Gastroesophageal reflux (GER) is a frequent normal phenomenon in children of any age. It is more common in infants, in whom the majority of episodes are short-lived and cause no other symptoms or complications, differentiating it from gastroesophageal reflux disease (GERD). The diagnosis and management of GER and GERD continue to be a challenge for the physician. Therefore, the aim of the Asociación Mexicana de Gastroenterología was to adapt international documents to facilitate their adoption by primary care physicians, with the goal of standardizing quality of care and reducing the number of diagnostic tests performed and inappropriate medication use.
The ADAPTE methodology was followed, and the recommendations were approved utilizing the Delphi strategy. The executive committee carried out the review of the guidelines, position papers, and international reviews that met the a priori quality criteria and possible applicability in a local context. The recommendations were taken from those sources and adapted, after which they were approved by the working group. The consensus consists of 25 statements and their supporting information on the diagnosis and treatment of GER and GERD in infants. The adapted document is the first systematic effort to provide an adequate consensus for use in Mexico, proposing a practical approach to and management of GER and GERD for healthcare providers.
El reflujo gastroesofágico (RGE), es un fenómeno frecuente y normal en niños de cualquier edad, siendo más común en lactantes, donde la mayoría de los episodios son breves y no causan otros síntomas ni complicaciones, lo que lo diferencia de la Enfermedad por Reflujo Gastroesofágico (ERGE). El diagnóstico y manejo del RGE y la ERGE siguen siendo un desafío para el médico; es por esto qué la Asociación Mexicana de Gastroenterología tuvo como objetivo adaptar los documentos internacionales, para facilitar la adopción por parte de los profesionales de atención primaria, con la intención de estandarizar la calidad de la atención y reducir el número de pruebas diagnósticas y el uso inapropiado de medicamentos.
La metodología que se siguió fue ADAPTE y para la aprobación de las recomendaciones se utilizó la estrategia Delphi. El comité ejecutivo realizó la revisión de guías, documentos de posición y revisiones internacionales, que cumplían a priori, los criterios de calidad y posible aplicabilidad al contexto local, de los cuales se extrajeron y adecuaron las recomendaciones que posteriormente fueron aprobadas por el grupo de desarrollo. El consenso contiene 25 enunciados junto con sus consideraciones de sustento, para el diagnóstico y tratamiento del RGE y de la ERGE en lactantes. El documento de adaptación representa el primer esfuerzo sistemático por adecuar un consenso para su uso en el contexto nacional, propone un enfoque y manejo prácticos de RGE y ERGE para los proveedores de atención médica.
Gastroesophageal reflux (GER) refers to the retrograde and involuntary movement of the gastric content into the esophagus; when the reflux becomes visible (from the mouth, nose), it is called regurgitation. Regurgitation is distinguished from vomiting, which is defined as a reflex of the central nervous system involving autonomous and skeletal muscles that forcefully expel the gastric content through the mouth by means of coordinated movements of the small bowel, stomach, esophagus, and diaphragm. Regurgitation is also different from rumination, in which previously swallowed foods are returned to the pharynx and mouth, and once again chewed and swallowed. When regurgitation of the gastric content causes complications and contributes to tissue damage or inflammation (e.g., esophagitis, obstructive apnea, bronchospasm, pulmonary aspiration, feeding and swallowing difficulties, or failure to thrive), it is called gastroesophageal reflux disease (GERD).1 The position paper of the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) adds “bothersome symptoms” as a criterion for differentiating infant regurgitation from GERD.2–5 The challenge of that definition is the lack of quantitative methods for defining “problematic”. Infants cannot verbalize what they are experiencing, and so the variations in the interpretations of “problematic” by physicians and parents have resulted in unnecessary diagnostic tests and treatments carried out on many infants with regurgitation that do not have GERD.1
The diagnostic criteria for infant regurgitation, proposed by the Rome working group, in neonates and healthy infants from 3 weeks to 12 months of age, must include the following two characteristics:
- 1
Regurgitation 2 or more times per day for 3 or more weeks.
- 2
No retching, hematemesis, aspiration, apnea, failure to thrive, feeding or swallowing difficulties, or abnormal posturing.
Regurgitation of the stomach content into the esophagus, mouth and/or nose is common in infants and is within the normal range of expected behavior for healthy infants. Infant regurgitation is the most frequent gut-brain interaction disorder (formally known as functional gastrointestinal disorders) in the first year of life,6 with a maximum prevalence in the first 3 to 4 months of life, presenting in 41 to 73% of infants.7–9 A large number of those infants regurgitate more than 4 times per day. Prevalence decreases to 14% at 7 months of age and to under 5% after 12 months of age.7,9
Many of the symptoms attributed to reflux are nonspecific and difficult to distinguish from other causes, often making the distinction between infant regurgitation and GERD a serious challenge, especially in younger infants.2
Excessive regurgitation is one of the symptoms of GERD, but the terms regurgitation and GERD should not be interchanged.10 Even though regurgitation is a typical symptom of GERD in infants, it is rare in older children and adults.
