Helicobacter pylori is a Gram-negative bacillus that colonizes the gastric mucosa and infects more than half of the world population. Treatment consists of two antibiotics and a proton pump inhibitor (PPI) that favors the replication of the bacterium and enhances the activity of the antibiotics. Despite the importance of proton pump inhibitor use in treating H. pylori infection, the precise mechanisms through which PPIs affect the physiology of the bacterium are not yet understood.
AimOur aim was to compile information pertaining to the effect of PPIs on the physiology of H. pylori and the mechanisms through which they produce alterations in the bacterium.
MethodsA bibliographic search was conducted, utilizing the PubMed, Science Direct, and LILACS databases, and included preclinical and clinical original articles published in any language.
ResultsThe sulfenamide form of PPIs was shown to have effects on H. pylori, including the induction of structural changes, inhibition of bacterial growth, and interference with enzymes, such as urease, ATPases, and alcohol dehydrogenase.
ConclusionsThe binding of the sulfenamide form of PPIs to the bacterial structural and enzymatic components was the main mechanism through which H. pylori physiology was altered in vitro, but how they induce alterations in the bacterium was not established in the clinical studies analyzed.
Helicobacter pylori es un bacilo gramnegativo que coloniza la mucosa gástrica e infecta a más de la mitad de la población mundial. El tratamiento consta de dos antibióticos y un inhibidor de la bomba de protones (IBP) que favorece la replicación de la bacteria y potencia la actividad de los antibióticos. A pesar de la importancia del uso de los IBP en la terapia contra H. pylori, aún no son precisos los mecanismos por los cuales estos medicamentos ejercen un efecto sobre la fisiología de la bacteria.
ObjetivoRecopilar información sobre el efecto de los IBP sobre la fisiología de H. pylori y el mecanismo por el cual producen alteraciones en la bacteria.
MétodosSe realizó una búsqueda de bibliografía en PubMed, Science Direct y LILACS. Se incluyeron artículos originales preclínicos y clínicos publicados en cualquier idioma.
ResultadosLos IBP y su forma sulfenamida tienen efectos en H. pylori, incluyendo la inducción de cambios estructurales, la inhibición del crecimiento bacteriano, la interferencia con enzimas como la ureasa, ATPasas y alcohol deshidrogenasa.
ConclusionesLa unión de la forma sulfenamida de los IBP a componentes estructurales y enzimáticos bacterianos demostró ser el principal mecanismo por el que se altera la fisiología de H. pylori in vitro. En los estudios clínicos no se precisan los mecanismos por los que estos fármacos inducen alteraciones en la bacteria.
Helicobacter pylori (H. pylori) is a microaerophilic, flagellated Gram-negative bacterium that colonizes the epithelial surface of the human gastric mucosa.1 The worldwide prevalence of H. pylori infection is high, with differences among countries.2 Around 50% of the population presents with the bacterium and its prevalence varies between 85 and 95% in low-income countries and between 30 and 50% in high-income countries.3 Despite the high prevalence of H. pylori infection, only 10% of infected individuals have symptoms. This is due to various factors, such as genetic variability of the isolates and biologic and environmental factors of the host, such as type of diet, smoking, alcohol use, and antibiotic use.2,4
H. pylori is considered one of the main risk factors for developing gastric cancer (GC) and peptic ulcer (PU). Therefore, the World Health Organization (WHO), through the International Agency for Research on Cancer (IARC), classified it as a grade I carcinogen, with infected persons six-times more susceptible to developing GC.5 The development of this type of cancer is related to different virulence factors of the bacterium, among which the urease enzyme stands out as one of the most important. It favors the resistance of the bacterium to acid pH, through the hydrolysis of urea into carbon dioxide (CO₂) and ammonia (NH3). In this manner, H. pylori buffers its periplasmic pH and increases the gastric pH, facilitating its establishment in the host and the activation of the immune response and the subsequent oxidative stress that stimulate the malignant transformation of the epithelium.1
Due to the capacity of H. pylori to establish itself and multiply in the gastric mucosa, the current therapy for eradicating the bacterium consists of a proton pump inhibitor (PPI) (omeprazole, lansoprazole, pantoprazole, etc.) and at least two antibiotics (clarithromycin and amoxicillin, among others).1 PPIs suppress gastric acid secretion by blocking the H +/K + ATPase enzyme, also known as the gastric proton pump.1 These drugs are essential because they promote different changes in the microenvironment of the host, such as the increase in the gastric pH, favoring the replication of the microorganism and increasing susceptibility to antibiotics.6
Different in vitro studies have demonstrated the inhibition of urease activity with the administration of high doses of omeprazole and have shown that PPIs produce changes in the cellular structure and morphology of H. pylori.6 Mirshahi et al. reported the bactericidal and bacteriostatic effect of these medications on low and high bacterial densities, respectively, and the altered viability and recovery of H. pylori observed in cultures, after PPI exposure.7,8
Despite the wide use of PPIs in treating H. pylori infection and the changes described in vitro in the morphology, growth, and enzyme activity of the bacterium, the precise mechanisms or pathways through which PPIs produce these effects on H. pylori are not yet understood. In addition, it is not known whether they occur during the infection in the host or with indiscriminate PPI consumption, affecting the diagnosis and the success of the current therapy. On the other hand, there are no studies that synthesize all the effects PPIs have on the bacterium or define the concentrations at which changes are produced. Therefore, the aim of this review was to collect the information on the effects that PPIs have on the physiology of H. pylori, and the associated mechanisms involved.
