Hepatocellular carcinoma (HCC) is a primary malignant tumor of liver epithelial cells and is the most frequent primary liver cancer. The broadening of transplantation and resectability criteria has made therapeutic decisions more complex. Our aim was to describe the clinical and survival characteristics of patients with HCC treated through resection or liver transplantation at our hospital and identify the presence of factors that enable outcome prediction and facilitate therapeutic decision-making.
Materials and methodsPatients with HCC that underwent surgery with curative intent at the Hospital Universitario Marqués de Valdecilla, within the time frame of 2007 and 2017, were retrospectively identified. Survival, mortality, disease-free interval, and different outcome-related variables were analyzed.
ResultsNinety-six patients with a mean follow-up after surgery of 44 months were included. Overall mortality and recurrence were higher in the resection group. Mean survival was 51.4 months in the liver transplantation group and 37.5 months in the resection group, and the disease-free interval was 49.4 ± 37.2 and 27.4 ± 28.7 months, respectively (p = 0.002). The tumor burden score was statistically significant regarding risk for recurrence and specific mortality.
ConclusionsThere appears to be no patient subgroup in whom the results of surgical resection were superior or comparable to those of transplantation. Tumor burden determination could be a useful tool for patient subclassification and help guide therapeutic decision-making.
El carcinoma hepatocelular (CHC) es una neoplasia maligna primaria de células epiteliales hepáticas que constituye la neoplasia primaria hepática más frecuente. La ampliación de criterios de trasplante y resecabilidad han hecho más complejas las decisiones terapéuticas. Nos proponemos describir las características clínicas y supervivencia de los pacientes con CHC tratados mediante resección (RH) o trasplante hepático (THO) en nuestro Hospital, e identificar la presencia de factores que permitan predecir el pronóstico y facilitar las decisiones terapéuticas.
Material y métodosSe identificaron retrospectivamente los pacientes con CHC intervenidos con intención curativa en el Hospital Universitario Marqués de Valdecilla entre 2007 y 2017. Se analizaron la supervivencia, mortalidad, intervalo libre de enfermedad, así como distintas variables relacionadas con el pronóstico.
ResultadosSe registraron 96 pacientes con un seguimiento medio tras cirugía de 44 meses. La mortalidad global y la recidiva fueron superiores en el grupo de resección. La supervivencia media fue de 51.4 meses en el grupo de TOH y de 37.5 meses en el de resección y el tiempo libre de enfermedad alcanzó los 49.4 ± 37.2 y 27.4 ± 28.7 meses, respectivamente (p: 0.002). La carga tumoral, valorada a través del “tumor burden score”, presentó una relación significativa con el riesgo de recidiva y la mortalidad específica.
ConclusionesNo parece existir un subgrupo de pacientes en los que la resección quirúrgica presente resultados de supervivencia superiores o equiparables al trasplante. La carga tumoral podría ser una herramienta útil para subclasificar y ayudar a guiar las decisiones terapéuticas.
Hepatocellular carcinoma (HCC) is a primary malignant tumor of liver epithelial cells. It is the most frequent primary liver cancer and one of the most common causes of death in patients presenting with cirrhosis of the liver.1
Patients whose tumors are diagnosed at initial stages can benefit from radical treatments with curative intent that improve their survival. Such treatments are liver resection (LR), orthotopic liver transplantation (OLT), and percutaneous tumor ablation.2 Optimum candidate selection is crucial for limiting surgical morbidity and mortality.
OLT is the best curative option for patients with decompensated cirrhosis and HCC. The Milan criteria are widely validated and enable the selection of candidates for receiving an OLT.1–3 However, despite the fact that OLT is conceptually the oncologic treatment of choice in patients with HCC, its applicability is limited due to its potential impact on transplant waiting lists.4
Therefore, in recent years, new recommendations for indicating surgical treatments have been made. The 2018 clinical guidelines for HCC management from the European Association for the Study of the Liver (EASL) propose broadening the surgical resection criteria and evaluating their possible benefit in the clinical evolution of those patients.1 The fact that patients that slightly exceed the conventional Milan cirteria3 can also be candidates for OLT has been emphasized, as long as the broadening of the classic criteria does not significantly limit the access to transplantation of patients with indications other than HCC.4–6 On the other hand, the emergence of new therapeutic targets, fundamentally in systemic treatment, has greatly complicated therapeutic decision-making. One such novel concept is treatment stage migration (TSM), in which patients initially presenting with a lower stage of a disease that has a poor prognosis opt for treatments indicated for more advanced stages. As a result, there is an awakening interest in identifying factors that can help subclassify patients, ways to optimize tumor burden measurement, and factors that help improve outcome prediction.4–8
Thus, the aim of our study was to describe the clinical and survival characteristics in patients with HCC that were treated with curative intent through LR or OLT at our hospital. In addition, we endeavored to determine which factors influence survival or tumor recurrence, to aid in carrying out a more precise selection of patients with HCC that could benefit from treatment through surgical resection, compared with OLT.
Materials and methodsStudy design and inclusion criteriaWe conducted a retrospective observational study on patients diagnosed with HCC that underwent LR or OLT, within the time frame of January 2007 and December 2017, at the General Surgery Department of the Hospital Universitario Marqués de Valdecilla (HUMV), Santander, Spain. Patients transplanted for a different reason, in whom HCC was incidentally detected in the explanted organ, were excluded from the study, as well as patients with a preoperative diagnosis of HCC that underwent LR or OLT but whose diagnosis was not confirmed by anatomopathologic study of the surgical specimen or in whom a tumor of a different cellular strain was found.
The criteria for deciding the type of treatment in each patient (LR vs. OLT) were based on internationally accepted guidelines and recommendations.1–3,9,10 In general, the candidates for LR were patients with no cirrhosis or with one or 2 tumor lesions in a cirrhotic liver, with no signs of vascular invasion or extrahepatic spread, and that had no liver dysfunction (model end-stage liver disease [MELD] score below 12, Barcelona Clinic Liver Cancer [BCLC] very early stage or early stage 0/A classification), or clinically relevant portal hypertension (PHT)(thrombocytopenia below 100,000 μl and a pressure gradient above 10 mmHg). OLT was the first-choice treatment for the patients with HCC that met the Milan criteria (one nodule ≤5 cm or up to 3 nodules ≤ than 3 cm, in the absence of vascular invasion or extrahepatic disease) and were not indicated for LR.1–4,9,10 The therapeutic decision was made by a multidisciplinary committee made up of surgeons, gastroenterologists, anesthetists, radiologists, etc.