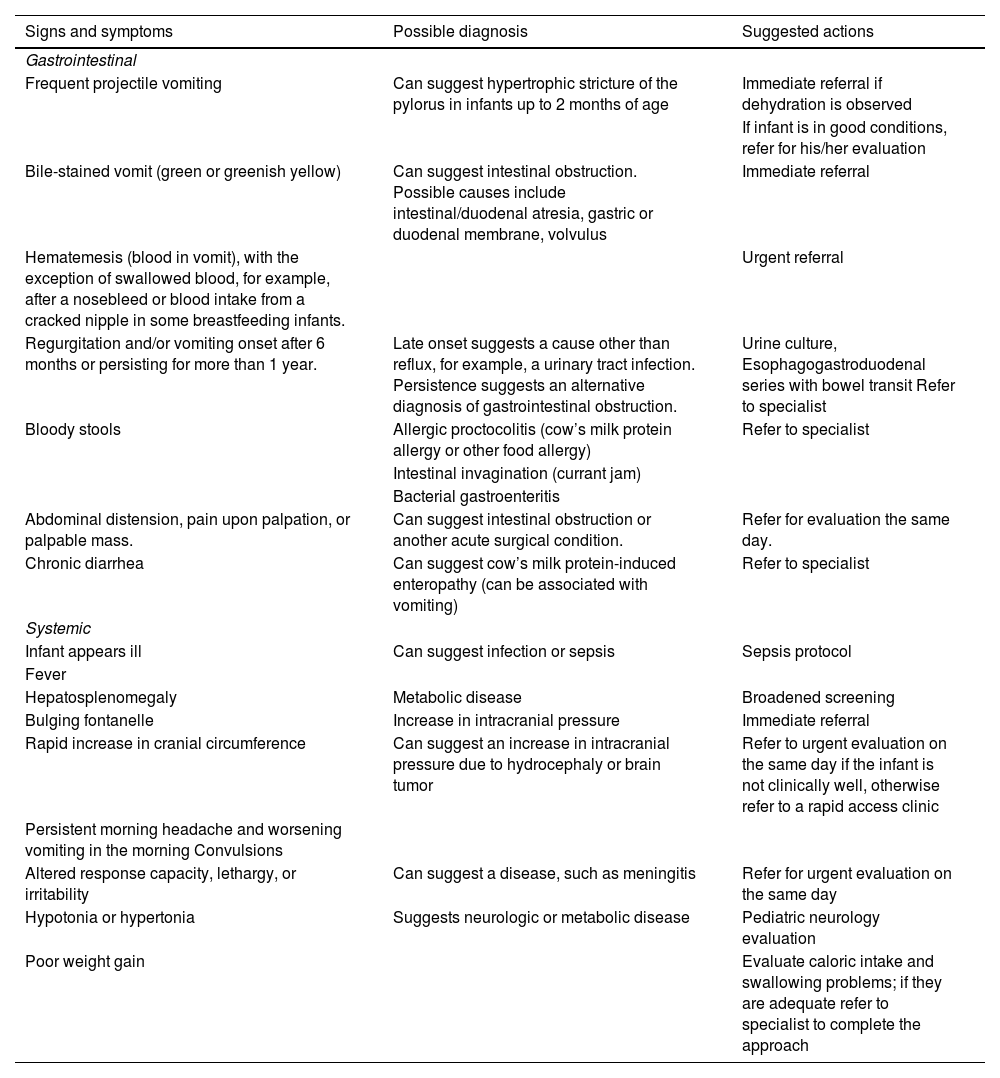
The presence of alarm signs should always be looked for in the patient with regurgitation (Table 1).2–5 Some of the characteristics suggestive of GERD include failure to thrive, irritability, feeding difficulty, sleep difficulty, crying episodes, and anemia. GERD in infants can also present with extraesophageal symptoms, such as cough, asphyxia, wheezing, and on rare occasions, apnea and/or a brief, resolved, unexplained event (BRUE), formerly known as an apparent life-threatening event (ALTE),11 but causality or temporal association have not been established in all subjects.12
Alarm signs.2–5
| Signs and symptoms | Possible diagnosis | Suggested actions |
|---|---|---|
| Gastrointestinal | ||
| Frequent projectile vomiting | Can suggest hypertrophic stricture of the pylorus in infants up to 2 months of age | Immediate referral if dehydration is observed |
| If infant is in good conditions, refer for his/her evaluation | ||
| Bile-stained vomit (green or greenish yellow) | Can suggest intestinal obstruction. Possible causes include intestinal/duodenal atresia, gastric or duodenal membrane, volvulus | Immediate referral |
| Hematemesis (blood in vomit), with the exception of swallowed blood, for example, after a nosebleed or blood intake from a cracked nipple in some breastfeeding infants. | Urgent referral | |
| Regurgitation and/or vomiting onset after 6 months or persisting for more than 1 year. | Late onset suggests a cause other than reflux, for example, a urinary tract infection. Persistence suggests an alternative diagnosis of gastrointestinal obstruction. | Urine culture, Esophagogastroduodenal series with bowel transit Refer to specialist |
| Bloody stools | Allergic proctocolitis (cow’s milk protein allergy or other food allergy) | Refer to specialist |
| Intestinal invagination (currant jam) | ||
| Bacterial gastroenteritis | ||
| Abdominal distension, pain upon palpation, or palpable mass. | Can suggest intestinal obstruction or another acute surgical condition. | Refer for evaluation the same day. |
| Chronic diarrhea | Can suggest cow’s milk protein-induced enteropathy (can be associated with vomiting) | Refer to specialist |
| Systemic | ||
| Infant appears ill | Can suggest infection or sepsis | Sepsis protocol |
| Fever | ||
| Hepatosplenomegaly | Metabolic disease | Broadened screening |
| Bulging fontanelle | Increase in intracranial pressure | Immediate referral |
| Rapid increase in cranial circumference | Can suggest an increase in intracranial pressure due to hydrocephaly or brain tumor | Refer to urgent evaluation on the same day if the infant is not clinically well, otherwise refer to a rapid access clinic |
| Persistent morning headache and worsening vomiting in the morning Convulsions | ||
| Altered response capacity, lethargy, or irritability | Can suggest a disease, such as meningitis | Refer for urgent evaluation on the same day |
| Hypotonia or hypertonia | Suggests neurologic or metabolic disease | Pediatric neurology evaluation |
| Poor weight gain | Evaluate caloric intake and swallowing problems; if they are adequate refer to specialist to complete the approach | |
There is a lack of correlation between crying, irritability, and GERD, and this disease is an uncommon cause of said behavior in otherwise healthy infants. Infant irritability or discomfort can accompany regurgitation and vomiting. However, in the absence of other alarm symptoms, the performance of additional diagnostic tests is not indicated.2–4
Excessive regurgitation rarely causes caloric insufficiency and malnutrition. Deficient weight gain is a crucial alarm sign that requires complete diagnostic study, with eventual hospitalization for performing diagnostic tests. Some infants may present with sucking or swallowing alterations; they have no apparent malformations and can be diagnosed with “nonorganic failure to thrive”, a disorder sometimes attributed to social/sensory deprivation or socioeconomic or primary maternal-child problems. Some reports state that deficient weight gain, feeding refusal, back arching, irritability, and sleep disturbances may or may not be related to GERD.2,13,14
MethodologyIn January 2022, the Asociación Mexicana de Gastroenterología put together the working group for the development of the present consensus. Pediatric gastroenterologists, pediatric endoscopists, pediatric neurogastroenterologists, and an international board-certified lactation consultant (IBCLC) participated.