MethodologySearch strategyThis systematic review was carried out following the recommendations of the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, defining the changes in the physiology of H. pylori as the problem of interest, PPIs with no comparison as the intervention to be analyzed, and defining the mechanisms through which PPIs produce changes in the physiology of PPIs. Those items were then grouped into the following question: What is the mechanism through which PPIs produce changes in the physiology of H. pylori?
Articles were systematically identified in PubMed (the National Library of Medicine, Bethesda, MD, USA), Science Direct, and LILACS (Literatura Latinoamericana de Información en Ciencias de la Salud). The following set of keywords in English were also defined: growth, metabolism, nutrition, enzyme activity, and the following MeSH (Medical Subject Headings) terms were utilized: «proton pump inhibitors», «growth». This terms-based approach enabled homogeneity in the search strategy for different languages. To guarantee study reproducibility, the search strategies for each database were employed by the authors of the review at different times, and said strategies are shown in Table 1.
Search strategy
| Database | Search strategy |
|---|---|
| ScienceDirect | Title “Helicobacter pylori” OR “H. pylori” |
| Title, abstract, or author-specified keywords: (“proton pump inhibitors” OR “proton pump inhibitors” OR PPI OR omeprazole OR esomeprazole OR pantoprazole OR rabeprazole OR lansoprazole) AND (Growth) | |
| (“proton pump inhibitors” OR “proton pump inhibitors” OR PPI OR omeprazole OR esomeprazole OR pantoprazole OR rabeprazole OR lansoprazole) AND (metabolism) | |
| (“proton pump inhibitors” OR “proton pump inhibitors” OR PPI OR omeprazole OR esomeprazole OR pantoprazole OR rabeprazole OR lansoprazole) AND (“enzyme activity”) | |
| (“proton pump inhibitors” OR “proton pump inhibitors” OR PPI OR omeprazole OR esomeprazole OR pantoprazole OR rabeprazole OR lansoprazole) AND (enzyme) | |
| (“proton pump inhibitors” OR “proton pump inhibitors” OR PPI OR omeprazole OR esomeprazole OR pantoprazole OR rabeprazole OR lansoprazole) AND (nutrition) | |
| Lilacs | (ti:(“Helicobacter pylori”)) AND (“proton pump inhibitors”) AND (growth) |
| (ti:(“Helicobacter pylori”)) AND (“proton pump inhibitor”) AND (growth) | |
| (ti:(“Helicobacter pylori”)) AND (“PPI”) AND (growth) | |
| (ti:(“Helicobacter pylori”)) AND (omeprazole) AND (growth) | |
| (ti:(“Helicobacter pylori”)) AND (esomeprazole) AND (growth) | |
| (ti:(“Helicobacter pylori”)) AND (pantoprazole) AND (growth) | |
| (ti:(“Helicobacter pylori”)) AND (lansoprazole) AND (growth) | |
| (ti:(“Helicobacter pylori”)) AND (rabeprazole) AND (growth) | |
| (ti:(“Helicobacter pylori”)) AND (“proton pump inhibitors”) AND (metabolism) | |
| (ti:(“Helicobacter pylori”)) AND (“proton pump inhibitor”) AND (metabolism) | |
| (ti:(“Helicobacter pylori”)) AND (“PPI”) AND (metabolism) | |
| (ti:(“Helicobacter pylori”)) AND (omeprazole) AND (metabolism) | |
| (ti:(“Helicobacter pylori”)) AND (esomeprazole) AND (metabolism) | |
| (ti:(“Helicobacter pylori”)) AND (pantoprazole) AND (metabolism) | |
| (ti:(“Helicobacter pylori”)) AND (lansoprazole) AND (metabolism) | |
| (ti:(“Helicobacter pylori”)) AND (rabeprazole) AND (metabolism) | |
| (ti:(“Helicobacter pylori”)) AND (“proton pump inhibitors”) AND (nutrition) | |
| (ti:(“Helicobacter pylori”)) AND (“proton pump inhibitor”) AND (nutrition) | |
| (ti:(“Helicobacter pylori”)) AND (“PPI”) AND (nutrition) | |
| (ti:(“Helicobacter pylori”)) AND (omeprazole) AND (nutrition) | |
| (ti:(“Helicobacter pylori”)) AND (esomeprazole) AND (nutrition) | |
| (ti:(“Helicobacter pylori”)) AND (pantoprazole) AND (nutrition) | |
| (ti:(“Helicobacter pylori”)) AND (lansoprazole) AND (nutrition) | |
| (ti:(“Helicobacter pylori”)) AND (rabeprazole) AND (nutrition) | |
| (ti:(“Helicobacter pylori”)) AND (“proton pump inhibitors”) AND (“enzyme activity”) | |