Study variablesThe demographic characteristics of the study population, the presence of comorbidities, and the laboratory test results were evaluated. The etiology of the liver disease was also collected and the presence or absence of PHT, clinically or through portal hemodynamics (pressure ≥ 5 mmHg). Surgical risk was evaluated through the ASA classification and the physiologic POSSUM scoring system.11
The registered preoperative HCC-related variables were the imaging test that enabled the diagnosis to be made, lesion number and size, and the presence or absence of vascular invasion. Tumor burden was evaluated through the data provided by the imaging tests, through the tumor burden score (TBS), following the formula described by Sasaki et al. in 2018.12 Liver function was evaluated through the Child-Pugh classification and MELD score. The BCLC classification was utilized for tumor staging.8,9
The perioperative variables, including mortality, were registered according to the Clavien–Dindo classification13 and the data from the histologic study of the surgical specimens were reviewed.
To evaluate patient evolution, follow-up time, the presence of tumor recurrence, overall mortality, and tumor-related mortality were analyzed. Overall survival and specific survival in months and the disease-free interval (DFI) were also registered.
Statistical analysisThe quantitative variables were expressed as mean ± standard deviation (SD), median, and interquartile range, as appropriate.
Before performing the inferential statistical analysis, the Kolmogorov-Smirnov test was utilized to determine whether the continuous variables had a normal distribution. The Student’s t test for independent samples, with the Welch correction for unequal variances, and the Wilcoxon test were employed for statistical comparisons. The categorical variables were expressed as percentages and compared, utilizing the chi-square test or the Fisher’s exact test, if the expected frequencies hypothesis was verified. The Pearson’s r or the Spearman’s rho were used for the correlation analysis.
The survival analysis was carried out using the Kaplan–Meier test and the log-rank test. The automatic forward stepwise Cox regression model was used for the multivariate analysis.
The discriminative ability of the different variables was calculated through the area under the ROC curve (AUROC) and its 95% confidence interval (CI).
The level of significance was 0.05, with a 95% CI.
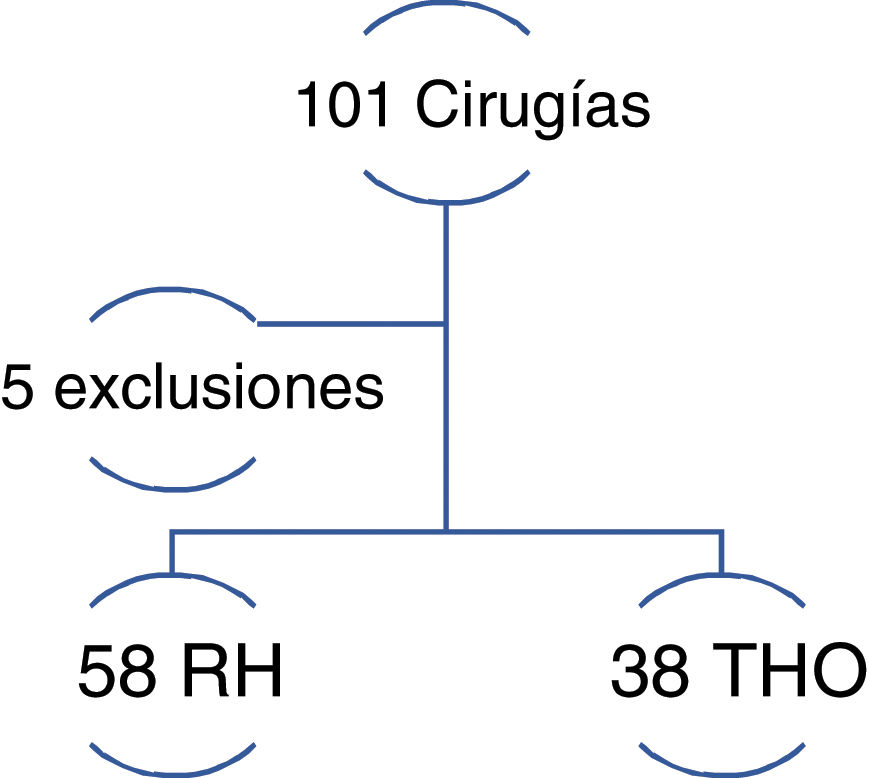
ResultsThe final sample was made up of 96 patients with HCC; 58 of them underwent LR and 38 underwent OLT (Fig. 1).
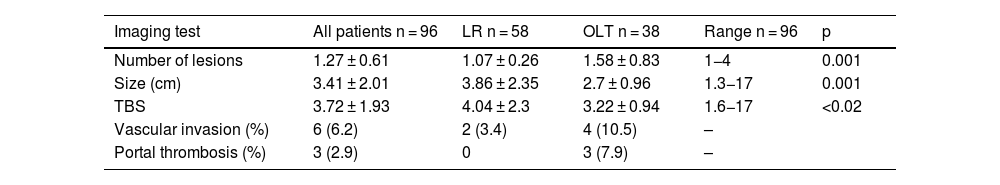
Table 1 shows the clinical and demographic characteristics of the study patients.
Baseline characteristics of the patients studied.