Taking into account the leading international guidelines, the ADAPTE methodology (Appendix A)15 was employed, enabling us to adapt them for their use in different organizational and cultural contexts. This process has been designed to ensure that the adapted guidelines answer specific relevant health questions that are adequate for the needs, priorities, legislation, policies, and resources of the targeted setting. This method is designed to be flexible, depending on the application. The transparent and explicit reporting of the adaptation process enhances the quality and validity of the adapted guidelines.
The Executive Committee first carried out a search on MEDLINE, PubMed, and EMBASE for guides, consensuses, and guidelines on the diagnosis and treatment of GER and GERD in infants and children, published within the time frame of January 2009 and October 2022, in English and Spanish. Utilizing the AGREE instrument,16 the next step entailed retrieving the most relevant recommendations that met the criteria and were related to the infant group, from each of the documents selected.2–5
This initial recommendation list was examined at the first virtual meeting, to identify specific undiscussed gaps or errors in the wording or interpretation of the selected topics. The content was then divided into six sections and the members were incorporated into an equal number of work teams, thus forming the working group.
Each team evaluated the evidence and wrote the justification for the statements. When pertinent, information from recent publications not included in the reference guidelines was added.
The statements and justifications were endorsed or modified by the broad consensus development working group in Delphi rounds,17 to maintain member anonymity and subsequently facilitate opinion expression. Each statement was evaluated using a 3-point Likert scale: 1) in complete agreement, 2) in partial agreement, and 3) in disagreement. The statements that reached a consensus (defined as agreement > 80%) were accepted and those that did not (agreement < 80%) were re-evaluated, either to be eliminated or to be reformulated by the members of the corresponding work teams and undergo a second anonymous voting round.
At a final meeting, each of the recommendations and justifications were reviewed. The final drafting of the document was carried out and reviewed by all participating members.
Diagnosis and diagnostic aidsDiagnosis was based on clinical history, physical examination, and complementary tests. Anamnesis is essential, but the lack of specificity of GERD symptoms can result in the need to perform additional tests.18
Statement 1. Diagnostic tests are not required for children under one year of age, in the absence of alarm signsLevel of agreement: in complete agreement 100%.
Diagnostic tests are ordered for ruling out complications of GERD or evaluating differential diagnoses.2,3
Statement 2. The use of esophagogastroduodenoscopy for diagnosing gastroesophageal reflux disease (GERD) is not recommended due to its low sensitivity and specificityLevel of agreement: in complete agreement 100%.
Esophagogastroduodenoscopy (EGD) is not needed for the routine evaluation of infants with GERD2,19,20 because the study does not reflect the frequency of reflux under physiologic conditions, and infants with or without GERD can have episodes of GER seen during the study, making it neither sensitive nor specific. In selected cases, such as infants vomiting bile or with little weight gain, an upper gastrointestinal series can be useful for identifying anatomic anomalies (esophageal stricture, hiatal hernia, intestinal malrotation, infantile hypertrophic pyloric stenosis, prepyloric/antroduodenal membranes, duodenal strictures, tracheoesophageal fistula) and in postoperative fundoplication patients.2–4,20
Statement 3. The routine use of scintigraphy is not recommended for the diagnosis of GERDLevel of agreement: in complete agreement 100%.
We do not recommend the use of scintigraphy for diagnosing GERD due to its low sensitivity and lack of standardization of the technique. It can be performed to confirm pulmonary aspiration in patients with refractory respiratory symptoms or with recurrent aspiration pneumonia, but it is not recommended for other reflux symptoms. It can also be used to rule out gastric emptying delay as a causal or precipitating factor of symptoms related to GERD.2–4
Statement 4. We do not recommend the use of ultrasound for the diagnosis of GERD in infants and childrenLevel of agreement: in complete agreement 100%.
Ultrasound is not considered a diagnostic tool for GERD in infants under one year of age. The imaging study is indicated for ruling out other conditions that can manifest with the presence of vomiting, such as infantile hypertrophic pyloric stenosis.21
Statement 5. We do not recommend the routine use of esophagogastroduodenoscopy (EGD) for the diagnosis of GERD in childrenLevel of agreement: in complete agreement 100%.
Evidence is insufficient for recommending the routine use of EGD with biopsy for the diagnosis of GERD in infants and children.2,3 Upper gastrointestinal endoscopy can be of diagnostic benefit in infants that do not respond to empiric treatment. When endoscopy is performed, biopsies should be taken of the esophagus, stomach, and duodenum because they can reveal clinically significant diseases, even when the macroscopic appearance of the mucosa is normal.22 EGD is useful for evaluating the esophageal mucosa and detecting complications of GERD (esophagitis, stricture, Barrett’s esophagus), for diagnosing conditions that predispose to GERD (e.g., hiatal hernia), or for diagnosing conditions that can simulate GERD (eosinophilic esophagitis, infectious esophagitis, etc.).2 In patients with extraesophageal manifestations of GERD, the main indication for EGD is to detect reflux simulators (such as eosinophilic esophagitis, esophageal candidiasis) and to treat esophageal obstructions resulting from coughing and aspiration. In the absence of erosive esophagitis, microscopic esophagitis is insufficient for diagnosing the presence of GERD.2,4 Nevertheless, GERD can exist even with a normal appearing esophageal mucosa and the absence of histologic abnormalities.2
Statement 6. We do not suggest the routine use of esophageal manometry for the diagnosis and evaluation of GERD in infantsLevel of agreement: in complete agreement 92.3%, in partial agreement 7.7%.
Studies of esophageal manometric pressure and lower esophageal sphincter function have been utilized for ruling out esophageal motility disorders, such as rumination syndrome and esophageal achalasia, whose symptoms can simulate those of GERD.23
Statement 7. Esophageal pH monitoring should not be routinely performed for diagnosing GERD in infantsLevel of agreement: in complete agreement 84.6%, in partial agreement 15.4%.
