbuvir (SOF) is a nucleotide analogue inhibitor of hepatitis C virus (HCV) NS5B polymerase. Velpatasvir (VEL) is a second-generation HCV NS5A inhibitor. The SOF/VEL combination has demonstrated high rates of sustained virologic response (SVR) in patients with diverse characteristics, with or without compensated cirrhosis, and has been shown to be pangenotypic and panfibrotic, as well as having a high barrier to resistance.1,2
There is also clinical evidence for recommending the use of SOF/VEL combination therapy in the treatment of HCV caused by all six genotypes, as confirmed in the ASTRAL-I,1 ASTRAL-2,3 and ASTRAL-33 phase III studies. Furthermore, there are real-world studies that have yielded good data on that pangenotypic combination.
The primary goal of hepatitis C treatment is to achieve an SVR at post-treatment week 12. SVR achievement at weeks 12 and 24 are indicators that the disease has been cured, since the results at both intervals have >99% agreement.4 In previously published studies with real-world data in patients treated with different direct-acting antiviral (DAA) agents, with or without ribavirin, high rates of SVR, between 86.4% and 99%, were achieved.5–7
The SOF/VEL combination was one of the first authorized therapies made available in public hospitals in Mexico, which is why registering real-world results is relevant. The aim of the present study was to evaluate the SVR in Mexican patients with HCV, 12 weeks after real-world treatment with SOF/VEL, in a multicenter setting.
Materials and methodsThe present retrospective review was carried out using data from patient records from 20 hospitals located in different cities in Mexico. All patients included had been diagnosed with HCV infection, and regardless of genotype and fibrosis stage, were treated with SOF/VEL or sofosbuvir/velpatasvir/ribavirin (SOF/VEL/RBV), for at least 12 weeks. Records with incomplete data or no assessment of SVR 12 weeks after treatment completion were excluded from the analysis.
The primary efficacy endpoint was the percentage of patients that achieved SVR at posttreatment week 12 (SVR12). The patients were divided into two groups for the analysis: treatment-experienced and treatment-naïve cases. Patient subgroups were evaluated according to their clinical characteristics (HCV genotype, fibrosis stage, presence of cirrhosis, human immunodeficiency virus [HIV] infection, and the DAA treatment received). The secondary endpoint was the percentage of patients that presented with adverse events.
Statistical analysisThe statistical analyses were performed using R 3.01. The chi-square test was employed for the categorical variables and the Kruskal-Wallis test for the continuous variables.
Ethical considerationsThe present research had a retrospective design, and so no type of experimentation was carried out on animals or humans. Given that no new molecules were involved, informed consent was not required for drug administration. The variables of each case were reviewed and reported, and patient data privacy was protected at all times. No personal patient data appear in the study, and due to the retrospective review of the medical records, authorization by an ethics committee was not required.
ResultsA total of 241 records were selected from nearly 300 records reviewed. Of those patients, 62 were treatment-experienced and 179 were treatment-naïve. Patient demographics and disease characteristics at baseline were balanced between groups and are summarized in Table 1.
Patient demographics and baseline characteristics.