| All patients (n = 96) | LR (n = 58) | OLT (n = 38) | Range (n = 96) | P (LT vs. OLT) | |
|---|---|---|---|---|---|
| Age | 62.3 ± 8.4 | 64.3 ± 8.8 | 59.2 ± 6.7 | 40−79 | 0.003 |
| Sex (men) | 88 (91.7%) | 53 (91.4%) | 35 (92.1%) | – | NS |
| Diabetes mellitus | 30 (31.3%) | 30 (31.3%) | 9 (23.6%) | – | NS |
| Charlson comorbidity index | 6.3 ± 1.8 | 6.3 ± 2.1 | 6.3 ± 1.4 | 2−15 | NS |
| Neutrophils (103/µl) | 3.53 ± 1.83 | 3.64 ± 1.48 | 3.37 ± 2.28 | 1.10−14.90 | NS |
| Lymphocytes (103/µl) | 1.74 ± 0.86 | 1.99 ± 0.82 | 1.37 ± 0.79 | 0.3−5 | 0.001 |
| Platelets (103/µl) | 147 ± 67 | 175.81 ± 56.85 | 103 ± 58 | 30−294 | 0.001 |
| NLR | 1.97 ± 1.79 | 1.74 ± 1.74 | 2.32 ± 1.83 | 0−11 | NS |
| PLR | 96.2 ± 79.4 | 108.1 ± 94.8 | 83.3 ± 45 | 21−270 | NS |
| Prothrombin activity (%) | 77.9 ± 16.4 | 84.79 ± 14.05 | 67.5 ± 14 | 33.1−107.2 | NS |
| Sodium (mEq/l) | 140.5 ± 2.7 | 141.3 ± 2.4 | 139.4 ± 2.8 | 134−146 | 0.01 |
| ALT (IU/l) | 48.7 ± 40.9 | 50.7 ± 47.45 | 45.8 ± 29.1 | 4−231 | NS |
| AST (IU/l) | 52.1 ± 38.9 | 46.7 ± 32.1 | 58.5 ± 45.3 | 15−229 | NS |
| GGT (IU/l) | 119.8 ± 115.8 | 122.2 ± 122.3 | 116.9 ± 109.2 | 13−586 | NS |
| ALP (IU/l) | 120.7 ± 58.7 | 106.8 ± 50.9 | 141.6 ± 63.9 | 37−302 | 0.005 |
| Bilirubin (mg/dl) | 1.5 ± 1.7 | 0.9 ± 0.7 | 2.1 ± 2.3 | 0.3−12.9 | 0.007 |
| Albumin (g/dl) | 4.0 ± 0.5 | 4.24 ± 0.36 | 3.72 ± 0.6 | 2.6−5 | 0.001 |
| AFP (ng/mL) | 93.6 ± 408 | 142.2 ± 515.6 | 16.72 ± 55.2 | 0.9−3158 | NS |
| PHT | 50 (52.1%) | 22 (40%) | 28 (84.8%) | – | 0.001 |
| MELD | 9.71 ± 3.87 | 8.35 ± 2.64 | 11.58 ± 4.5 | – | 0.001 |
| Child A/B/C (%) | 68.8/22.9/2.1 | 88.5/9.6/1.9 | 52.6/44.7/2.6 | – | 0.001 |
| BCLC 0/A/B (%) | 10.4/88.5/1 | 10.3/89.6/0 | 10.5/86.8/2.6 | – | NS |
| POSSUM | 15.5 ± 3.7 | 15.2 ± 4.6 | 15.4 ± 2.9 | 12−30 | NS |
| ASA | 2.66 ± 0.66 | 2.38 ± 0.52 | 3.08 ± 0.63 | 1−4 | 0.001 |
| CNB | 20 (20.8%) | 16 (27.6%) | 4 (10.5%) | – |
AFP: alpha-fetoprotein; ALP: alkaline phosphatase; ALT: alanine aminotransferase; ASA: American Society of Anesthesiologists scale; AST: aspartate aminotransferase; BCLC 0/A/B: Barcelona Clinic Liver Cancer classification 0/A/B; Child A/B/C: Child-Pugh classification in class A, B, or C; CNB: core needle biopsy of the liver before the intervention; GGT: gamma glutamyl transpeptidase; LR: liver resection; MELD: model for end-stage liver disease; NLR: neutrophil-to- lymphocyte ratio; NS: nonsignificant; OLT: orthotopic liver transplantation; PHT: portal hypertension; PLR: platelet-to-lymphocyte ratio; POSSUM: Portsmouth Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity.
The majority of the patients were men (91.7%) and the mean patient age was above 60 years. The patients that underwent LR were older, albeit their functional situation was more favorable. Patients that underwent LR had a better MELD score and a lower prevalence of PHT. Nevertheless, the BCLC staging classification showed no significant differences.
The ASA classification score was higher in the transplanted patients than in those that underwent resection. No differences were found through the POSSUM scoring system.
Computed axial tomography (CAT) was the imaging test that enabled the diagnosis to be made in the majority of cases. Diagnostic liver biopsy had to be performed on 20 patients.
All the transplanted patients presented with cirrhosis, whereas 10% of the patients that underwent LR did not present with the disease. Alcoholism was the most frequent etiology of liver disease, at about 30%, followed by hepatitis C virus (HCV) infection (20%). Likewise, approximately 20% of the patients presented with several causes of liver disease.
Table 2 shows the characteristics of the lesions detected in the imaging tests and the histologic study performed after the surgical intervention.
Characteristics of the neoplastic lesions in the imaging test and the histologic study.
| Imaging test | All patients n = 96 | LR n = 58 | OLT n = 38 | Range n = 96 | p |
|---|---|---|---|---|---|
| Number of lesions | 1.27 ± 0.61 | 1.07 ± 0.26 | 1.58 ± 0.83 | 1−4 | 0.001 |
| Size (cm) | 3.41 ± 2.01 | 3.86 ± 2.35 | 2.7 ± 0.96 | 1.3−17 | 0.001 |
| TBS | 3.72 ± 1.93 | 4.04 ± 2.3 | 3.22 ± 0.94 | 1.6−17 | <0.02 |
| Vascular invasion (%) | 6 (6.2) | 2 (3.4) | 4 (10.5) | – | |
| Portal thrombosis (%) | 3 (2.9) | 0 | 3 (7.9) | – |
| Histologic study | LR n = 58 | OLT n = 38 |
|---|---|---|
| Number of lesions | 1.10 ± 0.48 | 1.68 ± 1 |
| Size (cm) | 3.77 ± 2.23 | 3.02 ± 1.46 |
| TBS | 3.98 ± 2.19 | 3.57 ± 1.49 |
| Vascular invasion (%) | 16 (28.1) | 3 (8.3) |
| Satellitosis | 4 (6.9) | 3 (8.3) |
LR: liver resection; OLT: orthotopic liver transplantation; TBS: tumor burden score.
In the LR group, the surgical procedure of choice was nonanatomic hepatectomy (50 patients [86.2%]), whereas anatomic hepatectomy was carried out on fewer cases (8 patients [13.8%]).
Mean hospital stay of the transplanted patients was longer than that of the patients that underwent resection (26.8 ± 13.1 days vs. 11.6 ± 12.7 days). The OLT group also required a greater number of transfusions, at 94.7% of the transplanted patients, compared with 15.5% in the LR group.