Esophageal pH-monitoring is a quantitative measure of esophageal acid exposure, with established parameters and ranges. It measures the frequency and duration of episodes of acid reflux (pH < 4) through a series of parameters, such as the total number of reflux events in 24 hours, duration of the longest reflux event, or the most important total time with intraesophageal pH < 4 (reflux index: RI).
The pH-monitoring study enables the differentiation between physiologic reflux and pathologic reflux, considering the latter in infants, when the RI is above 10% or there are more than 100 acid reflux episodes in 24 hours, and in children above one year of age, when the RI is above 7% or there are more than 70 acid reflux episodes in 24 hours.13
Intraesophageal 24-h pH-monitoring has high sensitivity and specificity for diagnosing GERD. However, in the large majority of infants and children with reflux, pH-monitoring is not required for making the diagnosis. Indications13,24 for performing pH-monitoring are: 1) when there are symptoms suggestive of GER and progression is unfavorable despite establishing the correct treatment, 2) when it is relevant to determine the relation between GERD and extragastrointestinal symptoms, and 3) as an efficacy control of treatment, whether medical or surgical. Nevertheless, these indications should be individualized, according to the specific situation of each patient.
The main limitation of pH-monitoring is that it does not detect the presence of nonacid reflux, an entity that can appear in over half of infants with reflux. Likewise, it does not detect the extension of the reflex in the esophagus and it is difficult to establish the correlation between the symptoms experienced and the acid events, given that there is no consensus as to the time during which said events and symptoms are related, resulting in a low association between abnormal results of esophageal monitoring and GERD in this age group.25–27 In addition, the clinical symptoms of irritability, bradycardia, or desaturation episodes sometimes attributed to GERD in infants are poorly correlated with episodes of acid reflux.27
Despite those limitations, these studies can be useful in special rare situations, such as infants with mild episodes of severe symptoms (such as apnea, bradycardia, cough, or desaturation). In such a context, they are utilized together with the monitoring of respiratory rate, heart rate, or oxygen saturation to determine whether there is a temporal relation between reflux episodes and those events.28
Statement 8. Multichannel intraluminal impedance associated with pH monitoring (MII-pH) should not be used as the single tool for diagnosing GERD in infantsLevel of agreement: in complete agreement 84.6%, in partial agreement 7.7%, in disagreement 7.7%.
When esophageal reflux monitoring is performed, the ideal technique is to measure both the esophageal pH and the multichannel intraluminal impedance, on a single device, for 24 hours. Multichannel intraluminal impedance associated with pH monitoring (MII-pH) is recommended as the preferred technique for measuring gastroesophageal acid and nonacid reflux. Compared with pH-monitoring, impedance has the advantage of being independent of the pH value, and consequently is better adapted to measuring reflux, especially in the postprandial period when reflux is buffered, and for detecting the symptoms associated with nonacid or weak acid reflux. MII-pH is currently considered the gold standard for evaluating the symptoms of GERD.29 Its indications are the same as those for esophageal pH-monitoring. Currently, there are some indices that allow us to determine whether there is a correlation between reflux events and the symptoms recorded through statistical correlations, but normal reference values are not yet available.
Its indications are:
- •
To correlate symptoms with acid and nonacid reflux events.
- •
To clarify the role of acid reflux in the etiology of esophagitis and other signs and symptoms suggestive of GERD.
- •
To determine the efficacy of acid suppression.
Level of agreement: in complete agreement 92.3%, in partial agreement 7.7%.
A PPI diagnostic test is based on the hypothesis that the symptoms are related to acid. PPI use in controlled trials showed no symptom improvement compared with placebo. Regardless of trial duration, the administration of a PPI cannot be recommended for infants as a therapeutic test,30 nor are there sufficient data for recommending a PPI trial in patients with extraesophageal symptoms that are possibly related to GERD.22
TreatmentInitial treatment of GERD in pediatrics includes a combination of conservative measures, lifestyle changes, or dietary modifications. Pharmacologic measures can sometimes be used, and surgical treatment is rare (see Appendix B-Description of the algorithm).
Nonpharmacologic treatmentStatement 10. Infant regurgitation management should focus on parental education and supportLevel of agreement: in complete agreement 100%.
Parental education and support are essential, emphasizing that regurgitation is a physiologic and self-limited process and that symptoms are resolved in 98% of cases before the first year of life. Understanding the natural history of regurgitation can help reduce parental anxiety, medical referrals, and overtreatment.2–5
Statement 11. In children with regurgitation and GERD, we recommend promoting exclusive breastfeeding and avoiding its suspensionLevel of agreement: in complete agreement 100%.
Studies show that exclusively breastfed infants, especially between 2 and 6 months of age, have less probability of experiencing regurgitation, suggesting that maternal breastfeeding is a protective factor,7,31 as well as being associated with faster resolution.32 Some studies have shown that partially breastfed infants (i.e., infants that are both breastfed and bottle-fed with formula)7,31,33 have more reflux events, compared with exclusively breastfed infants, whereas other studies have reported no difference,34–37 as also occurs with the introduction of complementary feeding.31,37
Statement 12. in bottle-fed children (whether with breast milk or formula), evaluating weight gain, before modifying the volume and/or frequency of feeding, is suggested; in breastfed children, the recommendation is to instruct the mother in responsive feeding, through which she is able to recognize the signs of hunger and thirst, given that overfeeding can increase regurgitation frequencyLevel of agreement: in complete agreement 100%.