| (ti:(“Helicobacter pylori”)) AND (“proton pump inhibitor”) AND (“enzyme activity”) | |
| (ti:(“Helicobacter pylori”)) AND (“PPI”) AND (“enzyme activity”) | |
| (ti:(“Helicobacter pylori”)) AND (omeprazole) AND (“enzyme activity”) | |
| (ti:(“Helicobacter pylori”)) AND (esomeprazole) AND (“enzyme activity”) | |
| (ti:(“Helicobacter pylori”)) AND (pantoprazole) AND (“enzyme activity”) | |
| (ti:(“Helicobacter pylori”)) AND (lansoprazole) AND (“enzyme activity”) | |
| (ti:(“Helicobacter pylori”)) AND (rabeprazole) AND (“enzyme activity”) | |
| (ti:(“Helicobacter pylori”)) AND (“proton pump inhibitors”) AND (enzyme) | |
| (ti:(“Helicobacter pylori”)) AND (“proton pump inhibitor”) AND (enzyme) | |
| (ti:(“Helicobacter pylori”)) AND (“PPI”) AND (enzyme) | |
| (ti:(“Helicobacter pylori”)) AND (omeprazole) AND (enzyme) | |
| (ti:(“Helicobacter pylori”)) AND (esomeprazole) AND (enzyme) | |
| (ti:(“Helicobacter pylori”)) AND (pantoprazole) AND (enzyme) | |
| (ti:(“Helicobacter pylori”)) AND (lansoprazole) AND (enzyme) | |
| (ti:(“Helicobacter pylori”)) AND (rabeprazole) AND (enzyme) | |
| PubMed | (“Helicobacter pylori” [Title] OR "H.pylori" [Title]) AND (“proton pump inhibitors” [MeSH Terms] OR “proton pump inhibitors” [Tiab] OR "proton pump inhibitor" [Tiab] OR PPI [Tiab] OR omeprazole [Tiab] OR esomeprazole [Tiab] OR pantoprazole [Tiab] OR rabeprazole [Tiab] OR lansoprazole [Tiab]) AND (Growth [MeSH Terms] OR Growth [Tiab]) |
| (“Helicobacter pylori” [Title] OR “H. pylori” [Title]) AND (“proton pump inhibitors” [MeSH Terms] OR “proton pump inhibitors” [Tiab] OR "proton pump inhibitor" [Tiab] OR PPI [Tiab] OR omeprazole [Tiab] OR esomeprazole [Tiab] OR pantoprazole [Tiab] OR rabeprazole [Tiab] OR lansoprazole [Tiab]) AND (Metabolism [Tiab]) | |
| (“Helicobacter pylori” [Title] OR “H. pylori” [Title]) AND (“proton pump inhibitors” [MeSH Terms] OR “proton pump inhibitors” [Tiab] OR "proton pump inhibitor" [Tiab] OR PPI [Tiab] OR omeprazole [Tiab] OR esomeprazole [Tiab] OR pantoprazole [Tiab] OR rabeprazole [Tiab] OR lansoprazole [Tiab]) AND (enzymes [Tiab] OR "enzyme activity" [Tiab]) | |
| (“Helicobacter pylori” [Title] OR “H. pylori” [Title]) AND (“proton pump inhibitors” [MeSH Terms] OR “proton pump inhibitors” [Tiab] OR "proton pump inhibitor" [Tiab] OR PPI [Tiab] OR omeprazole [Tiab] OR esomeprazole [Tiab] OR pantoprazole [Tiab] OR rabeprazole [Tiab] OR lansoprazole [Tiab]) AND (nutrition [Tiab]) |
Original articles were selected, that had complete texts and were published in any language, within the time frame of 1990 to December 2022, to set the time period and guarantee reproducibility in the search at later times. The search was carried out from March 10 to 27, 2023. The articles whose titles and abstracts included the keywords and provided information of interest for developing the review were selected. As an additional strategy, the references of the selected articles were reviewed to obtain a larger number of bibliographic sources.
Exclusion criteriaNarrative reviews, systematic reviews, and duplicated articles were excluded, as well as articles with incomplete data or information that was irrelevant to our review aim, and articles whose complete texts were not available.
Evaluation of the methodological qualityTo evaluate methodological quality and possible biases of the studies included in this review, the checklist from the Joanna Briggs Institute was utilized. It was adapted for use regarding the articles included in the review and implemented independently by the authors of the present review.
Extraction of the variables and data analysisFor the data analysis, the following group of variables for each article was defined: type of PPI, concentration of each PPI utilized, exposure time, effect on growth, metabolism and/or enzyme activity of H. pylori, type of study (preclinical or clinical), methods, and results. To organize and structure the information obtained, the variables were placed in Excel tables, enabling specific data to be extracted from each study included in the review.