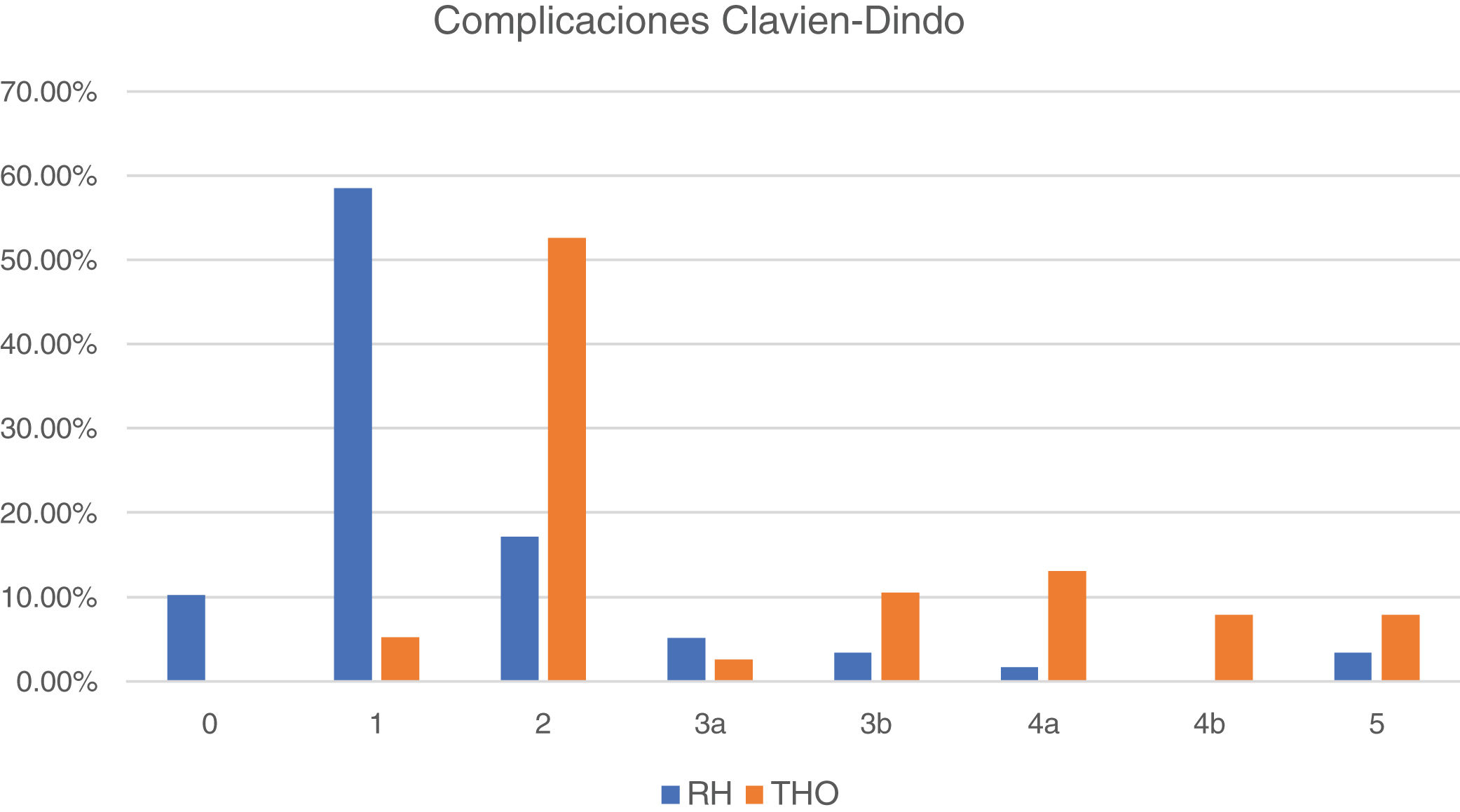
Fig. 2 shows the postoperative morbidity and mortality distribution in the 2 subgroups, according to the Clavien-Dindo classification. The large majority of complications in the patients that underwent resection were mild (classified as 0 and 1), whereas complications were classified as 2 in the majority of patients that underwent OLT and mild in only 5%.
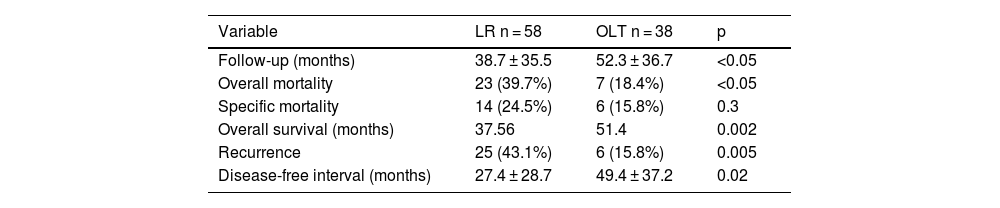
Survival and follow-upIn all the patients from both groups (LR and OLT), median overall survival was 74 months, and the DFI was 106 months. Table 3 summarizes the main data related to patient follow-up. Follow-up time, overall survival, and the DFI were longer in the OLT group, whereas recurrence and overall mortality were higher in the LR group.
Follow-up, survival, and mortality variables.
| Variable | LR n = 58 | OLT n = 38 | p |
|---|---|---|---|
| Follow-up (months) | 38.7 ± 35.5 | 52.3 ± 36.7 | <0.05 |
| Overall mortality | 23 (39.7%) | 7 (18.4%) | <0.05 |
| Specific mortality | 14 (24.5%) | 6 (15.8%) | 0.3 |
| Overall survival (months) | 37.56 | 51.4 | 0.002 |
| Recurrence | 25 (43.1%) | 6 (15.8%) | 0.005 |
| Disease-free interval (months) | 27.4 ± 28.7 | 49.4 ± 37.2 | 0.02 |
LR: liver resection; OLT: orthotopic liver resection.
Ten of the patients that underwent resection ended up having OLT, which was due to the identification of microvascular invasion in the surgical specimen, satellitosis, or to recurrence at follow-up. Those patients were kept in the LR group for the analyses results, carrying out an intention-to-treat analysis.