Early hunger signs are recognized when infants display behaviors, such as sticking out their tongue, touching their mouth with their hands, sucking their fist, and searching for the breast when awake. Crying is a late hunger sign and promotes aerophagia. Satiety signs are a relaxed body with open fists, voluntary release of the nipple, turning the head away or closing the lips when offered the breast, sucking slowly, or falling asleep at the breast.38 The ability of the infant to self-regulate milk consumption has been postulated as a factor associated with reduced reflux, which is also a reason why the manner in which infants are breastfed can influence the incidence of reflux. Directly breastfed infants have a greater capacity for self-regulating milk intake, compared with infants that have mixed feeding or are bottle-fed.31 An important effect of bottle feeding is that caretakers can ignore satiety signs, increasing the possibility of the infant’s receiving a larger volume of milk, which can result in a higher probability of developing reflux. Reflux can cause anxiety in both the infant and caregiver, leading to changes in the infant’s feeding behavior, even interrupting maternal breastfeeding.8 Breastfed infants with GERD can have feeding problems,39 which can lead the caretaker to utilize the bottle, in an attempt to alleviate the anxiety of the infant. In addition, exclusively breastfed infants often have fewer and shorter reflux episodes,7,8 with faster gastric emptying.4 These factors can lead to a lack of perception of reflux severity by the caregiver, and so it is reported less often.8
Infants with physiologic reflux have normal feeding behavior and generally feed frequently. In contrast, one of the concerning alarm signs in the evaluation of children with GERD is precisely food refusal, which can cause failure to thrive.
Statement 13. In breastfed infants that present with regurgitation, strict attention to the breastfeeding technique is recommendedLevel of agreement: in complete agreement 92.3%, in partial agreement 7.7%.
A complete evaluation carried out by trained personnel to assess the breastfeeding technique employed is important and includes positioning, attachment (latch-on), milk transfer, and effective sucking to optimize breastfeeding and prevent regurgitation.
The overproduction of maternal milk can cause symptoms of regurgitation, aerophagia due to superficial latching, gas, colic, explosive stools, choking, cough, crying during feeds and/or breast refusal.40
Aerophagia in the breastfeeding infant can promote regurgitation, especially in cases in which the feeding technique fails, such as shallow latch and inadequate seal around the breast due to poor attachment, and when there are anatomic problems, such as ankyloglossia, bubble palate, and short labial frenulum.41,42
Excessive volumes of milk during feeds can cause regurgitation, and so some documents2–4 recommend considering offering lower volumes and more frequent feeds, without incurring risks or extra costs. However, not all infants accept that, and with direct breastfeeding, the ingested volume cannot be measured, making other measures, such as choosing a position that does not exert intra-abdominal pressure, burping after feeding, and maintaining an upright position 15 to 20 minutes after feeding, possibly more useful. Keeping the infant in the same position for 30 minutes after feeding may help reduce regurgitation.43,44
Statement 14. In formula-fed infants, thickened formulas can be used to reduce visible regurgitationLevel of agreement: in complete agreement 84.6%, in partial agreement 15.4%.
Current guidelines for GERD recommend thickening as a first-line approach for treating GERD in infants and young children.2,3 It thickens the feeds, so they stay in the stomach longer, reducing the risk of regurgitation. MII-pH studies on otherwise healthy full-term infants have shown that thickeners decrease visible regurgitation by reducing the maximum height reached by the refluxate.45,46 Several studies utilizing different types of feeding thickeners, with trials varying from 1 to 8 weeks, showed no superiority of one over the other.45 The use of thickeners reduced reflux to 2 episodes/day and reduced parental anxiety most likely due to the placebo effect.47 Thickeners can sometimes cause diarrhea because of the increase in osmolality and weight gain from high caloric density. Paradoxically, the slowing of gastric emptying can increase reflux symptoms in some infants. There is no evidence that thickeners are useful in premature infants and some studies report a higher incidence of necrotizing enterocolitis.48
Commercial formulas are preferred to standard thickened formulas because of their better viscosity, digestibility, and nutritional balance. In the Mexican market, different anti-regurgitation (AR) formulas are available that contain pregelatinized starch from rice, tapioca, potato, and corn, as well as carob flour and xanthan gum, among others. However, as mentioned above, there are currently no clinical trials that compare one thickener with another. Oatmeal and rice cereals are an excellent option for thickening formulas because they are effective, accessible, and inexpensive, but they are not recommended for thickening breastmilk because its amylase dissolves them.
Statement 15. We do not recommend the routine use of extensively hydrolyzed formulas or amino acid-based formulas for managing infant physiologic reflux (regurgitation)Level of agreement: in complete agreement 84.6%, in partial agreement 15.4%.
Cow’s milk protein allergy (CMPA) is a common entity in pediatric consultation and shares symptoms with GERD, which can make the differential diagnosis difficult. Therefore, despite the lack of evidence supporting their routine use in the management of GERD symptoms, some guidelines suggest utilizing extensively hydrolyzed formulas or amino acid-based formulas for two to four weeks. If there is a favorable result, an oral challenge should be carried out to confirm or rule out the diagnosis of CMPA. This conduct is not justified in the infant with regurgitation. The elimination of cow’s milk protein from the maternal diet can aid in reducing reflux symptoms, in addition to improving esophageal acid exposure and mucosal integrity,49 and so breastfed infant patients can benefit from said strategy.
Statement 16. We do not recommend position changing as part of the management for improving regurgitationLevel of agreement: in complete agreement 92.3%, in disagreement 7.7%.
Due to the association of the prone and left lateral positions with a risk for crib death, those positions are not recommended.2,3,50 In addition, seated or semi-seated positions for infants under one year of age have not been shown to be efficacious in the management of GER, due to the increase in muscle tone and intra-abdominal pressure.51
Pharmacologic treatmentStatement 17. We suggest the use of alginates as an alternative to formula thickening for breastfed infants or as a therapeutic test in infants that persist with symptoms despite nonpharmacologic treatmentLevel of agreement: in complete agreement 92.3%, in disagreement 7.7%.
Alginates are natural polysaccharides isolated from brown seaweed. Alginate-based formulations act on GER through chemical and physical mechanisms. In the presence of gastric acid, they react forming a low-density viscous gel that tends to float, the so-called gel rafts. Upon finding that calcium increases the strength of the raft, pharmaceutical formulations have been developed with calcium carbonate. How calcium increases raft strength is attributed to its capacity to cross-link alginic acid polymers, enabling the gel to form an “eggbox” structure that gives it great strength. On the other hand, bicarbonate can also be added to alginates, which act as a carbon dioxide (CO2) production system, in such a way that the CO2 bubbles that form in the presence of gastric acid, remain trapped with the gel matrix, converting it into a foam that floats.52
Using aluminum-free formulations and considering the sodium content (variable according to different commercial products) is recommended for prolonged use, particularly in premature neonates and children with kidney disease.