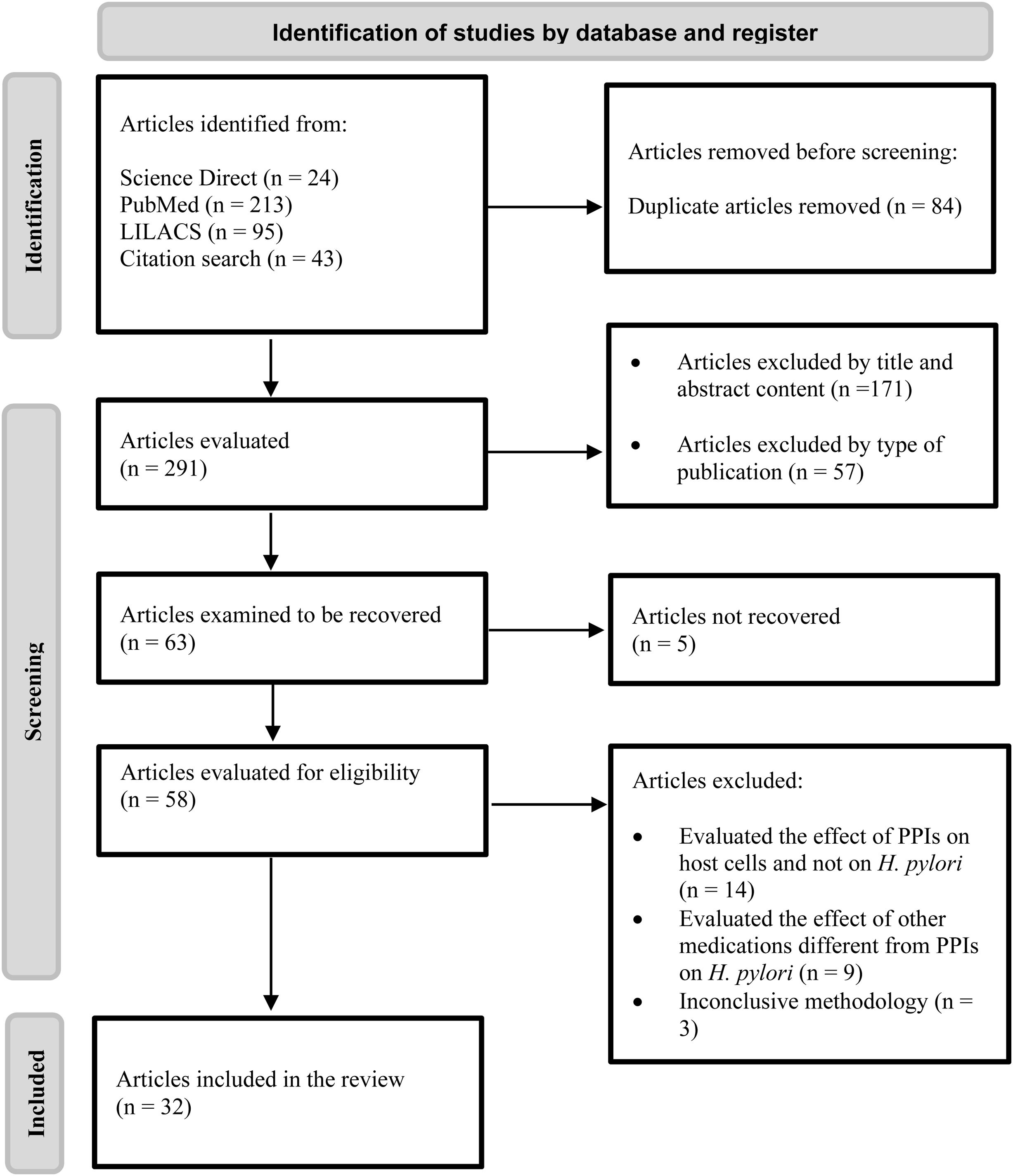
ResultsFollowing the search strategy, a total of 375 articles were identified. Twenty-four were identified from the Science Direct database, 213 from PubMed, 95 from LILACS, and 43 from other sources. Of the 375 articles, 84 were excluded because they were duplicates and 57 were excluded due to the type of article, after which 171 articles were excluded based on their titles and abstracts, leaving a total of 63 articles with complete texts to be read. Of those 63 articles, 31 were eliminated because they evaluated the effect of PPIs on the host cells and not on H. pylori, studied medications different from PPIs, had inconclusive methodologies and/or were not available for complete reading. Applying those criteria, 32 articles were obtained for the systematic review. Fig. 1 shows the flowgram that outlines the results, once the inclusion and exclusion criteria were applied. Table 2 shows the articles included in the review and the evaluated effect.
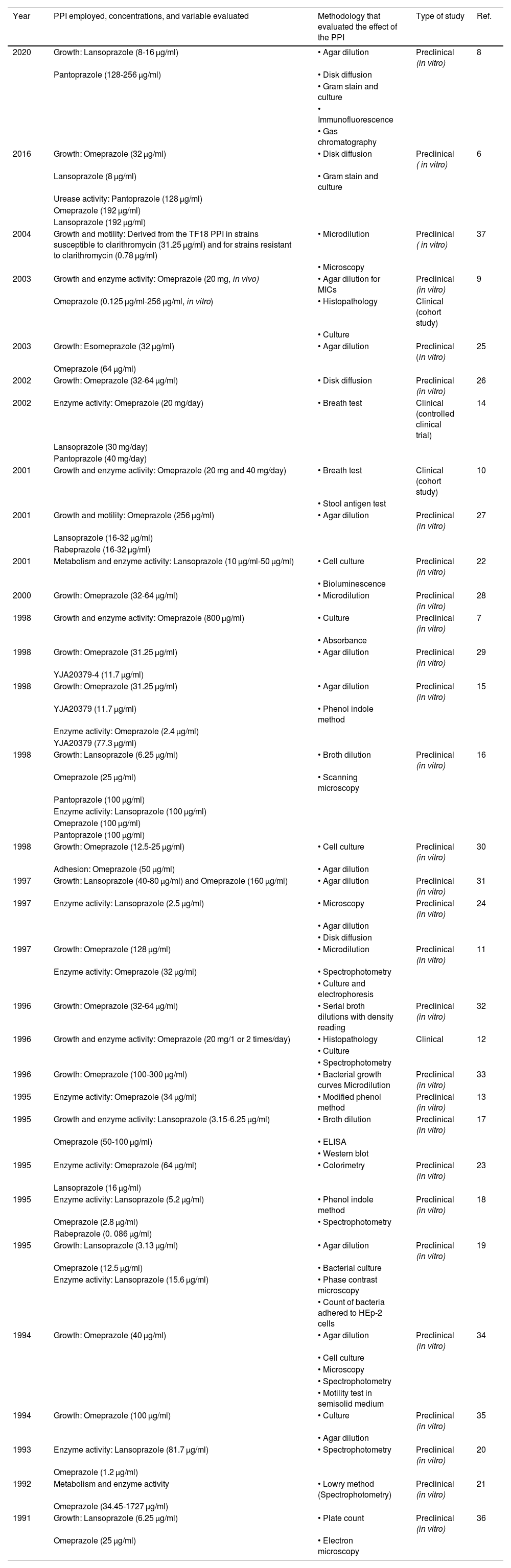
Articles included, and effect evaluated
| Year | PPI employed, concentrations, and variable evaluated | Methodology that evaluated the effect of the PPI | Type of study | Ref. |
|---|---|---|---|---|
| 2020 | Growth: Lansoprazole (8-16 μg/ml) | • Agar dilution | Preclinical (in vitro) | 8 |
| Pantoprazole (128-256 μg/ml) | • Disk diffusion | |||
| • Gram stain and culture | ||||
| • Immunofluorescence | ||||
| • Gas chromatography | ||||
| 2016 | Growth: Omeprazole (32 μg/ml) | • Disk diffusion | Preclinical ( in vitro) | 6 |
| Lansoprazole (8 μg/ml) | • Gram stain and culture | |||
| Urease activity: Pantoprazole (128 μg/ml) | ||||
| Omeprazole (192 μg/ml) | ||||
| Lansoprazole (192 μg/ml) | ||||
| 2004 | Growth and motility: Derived from the TF18 PPI in strains susceptible to clarithromycin (31.25 μg/ml) and for strains resistant to clarithromycin (0.78 μg/ml) | • Microdilution | Preclinical ( in vitro) | 37 |
| • Microscopy | ||||
| 2003 | Growth and enzyme activity: Omeprazole (20 mg, in vivo) | • Agar dilution for MICs | Preclinical (in vitro) | 9 |
| Omeprazole (0.125 μg/ml-256 μg/ml, in vitro) | • Histopathology | Clinical (cohort study) | ||
| • Culture | ||||
| 2003 | Growth: Esomeprazole (32 μg/ml) | • Agar dilution | Preclinical (in vitro) | 25 |
| Omeprazole (64 μg/ml) | ||||
| 2002 | Growth: Omeprazole (32-64 μg/ml) | • Disk diffusion | Preclinical (in vitro) | 26 |
| 2002 | Enzyme activity: Omeprazole (20 mg/day) | • Breath test | Clinical (controlled clinical trial) | 14 |
| Lansoprazole (30 mg/day) | ||||
| Pantoprazole (40 mg/day) | ||||
| 2001 | Growth and enzyme activity: Omeprazole (20 mg and 40 mg/day) | • Breath test | Clinical (cohort study) | 10 |
| • Stool antigen test | ||||
| 2001 | Growth and motility: Omeprazole (256 μg/ml) | • Agar dilution | Preclinical (in vitro) | 27 |
| Lansoprazole (16-32 μg/ml) | ||||
| Rabeprazole (16-32 μg/ml) | ||||
| 2001 | Metabolism and enzyme activity: Lansoprazole (10 μg/ml-50 μg/ml) | • Cell culture | Preclinical (in vitro) | 22 |
| • Bioluminescence | ||||
| 2000 | Growth: Omeprazole (32-64 μg/ml) | • Microdilution | Preclinical (in vitro) | 28 |
| 1998 | Growth and enzyme activity: Omeprazole (800 μg/ml) | • Culture | Preclinical (in vitro) | 7 |
| • Absorbance | ||||
| 1998 | Growth: Omeprazole (31.25 μg/ml) | • Agar dilution | Preclinical (in vitro) | 29 |
| YJA20379-4 (11.7 μg/ml) | ||||
| 1998 | Growth: Omeprazole (31.25 μg/ml) | • Agar dilution | Preclinical (in vitro) | 15 |
| YJA20379 (11.7 μg/ml) | • Phenol indole method | |||
| Enzyme activity: Omeprazole (2.4 μg/ml) | ||||
| YJA20379 (77.3 μg/ml) | ||||
| 1998 | Growth: Lansoprazole (6.25 μg/ml) | • Broth dilution | Preclinical (in vitro) | 16 |
| Omeprazole (25 μg/ml) | • Scanning microscopy | |||
| Pantoprazole (100 μg/ml) | ||||
| Enzyme activity: Lansoprazole (100 μg/ml) | ||||
| Omeprazole (100 μg/ml) | ||||
| Pantoprazole (100 μg/ml) | ||||
| 1998 | Growth: Omeprazole (12.5-25 μg/ml) | • Cell culture | Preclinical (in vitro) | 30 |
| Adhesion: Omeprazole (50 μg/ml) | • Agar dilution | |||
| 1997 | Growth: Lansoprazole (40-80 μg/ml) and Omeprazole (160 μg/ml) | • Agar dilution | Preclinical (in vitro) | 31 |