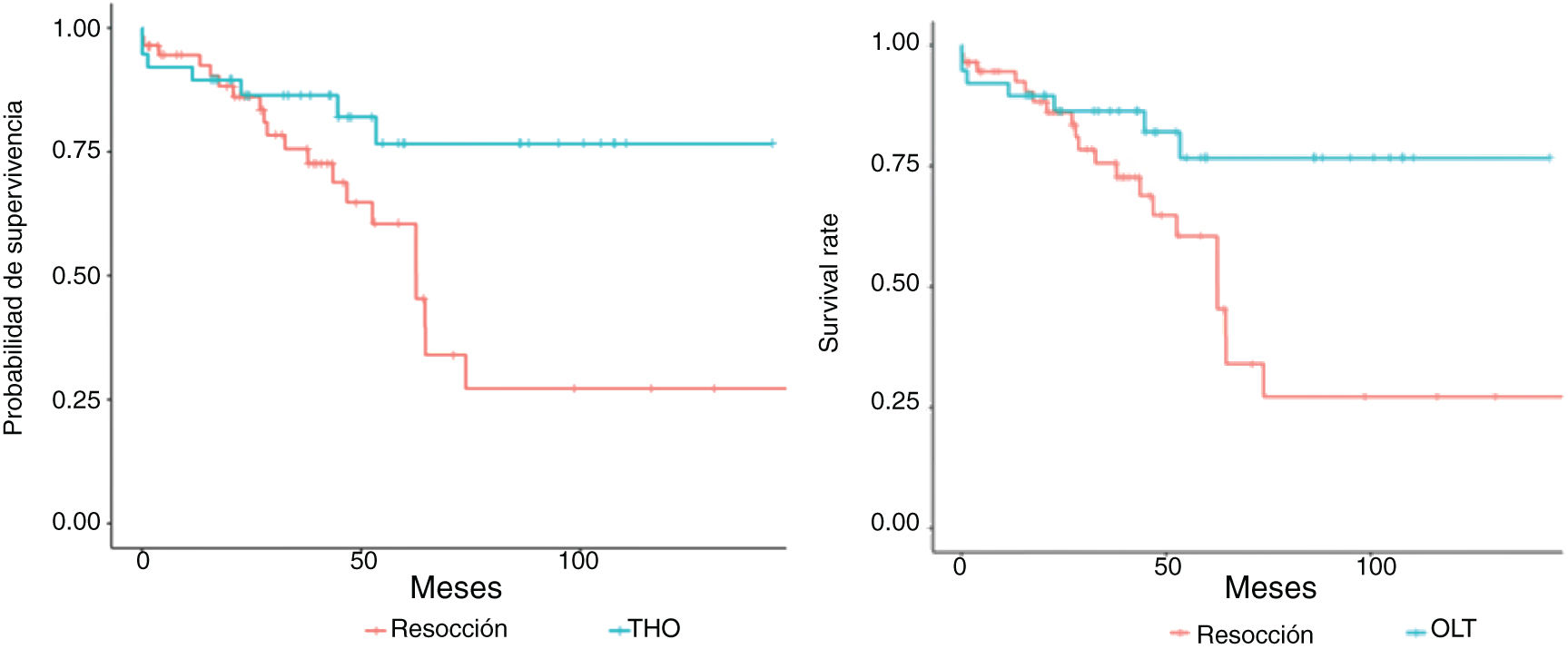
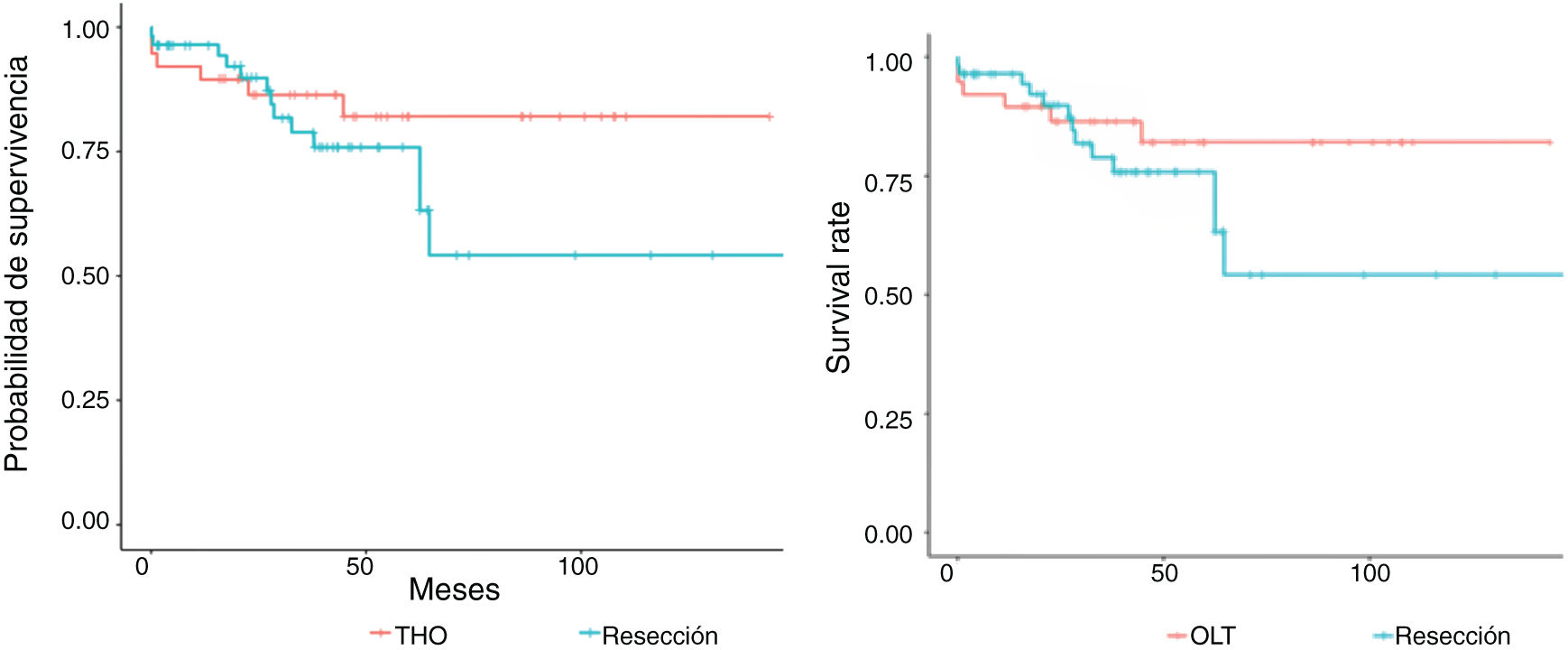
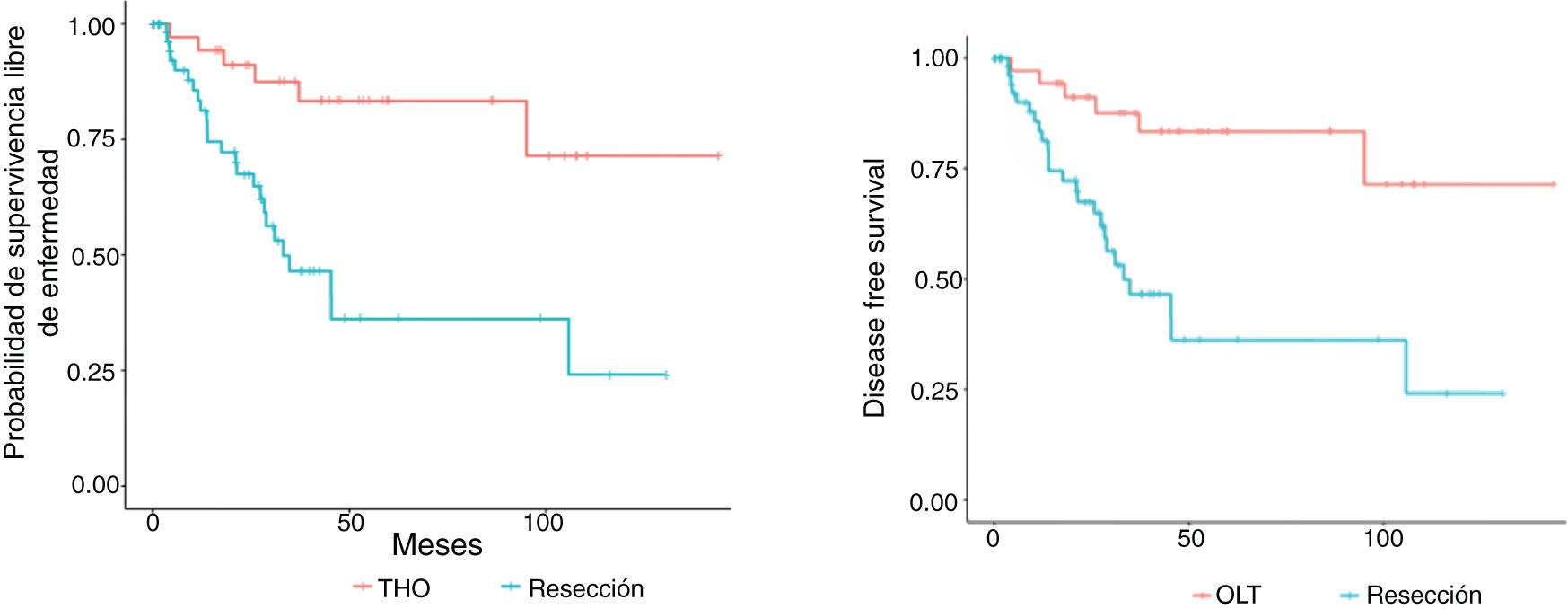
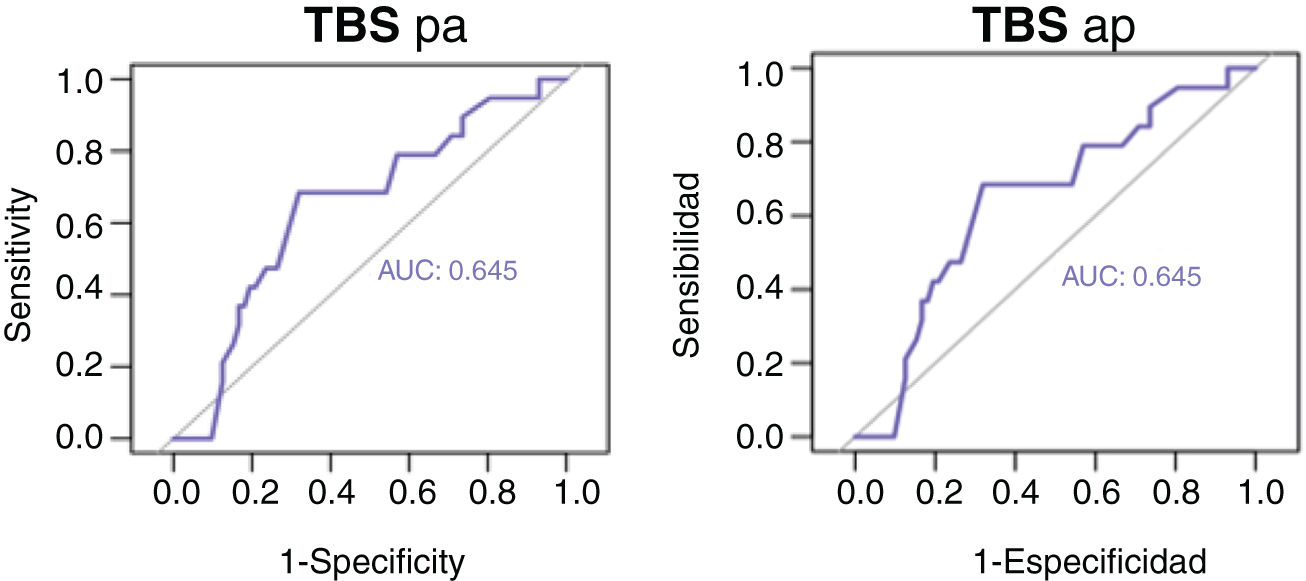
Figs. 3 and 4 show the overall survival and specific survival curves of the two groups and Fig. 5 shows the disease-free survival. The overall survival probability during the first year was about 90% in the two groups, and at 86% in the second year. At the third year, survival reached 75% in the patients that underwent resection and 82% in the transplanted patients (p < 0.03). Specific survival was similar in the LR and OLT groups, reaching 96.5% and 89.5% the first year and 89.8% and 86.4% at 2 years, respectively (p = 0.3).
Lastly, disease-free survival was shorter in the patients with LR, especially at the second and third years, in which the survival probability was 67.5% and 46.5%, compared with 91.1% and 87.5% in the patients with OLT (p = 0.03).
Analysis of the risk factors associated with survivalOverall mortalityThe risk for mortality was double in the univariate analysis of the LR group (HR: 2.68 [1.12–6.33]; p = 0.025), compared with the OLT group. Increased AFP (HR: 1.001 [1–1.002]; p < 0.01) and the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were also associated with a worse outcome (HR: 1.18 [1.01–1.37]; p < 0.05 and HR: 1.001 [1–1.01]; p < 0.05, respectively). In contrast, the presence of PHT (0.44 [0.26−0.94]; p < 0.05) and the number of lesions (HR: 0.26 [0.07−0.98]; p < 0.05) were associated with a better outcome.
When the analysis was restricted to patients with BCLC stage 0, there was no relation between LR or OLT and overall mortality. However, in patients with BCLC stage A, OLT had a favorable influence on survival.