A systematic review on the efficacy and safety of liquid alginate-based formulations for reducing GER in neonates and infants that includes two studies suggests there is significant symptom improvement with said formulations as intervention. No significant adverse events were observed, signifying that this treatment option is generally safe for use in infants.53
The study by Salvatore et al.54 suggests that alginate can reduce symptoms related to GER in infants, can reduce episodes of acid, nonacid reflux measured through MII-pH. Nevertheless, those authors express the need for conducting randomized, double blind, placebo-controlled trials to confirm their findings, concluding that alginate is a treatment option for symptoms related to GER in infants.
Statement 18. We do not recommend the routine use of prokinetics in the treatment of infants with regurgitationLevel of agreement: in complete agreement 92.3%, in disagreement 7.7%.
Despite the wide use of prokinetics in pediatrics for the treatment of GERD, there is still not enough evidence to confirm its efficacy. Systematic reviews on metoclopramide, domperidone, and cisapride have not found solid proof of their efficacy,55–57 and the international guidelines do not recommend their routine use. These medications have potentially serious adverse effects. For example, metoclopramide is associated with extrapyramidal neurologic symptoms and cisapride and domperidone are implicated in cardiac arrythmias. Therefore, adding a drug with debatable efficacy and possible adverse effects is questionable and the risk for adverse effects outweighs the benefit.58
Statement 19. PPIs and histamine H2 receptor antagonists (H2RAs) are not recommended for healthy infants that present with crying or irritability with/without regurgitationLevel of agreement: in complete agreement 100%.
Acid secretion inhibitors, such as the H2 receptor blockers and PPIs have been used for managing irritability in infants with suspected GERD, contrary to international recommendations.59,60
A 2015 systematic review61 and its 2022 update62 conclude that available data suggest that PPIs are not efficacious for treating crying and irritability in infants and their use has been related to an increase in infections, particularly diarrhea caused by Clostridioides difficile, as well as an increase in the risk of fractures.
A prospective study that simultaneously evaluated restlessness, crying, irritability, and GER through symptom quantification and evaluation based on the Face, Legs, Activity, Cry, Controllability (FLACC) scale and MII-pH found a temporal association between crying and GER in half of the episodes of irritability. No significant differences were observed in the FLACC score between episodes of irritability associated with or without GER, and nonacid reflux was perceived to be at least as painful as acid reflux. These results emphasize that acid inhibitors should not be started in infants that present with crying unless there is a clear association with acid GER.63
Statement 20. We recommend PPI use as first-line treatment in reflux related to erosive esophagitisLevel of agreement: in complete agreement 100%.
Data on the prevalence and severity of erosive esophagitis (EE) in young children is limited. The prevalence of EE confirmed by endoscopy and biopsy was reported at 29% in a study on 209 patients with GERD, whose age ranged from 18 months to 10 years and who had no neurologic alterations or congenital esophageal malformations.64 A retrospective review from the Pediatric Endoscopy Database System-Clinical Outcomes Research Initiative (PEDS-CORI) showed that 12.4% of the 7,188 children under 18 years of age that underwent endoscopy presented with EE.65 A retrospective cross-sectional study that collected information from 12 pediatric hospitals in the United States also reported that 9.5% of children one year of age and 7.6% of children 2 years of age had EE.65 Esophagitis can manifest as irritability, nausea, and feeding aversion; it rarely manifests as hematemesis or melena, anemia, weight loss, failure to thrive, or Sandifer syndrome. There is evidence of endoscopic and/or histologic remission, with symptom improvement, in patients that received PPIs.66–68
Statement 21. H2RAs can be used in cases in which PPIs are not available or are contraindicatedLevel of agreement: in complete agreement 84.6%, in partial agreement 15.4%.
Although it is clear that H2RAs can be beneficial and efficacious in EE or frank gastritis, the majority of infants with GERD do not present with those complications, and so the decision to treat all infants with GERD with H2RAs should not be precipitated, especially given the risks associated with their use. There is no good correlation between symptoms and the presence of reflux esophagitis, nor can any sign or symptom predict which infants would benefit from H2RA use.69,70
Among the H2RAs, cimetidine, ranitidine, famotidine, and nizatidine have traditionally been used in the majority of countries. Famotidine and nizatidine have been authorized for use in children in the United States but not in Europe.
Tachyphylaxis is an important disadvantage that seriously restricts their long-term use.71
Starting in 2019, the US Food and Drug Administration (FDA) found levels of N-Nitrosodimethylamine (NDMA) that exceeded acceptable ingestion limits in many medication batches, including ranitidine. This resulted in the generalized removal of several products and caused concern in patients and physicians, given that NDMA was classified as a possible carcinogen for humans. NDMA can form as a result of ranitidine degradation, especially after the expiration date, but it can also form from ranitidine inside the body. As a precaution, in April 2020, both the FDA and the European Medicines Agency (EMA) ordered manufacturers to remove all prescription and over-the-counter ranitidine products from the market because NDMA levels can increase over time if the medication is stored above room temperature, reaching dangerous levels.72
This same recommendation was adopted in Mexico and ranitidine was removed from the market. Currently there are no formulations of H2RAs for infants.
Surgical treatment and new therapeutic optionsStatement 22. Before performing antireflux surgery, we suggest a detailed evaluation with other diagnostic methods that rule out complications and GERD secondary to another diseaseLevel of agreement: in complete agreement 100%.
Antireflux surgery primarily includes fundoplication, as well as other procedures designed to reduce the passage of gastric content into the esophagus. Indications for surgery have not been clearly established, success and failure rates vary widely, and complications frequently occur. Diagnostic tests and surgical considerations depend on the clinical presentation of the patient with the goal of ruling out conditions that present with symptoms of GERD, and in some cases, demonstrate its presence.2–5
Statement 23. In patients with GERD that do not respond to medical treatment, evaluation by a pediatric gastroenterologist is recommended, for considering other therapeutic options, such as transpyloric tube feeding, before surgical treatmentLevel of agreement: in complete agreement 84.6%, in partial agreement 15.4%.