| 1997 | Enzyme activity: Lansoprazole (2.5 μg/ml) | • Microscopy | Preclinical (in vitro) | 24 |
| • Agar dilution | ||||
| • Disk diffusion | ||||
| 1997 | Growth: Omeprazole (128 μg/ml) | • Microdilution | Preclinical (in vitro) | 11 |
| Enzyme activity: Omeprazole (32 μg/ml) | • Spectrophotometry | |||
| • Culture and electrophoresis | ||||
| 1996 | Growth: Omeprazole (32-64 μg/ml) | • Serial broth dilutions with density reading | Preclinical (in vitro) | 32 |
| 1996 | Growth and enzyme activity: Omeprazole (20 mg/1 or 2 times/day) | • Histopathology | Clinical | 12 |
| • Culture | ||||
| • Spectrophotometry | ||||
| 1996 | Growth: Omeprazole (100-300 μg/ml) | • Bacterial growth curves Microdilution | Preclinical (in vitro) | 33 |
| 1995 | Enzyme activity: Omeprazole (34 μg/ml) | • Modified phenol method | Preclinical (in vitro) | 13 |
| 1995 | Growth and enzyme activity: Lansoprazole (3.15-6.25 μg/ml) | • Broth dilution | Preclinical (in vitro) | 17 |
| Omeprazole (50-100 μg/ml) | • ELISA | |||
| • Western blot | ||||
| 1995 | Enzyme activity: Omeprazole (64 μg/ml) | • Colorimetry | Preclinical (in vitro) | 23 |
| Lansoprazole (16 μg/ml) | ||||
| 1995 | Enzyme activity: Lansoprazole (5.2 μg/ml) | • Phenol indole method | Preclinical (in vitro) | 18 |
| Omeprazole (2.8 μg/ml) | • Spectrophotometry | |||
| Rabeprazole (0. 086 μg/ml) | ||||
| 1995 | Growth: Lansoprazole (3.13 μg/ml) | • Agar dilution | Preclinical (in vitro) | 19 |
| Omeprazole (12.5 μg/ml) | • Bacterial culture | |||
| Enzyme activity: Lansoprazole (15.6 μg/ml) | • Phase contrast microscopy | |||
| • Count of bacteria adhered to HEp-2 cells | ||||
| 1994 | Growth: Omeprazole (40 μg/ml) | • Agar dilution | Preclinical (in vitro) | 34 |
| • Cell culture | ||||
| • Microscopy | ||||
| • Spectrophotometry | ||||
| • Motility test in semisolid medium | ||||
| 1994 | Growth: Omeprazole (100 μg/ml) | • Culture | Preclinical (in vitro) | 35 |
| • Agar dilution | ||||
| 1993 | Enzyme activity: Lansoprazole (81.7 μg/ml) | • Spectrophotometry | Preclinical (in vitro) | 20 |
| Omeprazole (1.2 μg/ml) | ||||
| 1992 | Metabolism and enzyme activity | • Lowry method (Spectrophotometry) | Preclinical (in vitro) | 21 |
| Omeprazole (34.45-1727 μg/ml) | ||||
| 1991 | Growth: Lansoprazole (6.25 μg/ml) | • Plate count | Preclinical (in vitro) | 36 |
| Omeprazole (25 μg/ml) | • Electron microscopy |
Of the 32 articles included in the systematic review, 18 reported that PPIs had an effect on the in vitro enzyme activity of H. pylori and 14 of those showed that PPIs inhibited the enzyme activity of urease.6,7,9–20 Only one study evaluated the effect on the urease and ATPase enzymes. Three studies reported that PPIs in vitro inhibited the activity of other important enzymes for H. pylori, such as the ATPases, alcohol dehydrogenase (ADH), NADPH-quinone oxidoreductase, and pyruvate-flavodoxin oxidoreductase, enzymes that participate in the metabolic processes of the bacterium.21–23
Omeprazole was the most widely evaluated PPI, with seven studies,7,9–13,21 followed by lansoprazole with two articles.22,24 Four studies evaluated those two drugs together, and to a lesser degree, the rest of the articles included rabeprazole, pantoprazole, and esomeprazole, among other PPIs.6,14–20 The most widely used methods for measuring the urease activity of H. pylori were the colorimetric techniques, such as the modified phenol method and the indophenol method that measure the level of ammonia produced by the enzyme.7,11–13,15,16,18,20 The mechanism by which that function is altered might be related to the capacity of PPIs to block the sulfhydryl (SH) residues in cysteine from the active urease site.6,7,11,16–18,20
The concentrations of the PPIs used for evaluating urease activity in vitro were heterogeneous. Omeprazole concentrations ranged from 1.2 to 800 μg/ml, whereas lansoprazole concentrations ranged from 1.7 to 192 μg/ml.6,7,9–20,24 In the clinical trials, the dose of 20 mg of omeprazole once or twice a day and 30 mg/day of lansoprazole increased the number of false negatives in the rapid urease test, as well as the serologic and molecular tests.9,12,14