| Treatment-experienced | Treatment-naïve | All | p value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age [median] | 62.5 | (range) 29 - 80 | 62 | (range) 18 - 85 | 62 | (range) 18 - 85 | 0.418 |
| Sex | |||||||
| Male | 22 | 35.5% | 54 | 30.2% | 76 | 31.5% | 0.438 |
| Female | 40 | 64.5% | 125 | 69.8% | 165 | 68.5% | |
| Genotype | |||||||
| 1 | 37 | 61.7% | 106 | 58.6% | 143 | 59.3% | 0.614 |
| 2 | 22 | 36.7% | 66 | 36.5% | 88 | 36.5% | |
| 3 | 1 | 1.7% | 9 | 5.0% | 10 | 4.1% | |
| Fibrosis (stage) | |||||||
| 0 | 9 | 15.0% | 15 | 8.3% | 24 | 10.0% | 0.224 |
| 1 | 3 | 5.0% | 19 | 10.5% | 22 | 9.1% | |
| 2 | 7 | 11.7% | 28 | 15.5% | 35 | 14.5% | |
| 3 | 3 | 5.0% | 18 | 9.9% | 21 | 8.7% | |
| 4 | 38 | 63.3% | 101 | 55.8% | 139 | 57.7% | |
| Previous Treatment | |||||||
| None | 0 | 0.0% | 181 | 100.0% | 181 | 75.1% | 0.000 |
| Peg-IFN | 54 | 90.0% | 0 | 0.0% | 54 | 22.4% | |
| Peg-IFN, RBV | 3 | 5.0% | 0 | 0.0% | 3 | 1.2% | |
| Peg-IFN, RBV, boceprevir | 2 | 3.3% | 0 | 0.0% | 2 | 0.8% | |
| Simeprevir | 1 | 1.7% | 0 | 0.0% | 1 | 0.4% | |
| Treatment Group | |||||||
| SOF/VEL | 57 | 95.0% | 174 | 96.1% | 231 | 95.9% | 0.720 |
| SOF/VEL/RBV | 3 | 5.0% | 7 | 3.9% | 10 | 4.1% | |
| Weeks of treatment | |||||||
| 12 weeks | 56 | 93.3% | 174 | 96.1% | 230 | 95.4% | 0.410 |
| 16 weeks | 1 | 1.7% | 0 | 0.0% | 1 | 0.4% | |
| 20 weeks | 0 | 0.0% | 1 | 0.6% | 1 | 0.4% | |
| 24 weeks | 3 | 5.0% | 6 | 3.3% | 9 | 3.7% | |
| Concomitant therapy | |||||||
| Yes | 23 | 38.3% | 79 | 43.6% | 102 | 42.3% | 0.470 |
| No | 37 | 61.7% | 102 | 56.4% | 139 | 57.7% | |
| CHILD-PUGH class | |||||||
| A | 26 | 68.4% | 71 | 71.7% | 97 | 70.8% | 0.930 |
| B | 11 | 28.9% | 25 | 25.3% | 36 | 26.3% | |
| C | 1 | 2.6% | 3 | 3.0% | 4 | 2.9% | |
| MELD score | |||||||
| < 14 | 38 | 100% | 91 | 91% | 129 | 93.5% | 0.105 |
| 14−24 | 0 | 0% | 9 | 9% | 9 | 6.5% | |
MELD: model for end-stage liver disease; Peg-INF: pegylated interferon; RBV: ribavirin; SOF/VEL: sofosbuvir/velpatasvir; SOF/VEL/RBV: sofosbuvir/velpatasvir/ribavirin.
Of the total of 241 cases, 230 patients (95.4%) were treated for 12 weeks, one patient was treated for 16 weeks, one patient was treated for 20 weeks, and 9 patients were treated for 24 weeks. Treatment duration was determined by the physician. At 12 weeks, after treatment was completed, 238 patients achieved SVR. The overall SVR achieved for the study patients was 98.8% (95% CI 97.35–100%, p < 0.00001). SVR was achieved in 99.4% of the treatment-naïve patients and 96.7% of the treatment-experienced patients. All patients with HIV, the patients that stopped therapy, and the patients treated with the SOF/VEL/RBV combination achieved SVR. Table 2 summarizes the SVR results. No statistically significant differences were observed between the groups analyzed.
Sustained virologic response.
| Characteristic | Treatment-experienced | Treatment-naïve | All | p value | Test | |||
|---|---|---|---|---|---|---|---|---|
| n/median | %/range | n/median | %/range | n/median | %/range | |||
| Pre-treament viral load | 2.993 × 105 | 9.7 × 101–1. 8 × 107 | 7.670 × 105 | 1.37–1.5 × 108 | 6 × 105 | 2 × 106–1.5 × 108 | 0.539 | Kruskal-Wallis |
| End of treatment viral load* | 0 | 0–4.7 × 105 | 0 | 0–9.9 × 104 | 0 | 0 –4.7 × 105 | 0.093 | Kruskal-Wallis |
| SVR* | 0 | 0–5.6 × 104 | 0 | 0–4. 8 × 104 | 0 | 0–5.6 × 104 | 0.754 | Kruskal-Wallis |
| NO | 2 | 3.3% | 1 | 0.6% | 3 | 1.2% | 0.149 | χ2 |
| YES | 58 | 96.7% | 180 | 99.4% | 238 | 98.8% | ||
| Suspended therapy | ||||||||
| Yes | 0 | 0.0% | 2 | 1.1% | 2 | 0.8% | 1 | χ2 |
| No | 62 | 100.0% | 177 | 98.9% | 239 | 99.2% | ||
SVR: sustained virologic response.