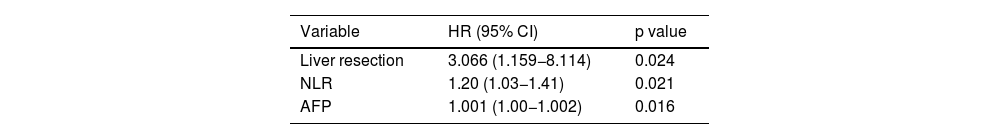
After carrying out the multivariate analysis, only LR, the NLR, and to a lesser degree, AFP levels, were still significant variables (Table 4).
Multivariate analysis of the factors that influenced overall survival in the group of patients studied.
| Variable | HR (95% CI) | p value |
|---|---|---|
| Liver resection | 3.066 (1.159−8.114) | 0.024 |
| NLR | 1.20 (1.03−1.41) | 0.021 |
| AFP | 1.001 (1.00−1.002) | 0.016 |
AFP: alpha-fetoprotein; HR: hazard ratio; NLR: neutrophil-to-lymphocyte ratio.
Upon performing the analyses by subgroup, the patients that underwent resection that presented with worse liver function (Child B and C) and a POSSUM score above 15, the outcome was worse (HR 9.97 [2.24–43.54]; p = 0.002 and HR: 4.51 [1.60–12.71]; p = 0.004, respectively).
In contrast, no variable in the OLT group reached statistical significance.
Specific mortalityThe univariate analysis showed that the presence of diabetes mellitus (DM) (HR 2.72 [1.1–6.71]; p = 0.03), and in the histologic study, lesions >5 cm (HR 2.74 [1.02–7.36]; p < 0.05), worsened patient specific survival.
As occurred with overall survival, the presence of PHT was a protective factor (HR: 0.25 [0.10−0.71]; p < 0.01), which, unlike the other variables, remained unchanged after the multivariate analysis (HR: 0.27 [95% CI 0.10−0.7]; p = 0.008). No type of association between the different variables and specific mortality could be established when the two groups of patients (LR or OLT) were analyzed separately.
Analysis of the risk factors associated with recurrenceIn the univariate analysis, LR (HR: 4.38 [1.79–10.8]; p = 0.001), DM (HR: 3.02 [1.47–6.2]; p = 0.003), radiologic (HR: 1.20 [1.08–1.38]; p < 0.02) and histologic (HR: 1.19 [1.05–1.35]; p < 0.01) tumor size and burden (measured by the TBS), and affected margins seen in the histologic study (HR: 3.69 [1.27–10.7]; p < 0.02) were associated with a greater recurrence probability. There was also a statistically significant and inversely proportional relation between both the preoperative tumor burden (radiologic DFI-TBS, r: −0.386, p < 0.001) and the postoperative tumor burden (pathologic anatomy DFI-TBS, r: −0.298, p = 0.003) to the DFI.
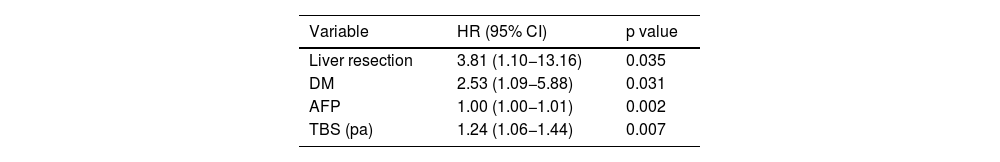
Nevertheless, after the multivariate analysis, only LR, DM, AFP levels, and histologic TBS were associated with recurrence (Table 5).
Multivariate analysis of the factors that influenced overall recurrence in the group of patients studied.
| Variable | HR (95% CI) | p value |
|---|---|---|
| Liver resection | 3.81 (1.10−13.16) | 0.035 |
| DM | 2.53 (1.09−5.88) | 0.031 |
| AFP | 1.00 (1.00−1.01) | 0.002 |
| TBS (pa) | 1.24 (1.06−1.44) | 0.007 |
AFP: alpha-fetoprotein; CI: confidence interval; DM: diabetes mellitus; HR: hazard ration; pa: pathologic anatomy; TBS: tumor burden score.
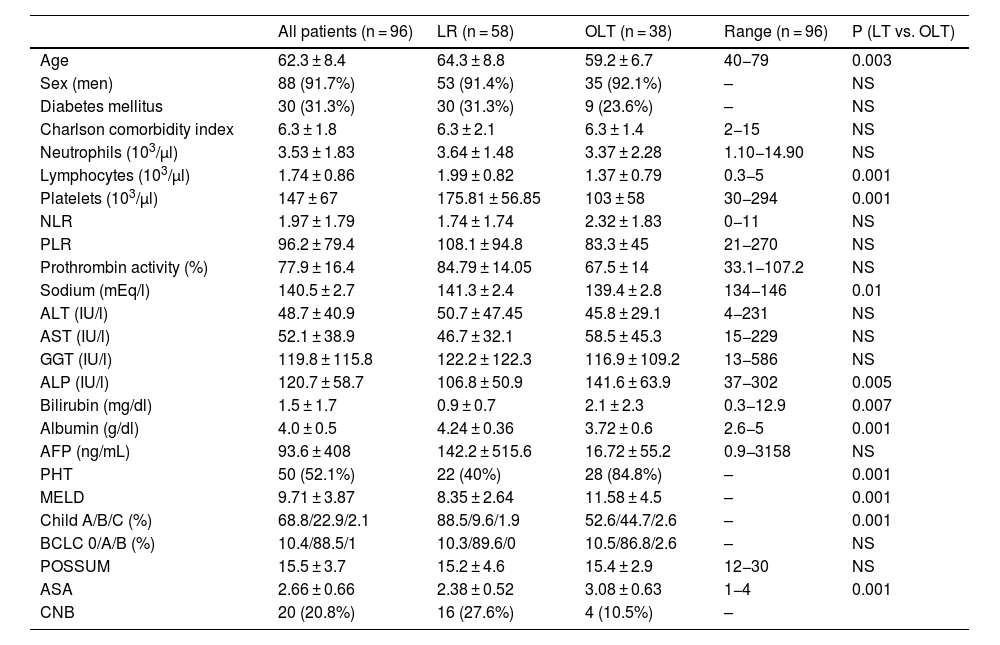
Lastly, the validity of the variables identified in the univariate and multivariate analyses for discriminating overall survival and specific survival, as well as the recurrence probability in the patients of our case series, was evaluated. After analyzing the different ROC curves, only histologic TBS could discriminate specific mortality, with an area under the curve (AUC) of 0.645 (95% CI; 0.508−0.783; p = 0.05), as shown in Fig. 6.