Effect of proton pump inhibitors on bacterial growthTwenty-five articles evaluated the effect of PPIs on the growth of H. pylori, and omeprazole exhibited the greatest effect. Twenty-one studies that conducted trials with omeprazole found minimal inhibitory concentrations (MICs) from 12.5 to 800 μg/ml, at 72 hours of exposure, mainly utilizing the agar dilution method.6,7,9,11,15–17,19,25–36 The second most widely evaluated PPI was lansoprazole, with 8 studies. The MICs were found to range from 3.13 to 40 μg/ml, with a mean of 17.9 μg/ml, using the agar dilution method.6,8,16,17,19,27,31,36 In only five studies, rabeprazole, pantoprazole, and esomeprazole were evaluated in combination with omeprazole and/or lansoprazole, but their effect was similar to that of the others.6,8,9,16,27
Ten of the authors did not specify the mechanism through which the PPIs exhibited bactericidal and bacteriostatic activity in H. pylori.7,9,10,12,17,19,27,29–31 Most of the authors coincided in the belief that said activity involved processes separate from urease inhibition that would be mediated by several factors. Among those factors was the binding of the sulfenamide compound in acid pH environments to different proteins and enzymes, such as the bacterial ATPases, or others, such as fumarate reductase and succinate-cytochrome c reductase involved in the respiratory chain.32–36 Lastly, other studies explained that the effect of PPIs on the growth and viability of H. pylori was related to the alteration in the cell membrane, a product of the modifications in the fatty acid content, leading to cell division inhibition, permeability changes, and bacterial lysis.6,8,11,16,26,28,37
Effect of proton pump inhibitors on metabolism or nutritionOnly two articles evaluated the effect of PPIs on important metabolic processes for H. pylori. The PPIs evaluated were omeprazole and lansoprazole, with ranges in concentration from 3.45 to 1,727 μg/ml and from 3 to 50 μg/ml, respectively.21,22 The methodology employed was different in the two studies. Nagata et al.22 based their analysis on bioluminescence for measuring cellular ATP production through the luciferin-luciferase method, and the polarography technique with oxygen electrodes for determining cellular oxygen production per minute. On the other hand, Roine et al.21 utilized colorimetric methods to evaluate ADH activity.
According to Nagata et al.,22 PPIs alter pyruvate-flavodoxin oxidoreductase activity, which intervenes in the decarboxylation and dehydrogenation of pyruvate, the main substrate for energy creation in H. pylori. In turn, it affects the production of NADPH that participates in the respiratory chain of the bacterium. Roine et al.21 described the action of PPIs on ADH, the enzyme that participates in the fermentation of carbohydrates to ethanol and intervenes in bacterial energy metabolism.
DiscussionPPIs are medications that have been patented since 1979 for the treatment of gastritis, peptic ulcer, and gastric reflux, among others. They selectively bind to the H +/K + ATPase pump of the gastric parietal cells, inhibiting hydrochloric acid secretion. Afterwards, when the relation between H. pylori and the development of gastric ulcer was established, PPIs were used as combined triple therapy with two antibiotics (clarithromycin, amoxicillin, or metronidazole).38 In the present review, studies were collected that showed the inhibiting effect of PPIs on the growth, enzyme activity, and metabolism of H. pylori. However, the heterogeneity of the concentrations utilized for the exposure of the bacterium to PPIs stood out. This is possibly due to the intrinsic characteristics of the methodology employed by each researcher, such as the strain utilized and the conditions of temperature, pH, and culture medium, among others.
Regarding the enzyme activity of H. pylori, urease is one of the most widely studied enzymes. It is necessary for colonizing and establishing H. pylori in the gastric mucosa, and so, is considered a potential target of the PPIs. The bactericidal and bacteriostatic effect of PPIs could be partially explained by the binding of these benzimidazole compounds to the urease active site.15,33 Even though some authors do not explain the mechanism by which PPIs affect the urease activity in H. pylori, others concur that this effect depends on both the dose of the drug and the gastric pH, because by being in their active form, these compounds bind to bacterial proteins, including urease.11,16–18,20