An alternative to antireflux surgery is transpyloric or jejunal feeding through nasojejunal or gastrojejunal tubes or jejunostomies that reduce reflux by bypassing the stomach.2 Efficacy is variable, and its utility is limited because the tube can easily be dislodged, along with other practical considerations.73
Statement 24. Surgical intervention is reserved for patients with refractory symptoms or potentially lethal complications and for chronic conditions with a significant risk for complications associated with GERD, despite optimum medical treatmentLevel of agreement: in complete agreement 100%.
Surgical indications for GERD in pediatrics are at times controversial, especially in infants, due to anatomic and physiologic peculiarities.74 In addition to there being no standardized evaluation of GERD in children that are programmed for surgery, diagnostic tests have limitations.
As previously described, EGD can be useful for ruling out anatomic abnormalities but its usefulness in diagnosing GERD is a subject of debate. The sensitivity of pH-monitoring is low in pediatric patients, given that clinical signs are poorly correlated to its results. Diagnostic yield is improved through MII-pH study, but normal values are not available in pediatrics. Upper gastrointestinal endoscopy does not always show validating pathologic findings of esophagitis for the diagnosis of GERD. Lastly, high-resolution esophageal manometry can be a useful tool, but it is available in very few centers.
Surgical indications for GERD result from an objective analysis of the patient that includes a clinical history specifying symptoms, the search for alarm signs, previous treatments carried out, patient characteristics, and associated diseases, such as esophageal atresia, congenital diaphragmatic hernia, bronchopulmonary dysplasia, and encephalopathy with associated neurologic decline that tends to be related to alterations in the swallow mechanism. Another group of patients are the infants that present with BRUE (previously known as ALTE) or crises of recurrent apnea.75
From the surgical perspective, Nissen fundoplication is the surgical technique of choice in the majority of operated patients, generally through the laparoscopic approach. Nevertheless, although some surgical centers perform hundreds of antireflux operations annually, others debate the need for and efficacy of fundoplication, and instead, promote better medical treatment, noninvasive feeding methods, and other surgical interventions, such as gastrostomy without fundoplication or gastrojejunal feeding.76
In patients with feeding problems due to alterations of the swallow mechanism, poor weight gain, or GERD, the placement of a percutaneous endoscopic gastrostomy (PEG) or surgical gastrostomy can be considered. However, the routine practice of adding fundoplication to those patients is no longer recommended because said procedure increases the risk for complications and does not improve reflux-related results.77–79
If bronchoaspiration-related lung disease is the indication for fundoplication, a comprehensive and multidisciplinary approach is very important because of the difficulty in differentiating retrograde bronchoaspiration (due to GERD) from anterograde bronchoaspiration (due to a swallow disorder). Even though fundoplication can reduce GER, it can also impede esophageal emptying and increase the possibility of aspiration. Transpyloric feeding or feeding through gastrostomy can also be considered in this group of patients.80
Other surgical procedures, such as radiofrequency ablation of the lower esophageal sphincter, endoscopic full-thickness serosa-to-serosa fundoplication, and total esophagogastric dissociation have been described mainly in adults and have not been evaluated in infants. At present, none of these techniques is recommended as primary therapy for GERD in children.2 Total esophagogastric dissociation can be adequate as a rescue procedure in children with neurologic impairment in whom fundoplication has failed, but because of the invasiveness of the procedure, a comprehensive evaluation is required, in order to make the appropriate diagnosis.
Antireflux procedures are more frequently performed in children during the period of life when regurgitation is normal and objective and physiologic measures of GERD are difficult to interpret. To identify significant results after surgery, indications should be clear and standardized. We must clarify the adequate study for infants and young children with GERD and better define “medical treatment failure” in that patient population.75
Statement 25. We recommend referring children with GERD to a pediatric gastroenterologist if- •
There are alarm signs and symptoms suggestive of an underlying gastrointestinal disease
- •
Patients do not respond to optimal treatment
- •
Pharmacologic treatment cannot be withdrawn after 6 to 12 months
Level of agreement: in complete agreement 100%.
Ethical considerationsBecause this is a review document with no patient participation, no informed consent or ethics committee approval was required. Intellectual property is respected, credit is given to the authors of the sources utilized in the present document, and all applicable norms and regulations are met.
The process has been designed to ensure that the adapted guidelines answer specific relevant health questions that must also be adequate for the needs, priorities, legislation, policies, and resources of the targeted setting. This method is designed to be flexible, depending on the application. The transparent and explicit reporting of the adaptation process will enhance the quality and validity of the adapted guidelines.
The methodological framework defined by the ADAPTE collaboration consists of three main phases: 1) The set-up phase establishes the areas to be completed before the adaptation process, as such; for example, identifying the resources and skills needed for the task; 2) The adaptation phase helps the users advance from the identification of the clinical theme to the identification of specific health questions, to the search for and evaluation of the guidelines, to the selection of the appropriate guidelines, and to the preparation of the drafting of the adapted guidelines; and 3) The finalization phase includes the external review of the adapted guidelines, with feedback from the different groups impacted by the guidelines, consulting with the developers of the source guidelines utilized in the adaptation process. In addition, a process of guideline review and updating is established and the final document is produced.