In the sub-analysis of the 137 patients with cirrhosis (F4), 97 (70%) were classified as Child-Pugh A, 36 (26.3%) as Child-Pugh B, and 4 patients (2.9%) as Child-Pugh C, signifying that the majority (70%) of patients had compensated cirrhosis and 30% had decompensated cirrhosis at the time of the decision to treat them. The SVR in the cirrhosis group was 98.6%. Once in treatment, any patient with compensated cirrhosis was reported as decompensated. SOF plus VEL is a well-known panfibrotic treatment that can be used in decompensated cirrhosis.
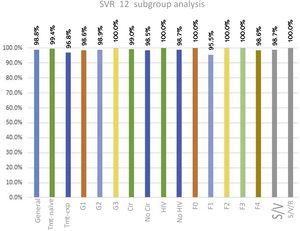
Three patients did not achieve SVR. Two of those patients had cirrhosis (Child-Pugh A, MELD score 8, and fibrosis stage 4) and the third patient did not present with cirrhosis but had previously been treated with peg-IFN. Two of those patients had genotype 1 and the third patient had genotype 2. Fig. 1 shows the response rates by patient subgroup, overall response, genotype, presence or absence of cirrhosis, HIV status, treatment-naïve cases, treatment-experienced cases, and fibrosis stage.
Sustained virologic response, both overall and by subgroup, at 12 weeks.
Cir: Cirrhosis; No Cir: No cirrhosis; HIV: human immunodeficiency virus; S/V: sofosbuvir/velpatasvir; S/V/R: sofosbuvir/velpatasvir/ribavirin; SVR: sustained virologic response; Tmt-exp: treatment-experienced; Tmt-naïve: treatment naïve.
During the treatment period, no serious adverse events were reported, confirming the good safety profile of the treatment. Adverse events were reported in 21.6% of the patients that were treated. The most frequent adverse events, in at least 5% of the population analyzed, were fatigue, headache, and insomnia. Table 3 shows all adverse events reported in each group and in the entire study population.
| Characteristic | Treatment-experienced | Treatment-naïve | All | |||
|---|---|---|---|---|---|---|
| 13 of 60 | 39 of 181 | 52 of 241 | ||||
| n | % | n | % | n | % | |
| Serious adverse events | 0 | 0% | 0 | 0% | 0 | 0% |
| Fatigue | 14 | 23.3% | 37 | 20.4% | 51 | 21.2% |
| Headache | 8 | 13.3% | 34 | 18.8% | 42 | 17.4% |
| Insomnia | 3 | 5.0% | 10 | 5.5% | 13 | 5.4% |
| Nausea | 3 | 5.0% | 3 | 1.7% | 6 | 2.5% |
| Abdominal distention | 3 | 5.0% | 0 | 0.0% | 3 | 1.2% |
| Myalgia/arthralgia | 1 | 1.7% | 1 | 0.6% | 2 | 0.8% |
| Colic | 1 | 1.7% | 0 | 0.0% | 1 | 0.4% |
| Hyporexia | 1 | 1.7% | 0 | 0.0% | 1 | 0.4% |
| Cough | 0 | 0.0% | 1 | 0.6% | 1 | 0.4% |
| Heartburn | 1 | 1.7% | 0 | 0.0% | 1 | 0.4% |
| Anxiety | 1 | 1.7% | 0 | 0.0% | 1 | 0.4% |
| Diarrhea | 0 | 0.0% | 1 | 0.6% | 1 | 0.4% |
The data analyzed in the present study were obtained from the clinical medical records of 241 patients infected with HCV that received care at 20 hospitals in Mexico. They were treated with SOF/VEL, the first pangenotypic combination authorized and available in Mexico. RBV was added to the treatment of 10 patients.