Even though urease is related to the survival of H. pylori in the host, in vitro studies showed inhibition of growth in urease-deleted strains after exposure to PPIs, suggesting that the blocking of urease is not the only bactericidal effect of PPIs and there are probably other mechanisms involved in the antibacterial activity.13,17H. pylori ATPases were recently proposed as other possible PPI targets, with the P-type ATPase that is involved in pH maintenance, intracellular ion balance, and turgor pressure control in the bacterium, standing out.38,39 Others are the F-type ATPases that participate in proton translocation and are important for maintaining intracellular pH regulation and ATP synthesis.39 Even though bacterial F/P ATPase activity is important for H. pylori adaptation, in vitro studies suggest that the binding of PPIs to those enzymes is not involved in inhibiting the growth of the bacterium.6,32
On the other hand, Kumiko et al.22 propose that lansoprazole’s inhibitory activity is related to H. pylori respiratory chain alteration. Despite the fact that H. pylori does not have a complete tricarboxylic acid cycle, it utilizes succinate, α-ketoglutarate, isocitrate, and pyruvate as respiratory substrates, with pyruvate being the main source of energy. Lansoprazole interferes with pyruvate-flavodoxin oxidoreductase activity and affects the transfer of hydrogen atoms to NADP, through flavodoxin NADP oxidoreductase, impeding the formation of NADPH, which is the main electron donor in the H. pylori respiratory chain. This finally alters the energy metabolism, and consequently, bacterial growth. Therefore, a more profound study of those enzymes is essential for understanding the relation between the enzymatic and metabolic alterations and the bactericidal effect of PPIs in H. pylori.22
Even though there are not enough recent studies that associate bacterial ADH inhibition with H. pylori growth suppression, the evidence found shows that PPIs have an effect on ADH, an important enzyme in the fermentation of sugars to ethanol, which is a process carried out by the bacterium to obtain energy.11,18,21 By affecting the activity of ADH, omeprazole not only interferes with an energy route essential for H. pylori growth, but also decreases the formation of acetaldehyde, a compound that is related to gastric lesions. This would alternately explain the growth suppression of the bacterium and the resolution of gastric lesions after PPI administration.21
There was ample evidence on the capacity of PPIs to be protonated at acid pH, converting into the sulfenamide form that favors the binding to different bacterial proteins, both structural and enzymatic.11,16–18,20,21,25 This can be suggested as the main mechanism supporting the bactericidal and bacteriostatic action of PPIs on H. pylori, in vitro. Given that the sulfenamide compound is highly reactive, it is able to nonspecifically bind to the SH groups available in the bacterium that not only are present in enzymes like urease, but also in the ATPases, reductases, and the rest of the proteins necessary for different physiologic processes and bacterial survival. This action is reversible and mediated by characteristics, such as pH, dose, and drug exposure time.21–24
Other studies propose alternate processes that explain the inhibition of H. pylori growth by PPIs, including their capacity to interact and alter the composition of the bacterial cell membrane.8,11,16,26,28,37 Because they are compounds with lipophilic and cationic characteristics, PPIs are able to bind to anionic compounds, such as phospholipids and fatty acids. It has been pointed out that the antimicrobials that have those properties cause the separation and reordering of lipids, resulting in the formation of pores, cellular content leakage, and changes in the fluidity and permeability of the membrane, actions that enhance the death of the bacterium. When faced with such stress, adjusting the composition of the fatty acids of H. pylori is essential for maintaining the biophysical properties of the membrane. However, that additional energy expenditure is compensated by the decrease in cell division and growth, guaranteeing structural rigidity and resistance to lysis. Kadkhodaei et al.8 evaluated the capacity of the bacterium to be cultured after PPI treatment, finding variations in the fatty acid composition of the cell membrane and alterations in the growth of the bacterium that were reversible upon being cultured in cholesterol-rich media. That finding suggests an alternate manner of culturing for the recovery and detection of H. pylori in biopsies from patients that use PPIs.