| Phase | Tasks | Associated modules |
|---|---|---|
| Configuration | Adaptation process preparation | Definition of the goal |
| Establishing the general reference terms | ||
| Initital definiions regarding consensus | ||
| Adaptation | Clinical question determination | a) The population of interest and the characteristics of the disease/condition |
| b) The interventions of interest | ||
| c) The professionals at whom the clinical guidelines are directed | ||
| d) The expected outcomes, including those related to patients, the organization, or aspects of public health | ||
| e) The area and context in which the consensus will be implemented | ||
| The search for clinical guidelines and other documents | MEDLINE, PubMed, and Embase | |
| Evaluation | Evaluation of the methodological quality of the documents selected utilizing an instrument prepared by a group of international researchers called the AGREE (Appraisal of Guidelines Research & Evaluation) collaboration | |
| Selection | Documents that answer the previously established relevant clinical questions | |
| Drafting of the recommendations | Based on the discussions and analyses described in the previous phase, the first list of recommendations is formulated | |
| Finalization | External review | The final list of recommendations is sent for review by the consensus working group utilizing the Delphi method. The aim is to evaluate the degree of consensus to produce the final list of recommendations |
| Future review and update planning | The recommendations should be systematically and thoroughly reviewed at a maximum period of five years | |
| Final document |
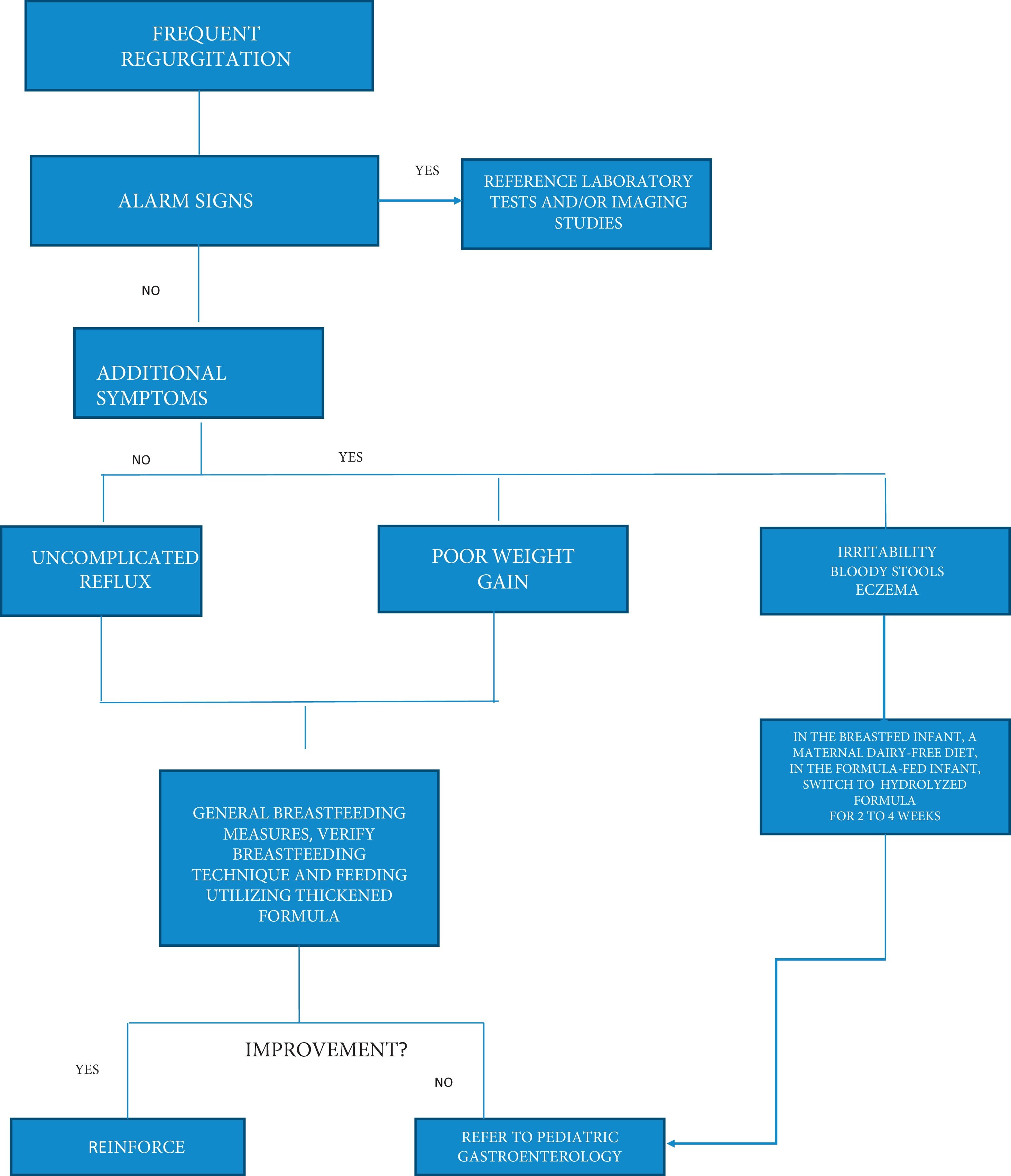
- 1
Diagnosis begins with a complete clinical history and complete physical examination. The first goal is to rule out the presence of “red flags” or “alarm signs”, eliminating the most frequent differential diagnoses that can present with similar histories and signs and symptoms. In the absence of alarm symptoms, no diagnostic tests at the primary healthcare level are recommended.
- 2
In infants with excessive regurgitation, in the absence of alarm signs and symptoms, with adequate weight gain, the explanation of why infants regurgitate (large proportional food volume, liquid feeding, supine position) and natural symptom progression (spontaneous improvement between 6 and 18 months, in the large majority) reduces parental anxiety and treatment demand. Regurgitation is rarely a reason to stop breastfeeding. In non-breastfed infants, the use of thickened formula is recommended, which will reduce regurgitation, and in turn, parental worry. The use of thickened formula can be evaluated in two weeks from its initiation, and if there is improvement, can be continued up to 6 months of age, or up to 12 months of age if the symptoms reappear with unthickened formula.
- 3
In children with low weight or poor weight gain, the recommendation is to first evaluate the caloric intake and whether there are swallowing problems. If caloric intake is adequate, causes of regurgitation and weight loss other than GERD should be evaluated, ordering complete blood count and blood chemistry. In specific cases of severe failure to thrive and/or delayed weight gain, additional evaluation to detect other diseases should be carried out.
- 4
In children with suspected CMPA, a test with extensively hydrolyzed formula is recommended for 2 to 4 weeks, especially in cases in which the infant has other symptoms suggestive or indicative of atopic diseases, such as atopic dermatitis. If the infant is breastfed, a maternal dairy-free diet is recommended for a period of 2 to 4 weeks, to evaluate its efficacy. If the infant is formula-fed, the formula should be changed to an extensively hydrolyzed formula and CMPA management followed, with a challenge after 2 to 4 weeks. The extensively hydrolyzed formula should be continued up to 12 months of age or for at least 6 months, whichever occurs first.
If nutritional management is unsuccessful, referral to a pediatric gastroenterologist is recommended.