DiscussionThe large majority of patients in our case series were men, and the mean patient age was somewhat above 60 years. More patients underwent resection than transplantation, most likely because age criteria are more demanding with respect to OLT than LR. Our results are similar to those described in other case series, in which the mean age of transplant recipients is between 50 and 64 years,14 and around 58 years15 for LR, albeit the number of men is lower (60–70%).
The patients that underwent LR had a more favorable functional situation and a lower frequency of PHT than those that underwent transplantation. In fact, all the transplanted patients presented with cirrhosis, whereas 10% of the patients that underwent resection did not. That could be related to the higher number of complications and worse survival in the patients that had LR that presented with poor liver functionality and PHT, making OLT the preferred procedure. Moreover, OLT can resolve the existing liver disorder, which is why it is indicated in patients with greater functional deterioration.1–8
The main cause of liver disease in our patients was alcohol, followed by HCV infection, coinciding with the most frequent causes of cirrhosis in our environment.2,5
The patients in the OLT group presented with a higher number of lesions, even though tumor size and burden measured by the TBS were greater in the LR group. That could be due to the fact that, according to the BCLC criteria, the presence of more than one lesion makes OLT the preferable option if the patient meets the Milan criteria, whereas when there is a single lesion, resection can be suggested regardless of the size of the tumor, if there is a sufficient amount of healthy parenchyma.1,2,6
Almost one-third of the patients that underwent resection had histologic signs of microvascular invasion, close to 10% presented with satellitosis, figures similar to those described by other authors.15,16
As was expected, the patients that underwent transplantation had longer hospital stay, a greater need for blood transfusions, and a higher number of severe complications. Three transplanted patients died in the postoperative period (7.8%), whereas two patients died after resection (3.4%). Those data are quite similar to the results of the study by Calatayud et al.,15 in which perioperative mortality was 2.8% and the percentage of severe complications was above 23% in the patients that underwent resection.15,16
Follow-up time, overall survival, and DFI were greater in the transplanted patients, whereas recurrence and overall mortality were higher in the LR group. In fact, the multivariate analysis showed that the patients that underwent LR had a 4-times greater risk for recurrence than the transplanted patients and 3-times the probability of death. But importantly, the survival probability in the first 3 years was very similar between the two treatments (around 80%), with the difference increasing in later years. In addition, there were no significant changes in the specific survival curves, which could be related to the higher recurrence rate in the LR group. Transplantation eliminates micrometastases; resection cannot always remove them, resulting in their progression in the liver remnant, after surgery.17 It could also be related to the lower complication rate associated with the liver disease itself, which in the case of OLT, would no longer be present because the transplantation would have resolved the liver disorder.6 It should also be pointed out that ten of the patients treated through LR (17%) had to undergo transplantation later, because of histologic poor prognosis criteria, which could at least partially explain our findings. At any rate, our data are consistent with those described in the literature, in which median survival is around 3 years, and the survival probability reaches 50–70% of the patients with BCLC stage A at 5 years,1,6 the most frequent stage in our patient sample.
One of our aims in the present study was to determine which factors influenced survival and tumor recurrence, in our patients. In addition to the type of surgical treatment (LR or OLT), the relation between the NLR and AFP levels also appeared to have an influence. In contrast, the number of lesions and the presence of PHT were protective factors. That could be related to the lower number of lesions and the lower frequency of PHT in the patients that underwent LR, which as we have stated, is accompanied by an increase in the risk of mortality.
Surgical treatment through LR also independently influenced the probability of tumor recurrence. Additionally, the presence of diabetes mellitus, AFP levels, and the TBS calculated with the histologic data, remained statistically significant as independent risk factors for recurrence in the multivariate analysis. The greater recurrence probability after resection could help explain the lower long-term survival in those patients. Both diabetes and obesity increase the risk for certain types of cancer, including HCC.18–20 The relation between AFP levels and the risk for recurrence are well known. In fact, the majority of research groups utilize AFP values above 1,000 ng/mL as an exclusion criterion for performing transplantation, due to the high risk for recurrence.8 Nevertheless, to the best of our knowledge, there are no studies relating tumor burden, determined through the TBS, to recurrence in HCC. The TBS, described by Sasaki et al.12 in 2018, was designed to evaluate tumor burden in surgery for liver metastases due to colorectal cancer. In a retrospective multicenter analysis on more than 1,000 patients operated on for HCC through resection with curative intent, within the time frame of 2000 and 2017, Tsilimigras et al.21 confirmed that patients with a low TBS and BCLC stage B disease had higher survival than patients with stage A disease and a high TBS.
The results of our study, despite its small sample size, concur with the aforementioned analysis, given that the TBS was shown to be an independent risk factor for specific mortality that could discriminate specific mortality due to HCC, with an AUC of 0.654. It also had an inversely proportional relation to the DFI.
Our study has the limitations inherent in retrospective observational analyses, and so the existence of certain biases (selection, assignment, etc.) cannot be ruled out. Moreover, the study was conducted at a referral center in northern Spain, thus its results cannot be extrapolated to populations of other regions or countries. Lastly, we cannot rule out the fact that, due to its sample size, the study does not have the sufficient statistical power to demonstrate the existence of significant differences between overall mortality and specific mortality (a type II error).
In conclusion, in our cohort of patients, LR had a higher risk of mortality and recurrence in the long term than transplantation. Measuring tumor burden through the TBS could be an adequate method for helping subclassify patients with HCC and guide treatment in the current context, in which therapeutic decision-making is increasingly more complex. At any rate, more studies are needed to further evaluate and validate the TBS in the management of patients with HCC.
Ethical considerationsThe present study was approved by the clinical research ethics committee (CREC) of Cantabria, fulfilling all protocols.
Protection of persons and animals. The authors declare that no experiments were conducted on humans or animals for this study.
Data confidentiality. The authors declare that they have followed the protocols of their work center on the publication of patient data and have preserved patient data anonymity.
Right to privacy and informed consent. Informed consent was not requested for the publication of this work, given its retrospective nature and the absence of personal data that could identify the patients.
Financial disclosureNo specific grants were received from public or private sector agencies, in relation to this study.
Conflict of interestThe authors declare that there is no conflict of interest.
See related content in DOI: 10.1016/j.rgmxen.2023.11.002, Moctezuma-Velazquez, C. Liver transplantation or resection for early hepatocellular carcinoma: More questions than answers Rev Gastroenterol Mex. 2024; 319–322.