Additional trials demonstrate that after PPI exposure, other functions, such as H. pylori motility, are altered.19,37 Tsutsui et al.40 described the H. pylori flagella and spiral shapes of the microorganism as important virulence factors in the colonization of the gastric mucosa and suggest the possibility that PPI derivatives, such as the rabeprazole thioether, bind to those structures or to molecules present on the bacterial surface, interfering with its virulence and motility. However, those effects do not appear to be related to inhibiting the growth of the bacterium.40,41 Escoffier et al. demonstrated the negative effect of pantoprazole on the motility of human spermatozoids, revealing that pantoprazole inhibited the non-gastric H +/K + ATPase of the spermatozoids and altered the exchange of ions through the membrane. That study provided evidence on the capacity PPIs have to alter not only bacterial components and enzymes, but also the structures of different organisms.42
Even when in vitro studies propose different mechanisms for explaining the effects of PPIs on H. pylori, studies conducted on persons do not clarify the reasons why they have an effect on the physiology of the bacterium. Unlike trials on patients, in vitro trials guarantee constant exposure of the bacterium to the medication. In addition, controlling factors in the host, such as peristaltic movements, pH, and the transport of substances over the gastric mucosa, all of which are involved in the behavior of H. pylori, is difficult in clinical trials.30
The present review includes few clinical trials but showed that PPIs affect the growth, morphology, and urease activity of H. pylori, leading to false negative results with the diagnostic methods employed to detect this microorganism, such as the rapid urease test, urea breath test, biopsy culture, and histopathology. Therefore, we recommend the cessation of PPI use at least two weeks before performing those tests.9,10,12,14 Throughout this review, we have underlined the importance of conducting new in vivo studies and clinical trials that will enable the effects of PPIs on H. pylori, and their possible repercussion on the treatment and diagnosis of gastric diseases caused by the bacterium, to be described in detail.
ConclusionsPPIs produce multiple effects on the physiology of H. pylori, in vitro, such as inhibiting urease activity, interacting with ATPases, and interfering with ADH and other important enzymes in the respiratory chain, in addition to modifying the composition of the cell membrane and affecting bacterial motility. The primary mechanism is related to the capacity of PPIs to be converted into sulfenamide, which nonspecifically binds to different bacterial enzymes and proteins, affecting both structural components and essential physiologic processes of the bacterium.
The studies concur that there is a greater effect induced by lansoprazole and omeprazole, given the inversely proportional relation between the dose employed and the effect on H. pylori. Even though PPIs have a broad spectrum of effects on the physiology of H. pylori, in vitro, the exact translation of those findings to clinical practice requires greater understanding of the mechanisms involved in the effects PPIs have on the bacterium in vivo and the consideration of the complexities of the gastric environment of the host. Thus, additional studies are needed, to clarify the details of those effects and their clinical relevance.
Financial disclosureThis study was financed by the Comité Para el Desarrollo de la Investigación (CODI) Universidad de Antioquia, with code 2020–34042.
The authors declare that there is no conflict of interest.