Most of the patients had HCV genotypes 1 and 2. Only 10 of them (4.1%) had genotype 3, and so the results detected in that subgroup should be analyzed with caution.
The SOF/VEL combination in patients with HCV proved to be highly effective in all the cases, even in the subgroups evaluated. Of the 241 patients treated, only 3 did not achieve SVR, resulting in a 98.76% rate (95% CI 97.35–100%, p < 0.00001). The results of the current study confirm the high rate of SVR achieved with SOF/VEL treatment.
In the real-world data study conducted by Chirino et al.,5 96% of 81 patients with a predominance of genotype 1b (70.4%), treated with diverse second-wave DAAs, achieved SVR. The overall SVR rate in the present study was higher, but the proportion of patients with genotype 1 was slightly lower.
Wyles et al.8 studied patients coinfected with HCV and HIV. The overall SVR results indicated that 95% (95% CI 89–99%) of patients achieved SVR, whereas patients with genotypes 1, 2, 3, and 4 achieved SVR in 95% (87–99%), 100% (72–100%), 92% (62–100%), and 100% (48–100%) of the cases, respectively. In the current analysis, all patients with HCV and HIV coinfection achieved SVR12.
One of the limitations of the present study was the fact that when the data were collected for the same patient characteristic, some of the subgroups were small, making it difficult to draw solid conclusions. For example, only 10 patients with genotype 3 were analyzed, and all of them achieved SVR. However, genotype 3 has been reported to have a less favorable response to any of the treatments, even to last-generation DAAs. That could also be true for the subgroup of 22 patients with low fibrosis (F1). They had a slightly lower SVR response (95.5%), compared with the 139 patients with advanced fibrosis/cirrhosis (F4) that achieved an SVR rate of 98.6%. In most studies, patients with increased fibrosis are expected to have a lower response rate than patients with mild liver damage.
Considering those results, the WHO strategy for the elimination of HCV by the year 2030 should be mentioned. The goals are to reduce the number of new cases by 90% and to reduce mortality by 65%, given that hepatitis C can cause cirrhosis and hepatocellular carcinoma, leading to death. Two of the most relevant strategy goals are to diagnose 90% of infected persons and link them to medical care, with 80% receiving treatment.9,10
To achieve the elimination of HCV, the strategy for treating that disease must focus on high-risk groups and sectors. Timely intervention in those groups could result in a substantial reduction in the number of infected persons in other populations. Within that context, the oral administration of pangenotypic and panfibrotic SOF/VEL for a period of 12 weeks is an invaluable tool for achieving the WHO goals by 2030, due to its high SVR in eliminating the disease in high-risk groups, regardless of HCV genotype, as well as in patients with or without cirrhosis, and even in patients with decompensated cirrhosis.
ConclusionsThe overall SVR obtained in the present retrospective review concurs with the results from randomized clinical trials and from real-world studies conducted at different locations and in diverse populations. No significant differences were identified in the demographic characteristics of the groups analyzed or in the general results of SVR and safety. The SOF/VEL and SOF/VEL/RBV treatments were shown to be highly effective and very safe.
Financial disclosureGilead Sciences Mexico supported this publication with a grant for editorial services. Gilead Sciences Mexico did not participate in the retrospective data collection, study development, or the writing of this article.
Conflict of interestThe authors J.A. Velarde Ruiz Velasco, G. Castro Narro, and F. Higuera De La Tijera have been speakers for Gilead. J.L. Pérez Hernández has received support from Gilead to attend medical congresses. The rest of the authors have no conflicts of interest to declare.
We received support from the medical writer Joaquín Herrera Rojas and the statistician Wayra Paz Ballesteros, both independent consultants, for the publication of this study.
Please cite this article as: Pérez-Hernández JL, Arce-Salinas CA, Lehmann-Mendoza R, Torre-Delgadillo A, Castro-Narro GE, Cerda-Reyes E, et al. Sofosbuvir-velpatasvir en pacientes mexicanos con hepatitis C: una revisión retrospectiva. Revista de Gastroenterología de México. 2022;87:52–58.